Background: Minimal inhibitory concentration (MIC) measurements are usually
conducted using the agar plate dilution test or the microdilution test. Methods
for rapid evaluation of antimicrobial susceptibility using ATP measurements with
luciferin-luciferase reagents and a tetrazolium salt have been reported.
Material and methods: We recently measured the minimal inhibitory
concentrations (MICs) of various antimicrobial agents against 96 strains of Escherichia coli (1 standard strain and 95 clinically isolated strains) with Cell Counting Kit-8 containing a tetrazolium salt and an electron carrier using the Dry
Plate Eiken for MIC measurement and compared the results with that obtained
using the standard method.
Results: Agreement exceeded 90% for ampicillin, cefotiam, ceftazidime, flomoxef,
aztreonam, imipenem, meropenem, gentamicin, and levofloxacin, and complete
agreement exceeded 90% for cefotiam, ceftazidime, flomoxef, aztreonam,
imipenem, meropenem, and levofloxacin. Agreement was in the range of 78.1-
86.5% for piperacillin, cefazolin, cefpodoxime, amikacin, and minocycline with
complete agreement in the range 55.2-78.1%. Cefaclor had the lowest agreement
and complete agreement (42.7% and 38.5, respectively). For the antimicrobial
agents with low agreement (piperacillin, cefaclor, cefazolin, amikacin and
minocycline), MICs measured by the short-time method were often below onehalf
or one-fourth of that measured by the standard method, and regrowth of
the bacteria appeared to occur 6 hours after the start of the short-time method.
Conclusion: The results of this study indicate that measuring absorbance with
Cell Counting Kit-8 enables counting of viable bacteria. The use of this method
for measuring MICs (short-time method) allowed the results of the susceptibility
testing to be obtained in 6 hours, and the results correlated well with the results
obtained using the standard method. Therefore, this method appears useful for
the early detection of drug-resistant bacteria.
Keywords
Antimicrobial agents; Minimal inhibitory concentrations (MICs); Tetrazolium salt; Rapid test
Introduction
Tests commonly performed for evaluating antimicrobial susceptibility include the disk diffusion and the microdilution tests. Minimal inhibitory concentration (MIC) measurements are usually conducted using the agar plate dilution test [1] or the microdilution test [2]. With these tests, results are usually obtained in approximately 18-24 hours.
Methods for rapid evaluation of antimicrobial susceptibility using ATP measurements with luciferin-luciferase reagents [3-6] and a tetrazolium salt [7-14] have been reported. The ATP measurement method allows the results to be obtained in a short time and the method is useful for counting bacteria. We previously measured bacterial growth during exposure to antimicrobial agents by measuring ATP in bacteria to evaluate the antibacterial activities of agents and compared the results obtained by this method with the data obtained by the microdilution test, which has been adopted as the standard method by Clinical and Laboratory Standards Institute [15]. However, ATP measurements requires a special device, and thus, these measurement can only be performed in a limited number of facilities. Furthermore, ATP measurements cannot be conducted on many samples simultaneously. Therefore, the method using ATP measurements is not practical in daily clinical practice.
Cell Counting Kit-8 (Dojindo Laboratories, Japan) is composed of a combination of tetrazolium salt (2-(2-methoxy-4- nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2Htetrazolium, monosodium salt: WST-8) [16] and an electron carrier (1-methoxy-5-methylphenazinium methylsulfate:1- methoxy PMS). Cell Counting Kit-8 enables counting of viable cells as follows: PMS is reduced within the cells, causing it to move out of the cells into the medium, and WST-8 in the medium is reduced by the reduced PMS to an extent proportional to the number of viable cells. Measurement with Cell Counting Kit-8 only requires the use of an ordinary absorption spectrometer. The use of Cell Counting Kit-8 also enables macroscopic examination of discoloration. We used this Kit for measurement and compared the results obtained using this method (short-time method) with that obtained using the standard method in relation to the viable cell count and the agreement of MICs calculated for various agents against clinically isolated Escherichia coli strains.
