Keywords
Breast cancer; Mastectomy; Radiotherapy; Skin toxicity; High resolution ultrasound
Introduction
Breast cancer is the leading cause of death among women all over the world, and lifetime risk for a woman to develop breast cancer 12% and one out of every eight woman are at risk to develop breast cancer for a lifetime [1,2]. Although more contemporary surgical techniques are available, adjuvant radiotherapy (RT) is still effective today. Recent studies showed that RT targeted to the thorax wall and the local lymphatics improves disease-free survival and decreases local and regional recurrence ratios [3-6]. New RT techniques with limited radiation-induced side effects are effective and safe. However, therapeutic radiation damages the surrounding healthy tissue [7,8]. Among patients who received Postmastectomy Radiotherapy (PMRT), about 90% develop dermal toxicity during the treatment period [3,8,9].
Despite modern techniques and fractional schemes, erythema, edema, and fibrosis are still the most common side effects. The European Organization for Research and Treatment of Cancer (EORTC) reported that during a 10-year follow-up of 5000 patients who had received RT, 26.9% developed medium severity dermal fibrosis. Skin toxicity includes a spectrum ranging from erythema to drydesquamation to ulceration [10].
For the development of the toxicity fractional dose, beam energy and treatment techniques are radiotherapy-dependent factors while breast size, geometry, addition of hormonal therapy, and history of smoking compose the patient dependent factors. Pain and systemic infection related to dermal toxicity would preclude RT. They also prevent the treatment from being effective and promote the development of late skin fibrosis [6-10].
For RT techniques, enhanced homogeneity conformity and fractional schemes are used [11]. RT-induced dermal toxicity is an early phase of chronic toxicity. Continuity of treatment, dose-regulation awareness, and early recognition of dermal toxicity are important for treatment and dose regulation. Radiation Therapy Oncology Group (RTOG) scoring, a subjective method evaluates radiation toxicity and depends on an inspection and physical examination by a clinician [10].
Skin is composed of three layers: the epidermis, dermis, and hypodermis. The epidermis, the outmost layer, is very thin and hard to define sonographically. Beneath this outer layer, the dermis includes hair follicles, fat and sweat glands, nerve endings, blood, and lymph vessels. The hypodermis (subcutan fat), the deepest layer, is typically composed of nerves, lymphatics, large blood vessels, and adipose tissue [12,13] (Figure 1).
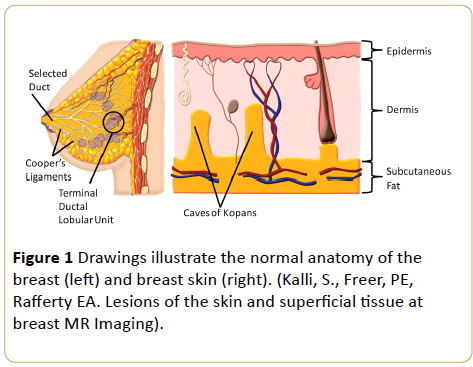
Figure 1: Drawings illustrate the normal anatomy of the breast (left) and breast skin (right). (Kalli, S., Freer, PE, Rafferty EA. Lesions of the skin and superficial tissue at breast MR Imaging).
The anatomical variation of skin thickness according to body site, gender, age and ethnic origin is an important parameter (0.7-2.3 mm). In skin thickness may vary between breast quadrants, especially in the inner quadrant of breast skin is thinner [14].
High-Resolution Ultrasonography (HRUS) is gaining more acceptance as a noninvasive and easily repeatable technique that detects focal lesions from dermal and subdermal tissues quickly and accurately [15]. In this study, HRUS, which provides a more objective and quantitative method based on the RTOG scoring system correlation of the RTOG grade with sonography findings, was used to compare healthy and recently irradiated tissues in a more sensitive, realistic, and quantitative way.
