Anitha Roy1*, Geetha R.V2 and Lakshmi T1
1Faculty of Pharmacology, Saveetha Dental College, Velappanchavady, Chennai-77
2Faculty of Microbiology, Saveetha Dental College, Velappanchavady, Chennai.-77
- Corresponding Author:
- Ms. Anitha Roy
Assistant Professor, Department of Pharmacology
Saveetha Dental College, Velappanchavady, Chennai-77
Email: anitharoypeeter@yahoo.co.in
Date of Submission: 04-09-2011 Date of Acceptance: 19-09-2011
Citation:Anitha Roy, Geetha R.V Lakshmi T,“Evaluation of Anti Mycotic Activity of Aqueous and Ethanolic Extracts of Aesculus hippocastanum- An In Vitro Study, ”, Int. J. Drug Dev. & Res., July-Sep 2011, 3(3):335-338
Copyright:© 2010 IJDDR, Anitha Roy et al. This is an open access paper distributed under the copyright agreement with Serials Publication, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords
Horse chestnut, agar well diffusion, antimycotic activity, zone of inhibition
Introduction
Antimicrobial activities of various herbs and spices in plant leaves, flowers, stems, roots, or fruits have been reported by many workers. [1] A wide variety of secondary metabolites, such as tannins, terpenoids, alkaloids, quinones and flavonoids are endowed with antimicrobial properties.[2, 3] The plant under present study, Aesculus hippocastanum also has some antimicrobial property.
Aesculus hippocastanum (family Hippocastanacae) is commonly known as Horse chestnut, which is native to Western Asia. Horse chest nut is also known as Spanish chestnut, buckeye, seven leaves tree, is a deciduous tree which grows up to a height of 35 meters with a large regular crown, five to seven digitate leaves and erect racemes of flowers with a yellow or reddish spot at the base of the white petals. The fruit is a spiny capsule containing up to three shiny, reddish brown seeds with a light-colored hilum. [4,5] It is indigenous to the mountains of Greece, Bulgaria, the Caucasus, northern Iran and the Himalayas[5]
The seeds have been used as an analgesic, antipyretic, narcotic, tonic, and vasoconstrictor. They have been used to treat backache, sunburn, neuralgia, rheumatism, whooping cough and hemorrhoids. [6, 7, 8] The bark has been used as a tonic, narcotic, antipyretic and induce sneezing. The flowers have been used as an anodyne, astringent, tonic and vulnerary[7]. The extracts of Horse chestnut have been traditionally employed both in the West and East for the treatment of peripheral vascular disorders including haemorrhoids, varicose veins, leg ulcers and bruises[9].It is used in the treatment for chronic venous insufficiency and peripheral edema[10]. It has antilipemic, expectorant, diuretic properties and antimicrobial activity. It is also used for the prevention of gastric ulcers, reduction of cerebral edema, reduction of cellulite, as adrenal stimulant, hypoglycemic agent, antithrombotic, antiinflammatory, and also for reduction of hematomas and inflammation from trauma or surgery.
Active Chemical Constituents of horse chest nut are coumarin derivatives like aesculin, fraxin, scopolin; flavonoids like quercetin, kaempferol, astragalin, isoquercetrin, rutin, leucocyanidine and essential oils like oleic acid, linoleic acid.Other constituents include amino acids (adenosine, adenine, guanine), allantoin, argyrin, carotin, choline, citric acid, epicatechin, leucodelphinidin, phyosterol, resin, scopoletin, tannin, and uric acid. [6,11,12]The principal extract and medicinal constituent of horse chestnut seed is aescin, a mixture of triterpenoid saponin glycosides. Its components include protoaescigenin, barringtogenol C, allantoin, sterols, leucocyanidin, leucodelphinidin, tannins, and alkanes.[5, 6, 12, 13] The present study was conducted to assess the antimycotic activity of aqueous and ethanolic extracts of Aesculus hippocastanum (horse chestnut) against different fungal strains.
Materials and Methods
Plant materials
Aqueous and ethanolic extracts of Aesculus hippocastanum was obtained from Green Chem Herbal Extract & Formulations, Bangalore.
Test microorganisms
Fungal strains used were Candida albicans, Aspergillus niger, Aspergillus fumigates, Mucor sps and Penicillium morneffi. The organisms were obtained from Department of Microbiology, Saveetha Medical College & Hospitals, and maintained in SDA slope at 4°C.
Methodology
The extracts were prepared in the following concentrations in sterile water.2.5 mg/ml, 5 mg/ml and 10 mg/ml, so that 50μl of extract of different concentrations delivers 125μg, 250μg and 500 μg respectively.
Anti mycotic Assay - Agar well Diffusion Technique
The extract at different concentrations was screened for their antimycotic (antifungal) activity against the selected fungal strains by Agar well diffusion method.[14,15] The fungal cultures were grown on Sabourauds destrose agar [Hi media M063]. The fungal growth from seven day old culture was washed, suspended in normal saline and then filtered through glass wool aseptically. The colony forming units (CFU/ml) of suspension of the fungus was determined and test inoculum was adjusted to 0.5 Mc Farland’s standard[16,17] and used for antifungal assay.100μl of the test inoculum were applied on the surface of the Sabourauds destrose agar plate and spread using sterile glass spreader. Wells were cut on the agar plates using sterile cork borer for different concentration of the extracts. 50μl of extract of different concentrations were loaded in to the wells and incubated for 48 h at 28ºC. As a positive control, fluconazole (10 mcg /disc) and amphotericin B (100 units /disc) were used. Zone of inhibition in mm were determined after 48 h. The test was performed in triplicate to minimize test error.