Material and Methods
Correlation between bacterial count and absorbance
Suspensions of random concentrations were prepared using 1 standard strain (ATCC25922) and 2 clinically isolated E. coli. Strains. Each suspension was serially diluted at a common ratio of 1:2. Each suspension was then applied at a volume of 100 μl to the microplate and mixed with Cell Counting Kit-8 (10 μl), followed by incubation for 2-5 hours. At the end of the incubation period, the absorbances were measured with a microplate reader (wavelength/reference wavelength: 450 nm//630 nm). Then, the correlations between the pre-culture viable bacterial count and absorbance were analyzed.
MIC measurement with the standard and shorttime methods
MICs of antimicrobial agents against 1 standard strain (ATCC25922) and 95 clinically isolated E. coli strains were measured with a Dry Plate Eiken (Eiken Chemical Co., Ltd). The antimicrobial agents used and their concentrations were as follows: ampicillin, 0.5-32 μl/ml; piperacillin, 0.5-64 μl/ml; cefazolin, 0.5-32 μl/ml; cefotiam, 0.5-32 μl/ml; ceftazidime, 0.5-32 μl/ml; cefaclor, 0.5-32 μl/ml; flomoxef, 0.5-32 μl/ml; cefpodoxime, 0.25-8 μl/ml; aztreonam, 0.5-32 μl/ml; imipenem, 0.5-16 μl/ml; meropenem, 0.5-16 μl/ml; gentamicin, 0.5-16 μl/ml; amikacin, 0.5-32 μl/ml; minocycline, 0.5-16 μl/ml and levofloxacin, 0.5-8 μl/ml.
For MIC measurement by the standard method, a bacterial suspension (5 × 105 colony forming units (CFUs) /ml) was prepared with Mueller-Hinton liquid medium and applied to each well of a microplate (100 μl/well), followed by 20 hours of incubation at 35°C to determine MIC. In the short-time method, Cell Counting Kit-8 (10 μl) was added to a microplate prepared in the same way as described for the standard method, followed by 6 hours of incubation at 35°C to measure the absorbance. In the measurement of MICs using the short-time method, absorbance values of over 0.100 after 6 hours of incubation were indicative of positive bacterial growth.
Results
Correlation between bacterial count and the absorbance value
Graphically presents the time-course of changes in the absorbance in relation to the pre-culture viable bacterial count. When the incubation time was short (2-3 hours), the absorbance was low, even though the density of the bacterial inoculum was high (Figure 1). When the density of the bacterial inoculum was 1.0 × 105 CFUs, a high absorbance was obtained after 4 hours of incubation, whereas when the density of the bacterial inoculum was 1.0 × 104 CFUs, a high absorbance was obtained after 5 hours of incubation. Thus, absorbance values measured with the Cell Counting Kit-8 were correlated with the pre-culture viable E. coli count, indicating that this method enables determination of the viable bacterial count within a short time.
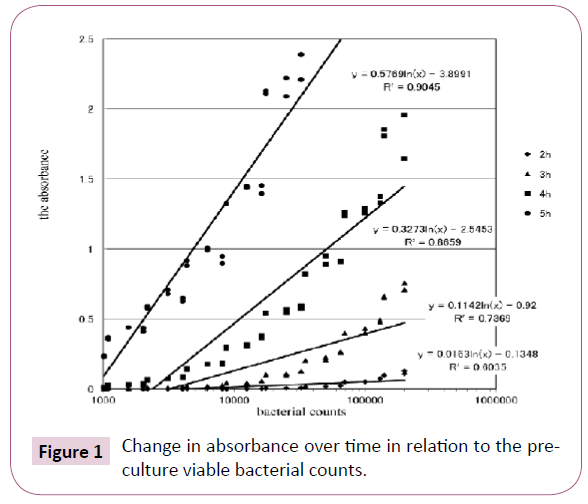
Figure 1: Change in absorbance over time in relation to the preculture viable bacterial counts.