Materials and Methods
Study participants were recruited from patients with breast cancer who were admitted to the Radiation Oncology Clinic for postoperative adjuvant radiation therapy between June 2009 and June 2012. A total of 58 patients who had received adjuvant radiotherapy of whom 31 had partial mastectomy and 27 had total mastectomy were included in this study. HRUS was performed on the patients to assess radiationinduced late skin toxicity. The ultrasound scans were blinded to the RTOG scores, and the skin thickness of the individually marked points on the irradiated chest wall was compared to the corresponding points on the non-irradiated breast (Table 1).
| Grade |
Grade 1 |
Grade 2 |
Grade 3 |
Grade 4 |
| |
Slight atrophy Pigmentation change, hair loss |
Patch atrophy; Modaretetelengiectasia, Total hair loss |
Marked atrophy, Gross telengiectasia |
Ulceration |
Table 1: NPS assessment scores for Mr. Rossi compared to the AD control group.
This study was approved by the Ethics Committee. All participants gave written informed consent before they were enrolled in the study. The study was performed in adherence to the tenets of the Declaration of Helsinki.
Radiotherapy Protocols
Thirty-one patients who had partial mastectomy had received radiotherapy in 25-28 fractions at 180-200cGy doses to the peripheral lymphatics and breast and 5-8 fractions at 10-16 Gy doses to the tumor bed. Twenty-seven patients who had total mastectomy received 25-28 fractions at 180-200 Gy doses daily and a total of 50-50.4 Gy doses to the peripheral lymphatics (supraclavicular and axillary regions) and the chest wall (as 1 cm thick bolus).
Wedges were used to optimize the homogeneity with 6 MV photon energy from the tangential medial and lateral areas under the guidance of International Commission on Radiation Units and Measurements 50 (ICRU-50) guidelines. Treatment planning was performed in the eclipse version 10. The linear accelerator VARIAN UNIQUE 2008 was used (Figure 2).
Figure 2: Radiotherapy treatment marks on the patient.
Ultrasound Examination
HRUS scanning began 6 months after the radiation therapy was completed. Skin thickness measurements were performed by a single radiologist who had 15 years of experience in breast sonography. Ultrasound examination was performed using a 13-18 MHz linear array high-resolution volumetric transducer (Toshiba Aplio, Japan 2012). Quantitative measurements of skin thickness were performed sonographically (Figure 3).
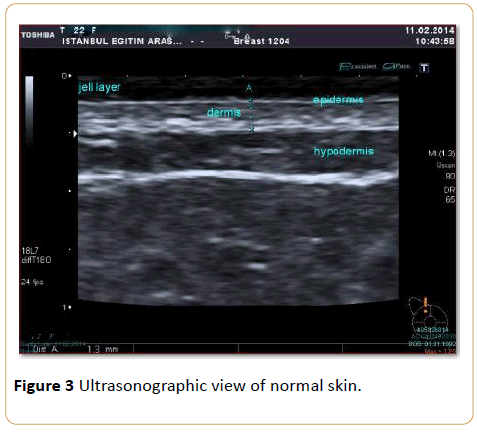
Figure 3: Ultrasonographic view of normal skin.
The breast quadrants were mapped by dividing each breast into 8 equal quadrants. The breast segments were numbered from 1 to 8 counterclockwise for the right breast and clockwise for the left breast. The axillary and supraclavicular regions were also mapped, and these regions composed the 9th and 10th areas, respectively. Thus, the irradiated breasts and contralateral normal breasts, which constituted the control group, were mapped symmetrically (Figure 4).
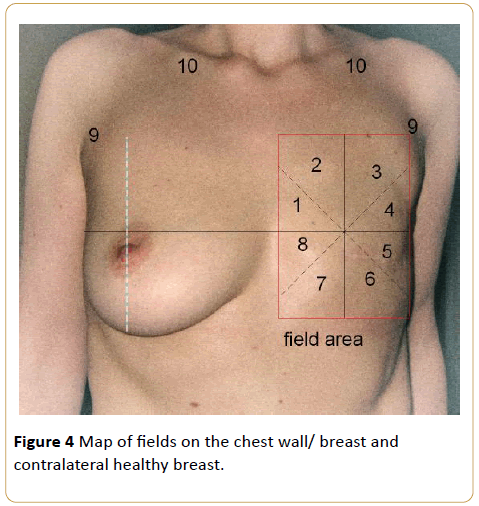
Figure 4: Map of fields on the chest wall/ breast and contralateral healthy breast.