Results and Discussion
The antimycotic activity of the extracts (Ethanolic and Aqueous) at different concentrations was screened by agar well diffusion technique and the zone of inhibition was measured in mm diameter. The results are given in the Table 1, Figure 1 and Figure 2
| Extract |
Concentration
[µg] |
Zone of inhibition
[in mm diameter] |
|
| |
|
B1 |
B2 |
B3 |
B4 |
B5 |
| Ethanolic |
125 |
14 |
12 |
14 |
10 |
11 |
| 250 |
18 |
17 |
16 |
13 |
14 |
| 500 |
22 |
19 |
20 |
14 |
22 |
| Aqueous |
125 |
9 |
7 |
9 |
7 |
10 |
| 250 |
12 |
14 |
12 |
10 |
14 |
| 500 |
16 |
21 |
15 |
12 |
16 |
| Fluconazole |
10mcg/disc |
21 |
21 |
20 |
19 |
20 |
| Amphotericin B |
100 units/disc |
20 |
20 |
19 |
18 |
18 |
Table 1. Antimycotic activity of Aqueous and Ethanolic extracts of A. hippocastanum
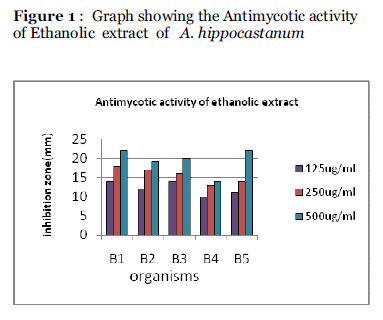
Figure 1 : Graph showing the Antimycotic activity of Ethanolic extract of A. hippocastanum
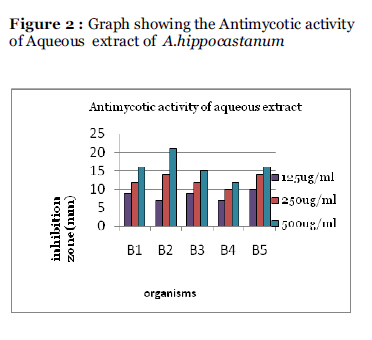
Figure 2: Graph showing the Antimycotic activity of Aqueous extract of A.hippocastanum.
Conclusion
The present study conclude the efficacy of Aesculus hippocastanum as a potent herb with antimycotic activity and may be used in treating diseases caused by the test organisms. Further studies are required to isolate, identify and elucidate the structure of the bioactive compound particularly responsible for the antimycotic activity of this medicinal plant.
Acknowledgement
The authors are thankful to Mr.R.Rajendran, Green Chem Herbal extracts and formulations Bangalore for providing us with the Ethanolic and Aqueous extracts of Aesculus hippocastanum (Horse chestnut) as a gift sample for our research work. We are also thankful, to the HOD Department of Microbiology, Saveetha Medical College and Hospitals for providing us with the fungal culture.
Conflict of Interest: NIL
Source of Support: NONE
5664
References
- Mau JL, Chen CP, Hsieh PC. Antimicrobial effect of extracts from Chinese chive, cinnamon, and corni fructus. J. Agric. Food Chem. 49, 2001, 183-188.
- Cowan, M.M. Plant Products as Antimicrobial Agents. Clin.Microbiol. Rev 1999. 12(4), 564-582
- Lewis, K.; Ausubel, F.M. Prospects for Plant- derived Antibacterials. Nat. Biotechnol 2006. 24(12), 1504-1507
- Bissett NG. Herbal drugs and phytopharmaceuticals. Stuttgart: Med harm CRC Press, 1994:566.
- Fleming T. PDR for herbal medicines. Montvale, NJ: Medical Economics Company, Inc., 1998.
- Peirce A. The American Pharmaceutical Association practical guide to natural medicines. New York: William Morrow and Company, Inc., 1999
- Schilcher H. Phytotherapy in paediatrics: handbook for physicians and pharmacists: with reference to commission E monographs of the Federal Department of Health in Germany: includes 100 commission E monographs and 15 ESCOP monographs. Stuttgart: medpharm Scientific Publishers, 1997:181.
- Evans WC, Saponins, cardioactive drugs and other steroids, Trease and Evans Pharmacognosy, 15th ed. London, WB Saunders 2002, page 304
- Sirtori CR, Aescin: pharmacology, pharmacokinetics and therapeutic profile, Pharmacol Res. 2001 Sep; 44 (3):183 93.
- Robbers JE, Tyler VE. Tyler's herbs of choice: the therapeutic use of phytomedicinals. New York: Haworth Herbal Press, 1999: x, 287
- Newall CA, Anderson LA, Phillipson JD. Herbal medicines: a guide for health-care professionals. London: Pharmaceutical Press, 1996: ix, 296.
- Schulz V, Hansel R, Tyler VE. Rational Phytotherapy: A Physicians' Guide to Herbal Medicine. Berlin: Springer, 1997:306.
- Connie R.Mahon., George Manuselis., Saunder’s Diagnostic Microbiology 2nd Edition.
- Collins, CH and Lyne, P.M 1976.Microbiological methods, London, Butterworths and co.288p
- J.G.Colle., A.G.Faster., B.P.Marmion., A.Simmons. Practical Microbiology [Mackie & Mc Cartney] 14th edition page851 – 852.
- Betty A.Forbes., Daniel F.Sahm., Alice S.Weissfeld. Bailey & Scott’s Diagnostic Microbiology 11th edition Mosby page 229 – 257