Illustrates the changes in absorbance over time in relation to the pre-culture viable bacterial counts. Even a bacterial suspension that exhibited low absorbance after 2 hours of incubation exhibited elevation of the absorbance value to over 0.1 after 5 hours of incubation (Figure 2).
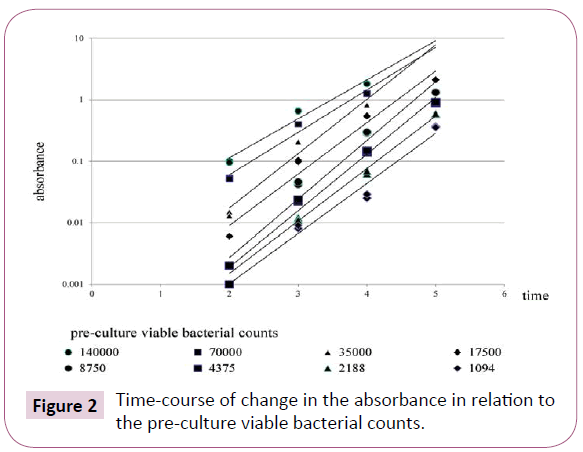
Figure 2: Time-course of change in the absorbance in relation to the pre-culture viable bacterial counts.
E. coli susceptibility to antimicrobial agents
The distribution of the susceptibility of 96 clinical E.coli isolates to antimicrobial agents (Table 1). The percentage of strains with susceptibility to each agent below the break point was as follows: ampicillin, 53.1%; piperacillin, 74.0%; cefazolin, 91.6%; cefotiam, 92.7%; ceftazidime, 97.9%; cefaclor, 89.6%; flomoxef, 99%; cefpodoxime, 89.6%; aztreonam, 96.8%; imipenem, 100%; meropenem, 100%; gentamicin, 89.5%; amikacin, 100%; minocycline, 85.5%; and levofloxacin, 71.9%.
| |
MIC range |
MIC50 |
MIC90 |
| ampicillin |
2?>32 |
4 |
>32 |
| piperacillin |
? 0.5?>64 |
4 |
>64 |
| cefaclor |
? 0.5?>32 |
1 |
8 |
| cefazolin |
? 0.5?>32 |
? 0.5 |
? 0.5 |
| cefotiam |
? 0.5?32 |
? 0.5 |
? 0.5 |
| ceftazidime |
? 0.5?>32 |
1 |
16 |
| flomoxef |
? 0.5?>32 |
? 0.5 |
? 0.5 |
| cefpodoxime |
? 0.25?>8 |
0.5 |
2 |
| aztreonam |
? 0.5?>32 |
? 0.5 |
? 0.5 |
| imipenem |
? 0.5?1 |
? 0.5 |
? 0.5 |
| meropenem |
? 0.5?1 |
? 0.5 |
? 0.5 |
| gentamicin |
? 0.5?2 |
? 0.5 |
16 |
| amikacin |
? 0.5?8 |
2 |
2 |
| minocycline |
? 0.5?>16 |
? 0.5 |
8 |
| levofloxacin |
? 0.5?>8 |
? 0.5 |
>8 |
Table 1: Distribution of MICs of 15 antimicrobial agents for 96 strains of E.coli.
MIC measurement with standard and short-time methods
MICs determined using a microplate were compared between the standard method and the short-time method. Complete agreement (the same MIC obtained using both methods) and agreement (MICs obtained by the two methods were within 1 log2 dilutions) were analyzed.
Agreement was over 90% for ampicillin, cefotiam, ceftazidime, flomoxef, aztreonam, imipenem, meropenem, gentamicin, and levofloxacin, and complete agreement was over 90% for cefotiam, ceftazidime, flomoxef, aztreonam, imipenem, meropenem, and levofloxacin (Table 2).