Ultrasonographic examination was performed as the patients lay in the supine position on the examination table, head turned to the opposite side, and the ipsilateral arm raised above the head (Figure 5).
Figure 5: Transducer position on the thick layer of gel on the skin.
The appropriate focus, frequency, and gain settings were made during the sonographic examination. Care was taken to achieve the optimal image by using optimal frequency and focus settings. A thick gel layer was placed between the skin and the ultrasound probe to obtain maximum image quality.
Beginning from the 1st quadrant of the irradiated breast, the skin thickness of all segments and then the skin thickness of the axillary and supraclavicular regions were measured. For every breast quadrant, along the transducer surface area the highest dimension of skin thickness was taken as the quadrant’s skin thickness, because even within a particular surface area of one transducer, the thickness values varied. Due to the boost radiotherapy to the tumor bed in the partial mastectomy patients and postoperative scar tissue in these areas, the measurements for this group were performed 2 cm around the boost area (Figure 6).
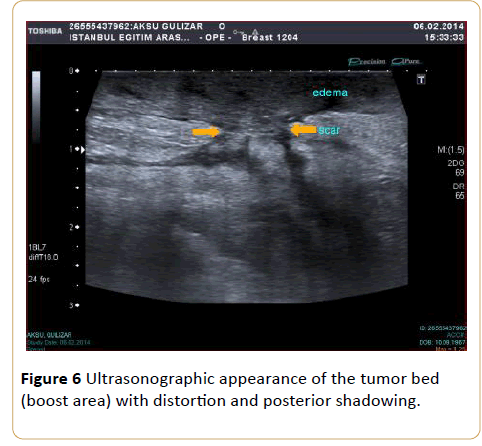
Figure 6: Ultrasonographic appearance of the tumor bed (boost area) with distortion and posterior shadowing.
In determining the thickness of the skin, the area between the thin echogenic line, which represents the epidermis, and the echogenic line, which is seen between the dermis and subcutaneous fatty tissue, was measured. Because the epidermis is very thin, skin thickness was considered the sum of the dermis and epidermis measurements. For every patient, 10 measurements of the irradiated breast and 10 measurements of the contralateral normal breast (for a total of 20 measurements) were performed.
Analytical and Statistical Methods
Descriptive statistics of the data mean, standard deviation, median lowest and highest values were used. Kolmogorov- Smirnov test was measured by the distribution of the variables. In the analysis of quantitative data, Mann-Whitney U test was used. Wilcoxon was used to analyze the repeated measures. Correlation analysis of Spearman correlation analysis was used. SPSS 22.0 software was used in the analysis. . Differences between groups of categorical variables were tested with chi-square analysis. Data were expressed as mean ± standard deviation (SD). p values <0.05 were accepted as significant.
Results
The mean age of patients was 51.4 ± 11.6 (29-80 years old), fifty-eight patients with breast cancer were enrolled in the study. Seventeen (29.3%) of the patients who had partial mastectomy on the right side, and 14 (24.2%) on the left side. Ten (17.2%) patients who had total mastectomy on the right side and 17 (29.3%) on the left side. The ultrasonographic screening period after radiation therapy was a minimum of 6 months and a maximum of 36 months. The mean time was 14.4 ± 8.3 months for all patients.
In the total mastectomy group, the skin thickness of the irradiated side and the control side was 1.0 ± 0.3 mm and 1.1 ± 0.3 mm, respectively (p>0.05). In the partial mastectomy group, the skin thickness of the irradiated side and the normal side was 1.3 ± 0.3 mm and 1.4 ± 0.3 mm, respectively (p>0.05). Both of the mastectomy groups, there were no significant differences between the skin thicknesses of any quadrants of the irradiated and normal sides including the 8 quadrants and the supraclavicular and axillary areas (p>0.05). In the partial and total mastectomy groups when compared each other there were no significant differences between the skin thicknesses of any quadrants (p>0.05).
A negative correlation was determined between the sonographic examination timing. In both groups, the skin thickness measured post-RT after 12th month was significantly lower than the measurements the post-RT before 12th month except the axillary and supraclavicular regions (quadrant 9-10). On the axillary and supraclavicular regions, there were no statistically significant differences between the quadrants of both groups p<0.05) (Figure 7).