| ? |
? -2 |
? -1 |
-1 |
0 |
1 |
? +1 |
? +2 |
% Complete agreement |
% Agreement |
| ampicillin |
2 |
2 |
2 |
84 |
5 |
1 |
0 |
87.5 |
94.8 |
| piperacillin |
6 |
8 |
15 |
53 |
11 |
3 |
0 |
55.2 |
82.3 |
| cefaclor |
14 |
41 |
4 |
37 |
0 |
0 |
0 |
38.5 |
42.7 |
| cefazolin |
2 |
18 |
17 |
58 |
0 |
1 |
0 |
60.4 |
78.1 |
| cefotiam |
0 |
2 |
2 |
92 |
0 |
0 |
0 |
95.8 |
97.9 |
| ceftazidime |
0 |
1 |
4 |
91 |
0 |
0 |
0 |
94.8 |
99 |
| flomoxef |
0 |
0 |
0 |
96 |
0 |
0 |
0 |
100 |
100 |
| cefpodoxime |
0 |
1 |
3 |
80 |
0 |
12 |
0 |
83.3 |
86.5 |
| aztreonam |
2 |
2 |
2 |
90 |
0 |
0 |
0 |
93.8 |
95.8 |
| imipenem |
0 |
2 |
0 |
93 |
0 |
1 |
0 |
96.9 |
96.9 |
| meropenem |
0 |
1 |
0 |
95 |
0 |
0 |
0 |
99 |
99 |
| gentamicin |
5 |
3 |
2 |
86 |
0 |
0 |
0 |
89.6 |
91.7 |
| amikacin |
4 |
14 |
2 |
75 |
0 |
1 |
0 |
78.1 |
80.2 |
| minocycline |
8 |
13 |
8 |
62 |
0 |
3 |
2 |
64.6 |
72.9 |
| levofloxacin |
1 |
2 |
0 |
90 |
1 |
2 |
0 |
93.8 |
94.8 |
Table 2: Distribution of differences in the MICs of 15 antimicrobial agents for 96 E.coli determined by the standard method versus the short-time method.
For piperacillin, cefazolin, cefpodoxime, amikacin and minocycline, the agreement and complete agreement were in the range 78.1-86.5% and 55.2-78.1%, respectively. Cefaclor had the lowest agreement and complete agreement (42.7 and 38.5%, respectively). For piperacillin, cefaclor, cefazolin, amikacin and minocycline for which low agreement was noted, MICs measured by the short-time method were often lower (below one-half or one-fourth) compared with that measured using the standard method, and it appeared that the bacteria started to regrow beginning 6 hours after the start of the short-time method (Table 2).
Discussion
Bacteria contain ATP and bacterial ATP levels are known to be correlated with the viable bacterial counts. It has been reported that this correlation can be utilized for obtaining the bacterial count in biofilms [17] and for evaluating the postantibiotic effect of antimicrobial agents [18]. Furthermore, reports have been published concerning evaluation of the susceptibility of Mycobacterium tuberculosis [3], as well as mycobacteria in general [19], using the ATP-bioluminescence method. Evaluation of the antimicrobial susceptibility of bacteria in general using this relationship has also been reported [4-6]. In our previous study, we observed an increase in ATP levels that reflected the increase in bacterial counts in the presence of antimicrobial agents, which could be measured by the chemiluminescence method, demonstrating the validity of this method for evaluating the efficacy of antimicrobial agents in 6 hours. This method, however, has shortcomings in that it requires a special device for ATP measurement and the measurement method is not simple.
3-(4,5-Di-methylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), a yellow tetrazole is reduced in the mitochondria of viable cells by dehydrogenase purple formazan. This color development is correlated with the viable cell count. Utilization of this relationship for evaluating the antimicrobial susceptibility of bacteria in general [7-9], Mycobacterium emphasis [10,11], and fungi [12-14] has been reported. 2,3-Bis-(2-methoxy-4-nitro- 5-sulfophenyl)-2H-tetrazolium-5-carboxanilide(XTT) is a reagent that can serve as a substitute for MTT. XTT has higher detection sensitivity than MTT and does not require solubilization. XTT has also been utilized for evaluating antimicrobial susceptibility [20].
Measurement with Cell Counting Kit-8 requires only addition of the reagent to the medium and measurement at a certain time. In Cell Counting Kit-8, NADH which is produced by dehydrogenase activities in bacteria is reduced the formazan by 1-Methoxy PMS. We expected that this method would be simpler than the conventional methods of measurement using MTT or chemiluminescence. The present study was undertaken to measure MICs with this reagent (superior to the MTT assay in terms of high sensitivity, low cytotoxicity, and other factors) by detecting bacterial growth.
Analysis of the changes in absorbance over time (before culture and at multiple points of time after the start of culture) in relation to the density of the bacterial inoculum revealed that the absorbance increased as the density of the bacterial inoculum and the incubation time increased. This suggests that in the presence of a certain amount of bacteria(e.g., 5 × 104), bacterial count is correlated with the absorbance even when the incubation time is approximately 4 or 5 hours. Considering that the amount of bacteria inoculated for measuring MIC with a microplate is 5 × 104, it appears that this method may enables judging whether bacteria will grow in the presence of an antimicrobial agent. We realized to have enough time (such as 4 or 5 hours) detecting the absorbance in the medium without antimicrobial agents, However we needed more long time to growing bacteria in the medium with antimicrobial agents, so we created at 6 hours. In the present study, this judgment could be made 6 hours after the start of the incubation, in view of the possible slowing of bacterial growth in the presence of each agent. In any event, the capability of this method to yield results within the same day appears advantageous for clinical applications.
Of the 15 antimicrobial agents tested, MIC consistency rates were high (over 90%) for nine drugs. Of the remaining six drugs, the consistency rate was between 78.1 and 86.5% for five drugs. These results suggest that this method can be utilized clinically as a drug susceptibility test. The consistency rate was below 50% only for cefaclor, suggesting that estimation of MIC of this drug using the short-time method may be difficult.
In the standard method for MIC measurement, the presence/ absence of macroscopic bacterial growth is examined at 20 hours, whereas in the short-time method, bacterial growth is examined on the basis of mitochondrial reduction of the reagent at 6 hours. Bacterial strains rated as +1 to +2 may exhibit growth when assayed by the short-time method, but may not grow to a level detectable by the standard method. It is possible that these bacteria initially grow to some extent, but then their growth subsequently slows. By contrast, the bacterial strains rated as −1 to −2 do not grow to a level detectable in 6 hours by the shorttime method, but exhibit detectable growth at 20 hours. For piperacillin, cefaclor, cefazolin, amikacin, and minocycline, for which low consistency rates were observed, the rating by the short-time method was lower by −1 to −2 (one-half or one-fourth) compared with that by the standard method. This indicates that these drugs can suppress bacterial growth for approximately 6 hours but not for 20 hours, suggesting that for such long-term suppression they should be used at higher concentrations.
It appears natural that there was a discrepancy in MICs obtained by the two methods depending on the bacterium−drug combination. There are reports of differences in the consistency rates from those yielded by the standard method depending on the bacterium and/or drugs [5-8].
Conclusion
In the present study, counting viable bacteria was possible by measuring absorbance with Cell Counting Kit-8. When this reagent was used for measuring MICs (short-time method), the results were obtained in 6 hours and were closely correlated with the results obtained using the standard method, thus indicating its usefulness in the early detection of drug-resistant microorganisms.
Conflict of Interest
The subject matter of this article does not involve any conflict of interest for us.
19485
References
- CLSI (2009) Performance Standards for Antimicrobial Disk Susceptibility Tests; Approved Standard CLSI document M02-A10. Clinical and Laboratory Standards Institute 12(3): 20.
- CLSI (2009) Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically. Clinical and Laboratory Standards Institute 15(7): 18.
- Nilsson LE, Hoffner SE, Ansehn S (1988) Rapid susceptibility testing of Mycobacterium tuberculosis by bioluminescence assay of mycobacterial ATP. Antimicrob. Agents Chemother 32(2): 120.
- Hattori N, Nakajima M, O’hara K, Sawai T (1998) Novel Antibiotic susceptibility tests by the ATP-Bioluminescence method using filamentous cell treatment. Antimicrob. Agents Chemother 42(8): 29.