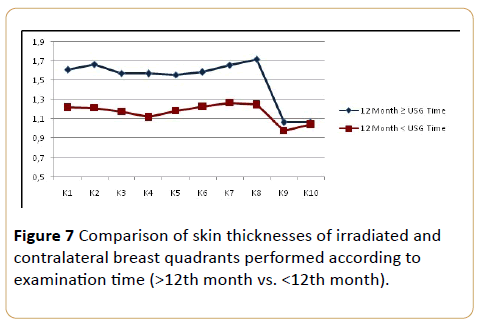
Figure 7: Comparison of skin thicknesses of irradiated and contralateral breast quadrants performed according to examination time (>12th month vs. <12th month).
In both groups, after the 12th month of radiation therapy, the skin thickness of the irradiated side was significantly lower than that of the contralateral healthy side (p<0.05) (Figure 8 and 9).
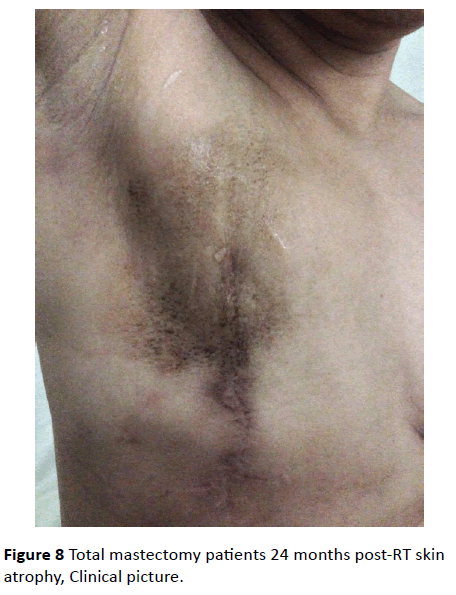
Figure 8: Total mastectomy patients 24 months post-RT skin atrophy, Clinical picture.
Figure 9: Total mastectomy patients 24 months post-RT skin atrophy, Sonographic view.
We also compared skin thickness for grade 1 and grade 2 of skin toxicityin this study. In the total mastectomy group, 5 patients (19%) had grade 2 and 22 patients (81%) had grade 1 skin toxicity according to the RTOG late toxicity scoring criteria. In the partial mastectomy group, 9 patients (29%) had grade 2 and 22 patients (71%) grade 1 skin toxicity. Among the total cases (n=58), 14 patients had grade 2 (24%), and 44 patients (76%) had grade 1 skin toxicity. No cases of grade 3 or 4 skin toxicity were found.
The mean skin thickness for patients who had grade 1 was 1.3 mm ( ± 0.2 mm), while for grade 2 it was 1.7 mm ( ± 0.7 mm). All patients with grade 1 and grade 2 toxicity, skin thickness in all quadrants were significantly higher in comparison with cases in grade 2 ( p=0.003) (Figure 10).
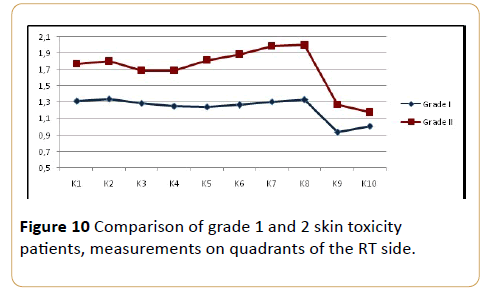
Figure 10: Comparison of grade 1 and 2 skin toxicity patients, measurements on quadrants of the RT side.
Discussion
Radiotherapy is applied to provide local disease control in surgically treated patients who had undergone either a total or partial mastectomy [16]. The most common side effect of RT is acute and chronic radiation injury of the skin. This wide spectrum of injury starts with erythema and goes to ulcerations. Chronic toxicity is fibrosis, which can cause cosmetic issues. Toxicity should be inspected by the clinician and staged according to the RTOG scoring system. Unfortunately, this method is subjective. We saw HRUS after radiotherapy can be done, especially with the use of more objective assessment to demonstrate skin fibrosis that develops in chronic phase. Many studies are in the literature depended on US and RTOG scoring in post-treatment evaluation of irradiated skin toxicity [3-9,12,17,18].
Huang et al. defined in a study that ultrasonographic measurements of fibrosis as late-onset skin toxicity are a quantitative and noninvasive method [19]. In the current study, dermal thickness was measured to evaluate late-phase skin effects using HRUS. In the early phase, especially in the dermal and subdermal tissue planes, residual parenchymal borders in the tumor bed are very hard to demarcate. Because of extensive inflammation and edema, dermal thickness measurements cannot be performed. Therefore, since no objective evaluations of early post-radiotherapy measurements of dermal thicknesses are available, acute toxicity findings cannot be used for comparison. This was a limiting factor of our study. In addition, patients who had axillary dissection sometimes developed more pronounced edema, particularly long lasting, which would also hide the presence of fibrosis. In the literature, several studies have evaluated radiotherapy-induced skin fibrosis in breast cancer cases that involved mastectomy [18].
In this study, we used HRUS as a quantitative examination tool to calculate the degree of skin fibrosis (with the RTOG system) induced by RT. Skin measurements on the corresponding quadrants of the patients’ healthy breasts were planned as individual-based comparisons. Sonographically measured breast skin thicknesses in these patient groups statistically decreased as an indication of fibrosis compared to the healthy breasts. Wang et al. study showed that 6 months after surgery there was a decrease in skin thickness indicating extensive fibrotic proliferation [3]. In our study, measurements made earlier than 12th postsurgical month yielded increased skin thicknesses compared with the control breast skin. A cause for this was thought to be long-lasting edema. In addition, in other studies, in postradiotherapy necks, the dermal thickness increased [19,20].
Leucht, in a study that involved ultrasonography, started measurements at 3 months after the cessation of RT and kept measuring periodically up to 8 years. He reported that although early measurements were much higher, after 2 years skin thicknesses declined with time [21]. It was apparent that as the time period between RT and sonographic examination increases, skin thicknesses decrease toward fibrosis. Wang et al. reported that as a late phase effect fibrosis was dominant after 1 year [3]. Warszowski claimed that fibrosis was a late complication of RT and appeared within 1-2 years of radiotherapy [19].
In the current study, except for the axilla and supraclavicular region, the skin measurements of the mastectomy region were statistically negatively correlated. The skin thickness measurements of the 8 quadrants, especially in the boost irradiated tumor bed areas, and the ratio of fibrosis was higher. In the partial mastectomy group our participants 17 tumors were located in the upper outer quadrant. The statistical positivity of the skin findings in the upper outer quadrant was related to the fact that these patients received high-dose radiation.
In the total mastectomy cases, while using bolus to increase the effective skin dose, half of the total dose (12-13 fractioned) was given with the bolus method, and the other (12-13 fraction) half was programmed without bolus to decrease toxicity. In the literature, there are reports that using the wedge or bolus method increases skin doses. Especially, with the bolus method, it has been reported that superficial doses could have been increased up to 40% [3].
According to the RTOG, complications during RT and within the first 6 months are called acute complications and those after 6 months are late complications [6]. These criteria provide a subjective guide for clinicians to score acute and late skin changes. In our study, 24% of the patients had grade 2 toxicity, and 76% had grade 1 skin toxicity. These results correlated with the literature [6,22,23]. In our patient group, no late phase, grade 3 and 4 skin toxicity was observed. In the partial mastectomy group, RTOG late phase grade 2 skin toxicity was more common (29%) than in the total mastectomy group (19%). This situation could be explained by the additional dose of RT targeted toward the tumor bed in the patients who underwent a partial mastectomy.
In the healthy contralateral axillary and supraclavicular region, the skin thicknesses in the grade 2 group were statistically higher than in the grade 1 group. Ongoing edema in these areas was anticipated as the cause of this difference. Skin thickness decreases as the time to sonographic examination increases. HRUS as an easy, inexpensive, noninvasive, and quantitative method requiring radiological experience is valuable for evaluating dermal thickness and acute and chronic complications caused by RT [3,12]. In this study, skin thickness in the acute phase was limited, and we could not evaluate acute effects. In recent years with evolving early diagnostic methods for breast cancer and evolution in treatable disease, the importance of cosmesis has increased, leading to oncoplastic methods. However, in spite of the advancements in radiotherapy techniques, skin toxicity is still a cosmetic problem [15,23-25].