- Manome I, Ikedo M, Saito Y, Ishii K, Kaku M (2003) Evaluation of a novel automated chemiluminescencent assay system for antimicrobial susceptibility testing. J Clin Microbiol 41(9): 174.
- Takakura R, Arita K, Kohara T, Nishino R (2003) Detection of MRSA with Antimicrobial Susceptibility by Using Chemiluminescent Assay.J Jpn Infect Dis 77(2): 28.
- Rahman M, Kuhn I, Rahman M, Olsson-Liljequist B, et al. (2004) Evaluation of a scanner-assisted colorimetric MIC method for susceptibility testing of gram-negative fermentative bacteria. Appl Environ Microbiol 70(8): 321.
- Gattringer R, Niks M, Ostertág R, Schwarz K, Medvedovic H, et al. (2002) Evaluation of MIDITECH automated colorimetric MIC reading for antimicrobial susceptibility testing. J Antimicrob Chemother 49(11): 28.
- Baker CN, Banerjee SN, Tenover FC (1994) Evaluation of Alamar colorimetric MIC method for antimicrobial susceptibility testing of gram-negative bacteria. J Clin Microbiol 32(3): 114.
- Franzblau SG, Witzig RS, McLaughlin JC, Torres P, Madico G, et al. (1998) Rapid, low-technology MIC determination with clinical Mycobacterium tuberculosis isolates by using the microplate Alamar Blue assay. J Clin Microbiol 36(1): 53.
- Yajko DM, Madej JJ, Lancaster MV, Sanders CA, Cawthon VL, et al. (1995) Colorimetric method for determining MICs of antimicrobial agents for Mycobacterium tuberculosis. J Clin Microbiol 33(5): 33.
- Pfaller MA, Barry AL (1994) Evaluation of a novel colorimetric broth microdilution method for antifungal susceptibility testing of yeast isolates. J Clin Microbiol 32(7): 24.
- Usefulness of a colorimetric method for testing antifungal drug susceptibilities of Aspergillus species to voriconazole,HideyoYamaguchiKatsuhisaUchidaKenjiNaginoToshiyukiMatsunaga
- Shehata AS, Mukherjee PK, Ghannoum MA (2008) Comparison between the standardized clinical and laboratory standards institute M38-A2 method and a 2,3-Bis(2-Methoxy-4-Nitro-5-[(Sulphenylamino)Carbonyl]-2H-tetrazolium hydroxide- based method for testing antifungal susceptibility of dermatophytes. J Clin Microbiol 46: 3668-3671.
- Yoshida M, Shiba K, Hosoya T (2005) Antimicrobial susceptibility testing by chemiluminescence ATP assay. Jpn J Chemother 53(1): 242.
- Brady AJ, Kearney P, Tunney MM (2007) Comparative evaluation of 2,3-bis [2-methyloxy-4-nitro-5-sulfophenyl]-2H-tetrazolium-5-carboxanilide (XTT) and 2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium, monosodium salt (WST-8) rapid colorimetric assays for antimicrobial susceptibility testing of staphylococci and ESBL-producing clinical isolates. J Microbiol Methods 71(10): 220.
- Kumon H, Ono N, Iida M, Nickel JC (1995) Combination effect of fosfomycin and ofloxacin against Pseudomonas growing in a biofilm. Antimicrob. Agents Chemother 39(6): 182.
- Mackenzie FM, Gould IM, Chapman DG, Jason D (1994) Postantibiotic effect of meropenem on members of family Enterobacteriaceae determined by five methods. Antimicrob. Agents Chemother 38(4): 18.
- Becker B, Lang HRM, Schimke D, Lammers A (1985) Evaluation of bioluminescence assay for rapid antimicrobial susceptibility testing of Mycobacteria. Eur J Clin Microbiol 4(8): 12.
- Antachopoulos C, Meletiadis J, Roilides E, Sein T, Walsh TJ (2006) Rapid susceptibility testing of medically important zygomycetes by XTT assay. J Clin Microbiol 44