After reconstructive surgery following mastectomy and postsurgical (adjuvan) radiotherapy, complications have been shown in some studies. In one study, in patients undergoing radiotherapy and those treated without RT, complications occurred in 51% and 14% of the patients, respectively [26]. In another study, the irradiated reconstructed breasts capsule contracture ratio was as high as 40% [27].
There are many variations of studies targeted at decreasing radiation-induced skin reactions. Topical agents, cremes, increase skin tolerance by hydrating the skin and reducing the factors that negatively affect skin reactions like chemotherapy and the smoking skin toxicity could be reduced [19,28-30]. Eliminating the risk factors, by decreasing early and late phase skin toxicity, will increase patient tolerance and affect psychosocial situation positively.
Conclusion
Skin toxicity is an important complication induced by radiotherapy in breast cancer treatment and increases morbidity. In recent medical practice, for evaluation and follow-up of these complications, the RTOG grading system has been used. HRUS in the evaluation of skin toxicity is a noninvasive, objective, and easy imaging method that provides quantitative results. Ä°n evaluation and follow-up of skin toxicity caused by breast and head-and-neck tumor radiation treatment, widespread use of ultrasonography with RTOG grading system could help patient follow-up and promote evolution of new reconstructive methods with better cosmetic results.
9943
References
- Howlader N, Noone AM, Krapcho M (2012) SEER Cancer Statistics Review, 1975–2009 (Vintage 2009 Populations), National Cancer Institute. Bethesda, USA.
- Zhou J, Zhang P, Osterman KS, Woodhouse SA, Schiff PB, et al. (2009) Implementation and validation of an ultrasonic tissue characterization technique for quantitative assessment of normal-tissue toxicity in radiation therapy. Med Phys 36: 1643-1650.
- Wong S, Kaur A, Back M, Lee KM, Baggarley S, et al. (2011) An ultrasonographic evaluation of skin thickness in breast cancer patients after postmastectomy radiation therapy. RadiatOncol 6: 9.
- Overgaard M (1999) Overview of randomized trials in high risk breast cancer patients treated with adjuvant systemic therapy with or without postmastectomy irradiation. Seminar RadiatOncol 9: 292-299.
- Fuller SA, Haybittle JL, Smith RE, Dobbs HJ (1992) Cardiac doses in postoperative breast irradiation. RadiotherOncol 25: 19-24.
- Paszat LF, Mackillop WJ, Groome PA (1999) Mortality from myocardial infarction following postlumpectomy radiotherapy for breast cancer: a population-based study in Ontario, Canada. Int J RadiatOncolBiolPhys 43: 755-762.
- Chen PY, Wallace M, Mitchell C, Grills I, Kestin L, et al. (2010) Four-year efficacy, cosmesis, and toxicity using three-dimensional conformal external beam radiation therapy to deliver accelerated partial breast irradiation. Int J RadiatOncolBiolPhys 76: 991-997.
- Porock D, Kristjanson L (1999) Skin reactions during radiotherapy for breast cancer: the use and impact of topical agents and dressings. Eur J Cancer Care (Engl) 8: 143-153.
- Collette S, Collette L, Budiharto T, Horiot JC, Poortmans PM, et al. (2008) Predictors of the risk of fibrosis at 10 years after breast conserving therapy for early breast cancer: a study based on the EORTC Trial 22881-10882 ‘boost versus no boost.’ Eur J Cancer 44: 2587-2599.
- Hoeller U, Tribius S, Kuhlmey A, Grader K, Fehlauer F, et al. (2003) Increasing the rate of late toxicity by changing the score? A comparison of RTOG/EORTC and LENT/SOMA scores. Int J RadiatOncolBiolPhys 55: 1013-1018.
- Kumar R, Sharma SC, Kapoor R, Singh R, Bhardawaj A (2012) Dosimetric evaluation of 3D conformal accelerated partial-breast irradiation vs. whole-breast irradiation: a comparative study. Int J Appl Basic Med Res 2: 52-57.
- Giess CS, Raza S, Birdwell RL (2011) Distinguishing breast skin lesions from superficial breast parenchymal lesions: diagnostic criteria, imaging characteristics, and pitfalls Radiographics 31: 1959-1972.
- Kalli S, Freer PE, Rafferty EA (2010) Lesions of the skin and superficial tissue at breast MR imaging. RadioGraphics 30: 1891-1913.
- Laurent A, Mistretta F, Bottigioli D (2007) Echographic measurement of skin thickness in adults by high frequency ultrasound to assess the appropriate microneedle length for intradermal delivery of vaccines. Vaccine 25: 6423-6430.
- Giovagnorio F, Andreoli C, De Cicco ML (1999) Color Doppler sonography of focal lesions of the skin and subcutaneous tissue. J Ultrasound Med 18: 89-93.
- Baker MR, Bader D, Hopewell JW (1989) The effects of single doses of x-rays on the mechanical properties of pig skin in vivo. Br J Radiol : 830-837.
- Yoshida EJ, Chen H, Torres M, Andic F, Liu HY, et al. (2012) Reliability of quantitative ultrasonic assessment of normal tissue toxicity in breast cancer radiotherapy. Int J RadiatOncolBiolPhys 82: 724-731.
- Huang YP, Zheng YP, Leung SF, Choi AP (2007) High-frequency ultrasound assessment of skin fibrosis: clinical results. Ultrasound Med Biol 8: 1191-1198.
- Gottlober P, Kerscher MJ, Korting HC, Peter RU (1997) Sonographic determination of cutaneous and subcutaneous fibrosis after accidental exposure to ionising radiation in the course of the Chernobyl nuclear power plant accident. Ultrasound Med Bio 23: 9-13.
- Warszawski A, Rottinger EM, Vogel R, Warszawski N (1997) 20MHZ ultrasonic imaging for quantitative assessment and documentation of early and late postradiation skin reactions in breast cancer patients. RadiotherOncol 47: 241-247.
- Leucht WJ, Rabe DR (1988) Sonographic findings following conservative surgery and irradiation for breast carcinoma. Ultrasound Med Biol 14: 27-41.
- Back M, Guerrieri M, Wratten C, Steigler A (2004) Impact of radiation therapy on acute toxicity in breast conservation therapy for early breast cancer. ClinOncol 16:12-16.
- Gorodetsky R, Mou XD, Fisher DR, Taylor JM, Withers HR (1990) Radiation effect in mouse skin: dose fractionation and would healing. Int J RadiatOncolBiolPhys 18: 1077-1081.
- Russell NS, Knaken H, Bruinvis IA, Hart AA, Begg AC, et al. (1994) Quantification of patient to patient variation of skin erythema developing as a response to radiotherapy. RadiotherOncol 30: 213-221.
- Nuutinen J, Latinen T, Turrunen M (1998) A dielectric method for measuring early and late reactions in irradiated human skin. RadiotherOncol 47: 249-254.
- Tallet AV, Salem N, Moutardier V, Ananian P, Braud AC, et al. (2003) Radiotherapy and immediate two-stage breast reconstruction with a tissue expander and implant. Complications and esthetic results. Int J RadiatOncolBiolPhys 57: 136-142.
- Behranwala KA, Dua RS, Ross GM, Ward A, Gui GP (2006) The influence of radiotherapy on capsule formation and aesthetic outcome after immediate breast reconstruction using biodimensional anatomical expander implants. J PlastReconstrAesthetSurg 59: 1043-1051.
- Schmuth M, Wimmer MA, Hofer S, Sztankay A (2002) Topical corticosteroid therapy for acute radiation dermatitis: a prospective, randomized, double-blind study. Br J Dermatol 146: 983-991.
- GozzoTde O, Panobianco MS, Clapis MJ, De Almeida (2010) Dermatological toxicity in women with breast cancer undergoing chemotherapy treatment. Rev Lat Am Enfermagem 18: 681-687.
- Di Franco R, Sammarco E, Calvanese MG, De Natale F, Falivene S, et al. (2013) Preventing the acute skin side effects in patients treated with radiotherapy for breast cancer: the use of corneometry in order to evaluate the protective effect of moisturizing creams. RadiatOncol 8: 57.















