Sara S McCoy1*, Vivek Nagaraja2, Scott Paviol3, Johann E Gudjonsson4 and J Michelle Kahlenberg1
1Department of Internal Medicine, Division of Rheumatology, University of Michigan, USA
2Department of Internal Medicine, Division of Rheumatology, University of Toledo, USA
3Department of Dermatology, Piedmont Healthcare, USA
4Department of Dermatology, University of Michigan, USA
Corresponding Author:
Sara S McCoy
Division of Rheumatology
University of Michigan Health Center, University of Michigan
300 North Ingalls Building Room 7C27, Ann Arbor, MI-48109, USA
Tel: 734-936-5561
Fax: 734-763-1253
E-mail: ssmccoy@med.umich.edu
Keywords
Psoriasis; Drug-induced; Hydroxychloroquine; Anti-malarial; Arthritis
Introduction
Systemic Anti-Malarials (SADs) are commonly used in rheumatology and are generally thought to have a low toxicity profile. SAD-induced psoriasis has been reported as an adverse effect in the literature. Although case reports are present in the literature, larger studies have failed to find a clear association between SADs and psoriasis exacerbation [1,2]. With rates of psoriasis as high as 8.5%, it is imperative that physicians are aware of this potential adverse effect prior to initiation of SADs [3]. We present a case of SAD-induced psoriasis, explore the potential mechanisms behind SAD-induced psoriasis, and perform a literature review.
Case Report
A 57-year old African American woman presented to the emergency department with sudden onset generalized rash. The patient reported 3 years of pain and swelling involving her hands, wrists, elbows, knees and feet. Over the past few years, the joint pain was partially responsive to brief courses of oral glucocorticoids provided by her primary care physician. The pain returned upon discontinuation of steroid therapy. One month prior to presentation, the patient was evaluated by an outside rheumatologist and diagnosed with Primary Sjogren’s syndrome based on the presence of a positive anti-SSA/Ro antibody. The patient did not have complaints of sicca. She was started on oral hydroxychloroquine (HCQ) for polyarthralgia. One week after initiation of HCQ, she developed a painful generalized rash prompting her admission to the hospital.
Upon further questioning, the patient described a long-standing, scaly rash that was erratically present on her elbows, calves, and back. Skin examination was notable for diffuse targetoid erythematous papules and plaques covering 80% of her body surface area (Figure 1A). Over her back she was noted to have 15-20 hyperkeratotic 1-2 cm plaques (Figure 1B). Fingernail examination was notable for scattered pitting, onycholysis and oil spotting. Musculoskeletal examination revealed synovitis of multiple joints including the bilateral 2nd through 5th proximal interphalangeal joints with the most pronounced synovitis in her left 3rd and 4th proximal interphalangeal joints. The left 3rd and 4th PIP had a Boutonnière’s deformity. The wrists were tender and warm bilaterally. The shoulders had reduced internal and external range of motion bilaterally. The ankles and metatarsal joints also demonstrated evidence of synovitis. She had a leukocytosis of 26,000 per μL (normal range, 4,000 to 11,000 per μL) with a neutrophilic predominance, elevated C-reactive protein elevated to 13.1 (normal range, 0.0-0.6 mg/dL), and a positive Anti-SSa antibody. Pertinent negative labs included immunofluorescent anti-nuclear antibody, rheumatoid factor, and anti-cyclic citrullinated peptide. X-rays of hands and feet were notable for periosteal reaction of the left third proximal phalanx (Figure 2). A biopsy of the plaque-like lesion of the back was consistent with psoriasis.
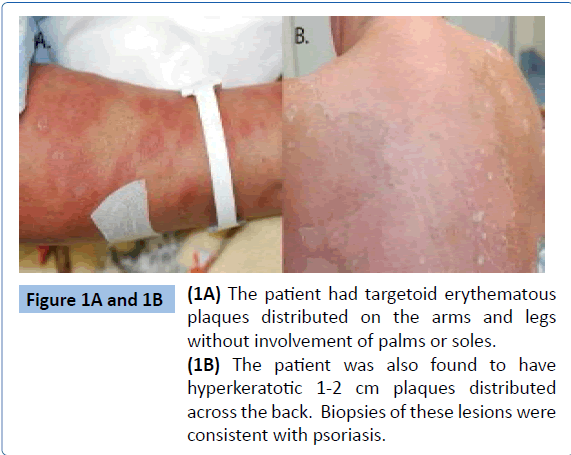
Figure 1A and 1B: (1A) The patient had targetoid erythematous plaques distributed on the arms and legs without involvement of palms or soles. (1B) The patient was also found to have hyperkeratotic 1-2 cm plaques distributed across the back. Biopsies of these lesions were consistent with psoriasis.
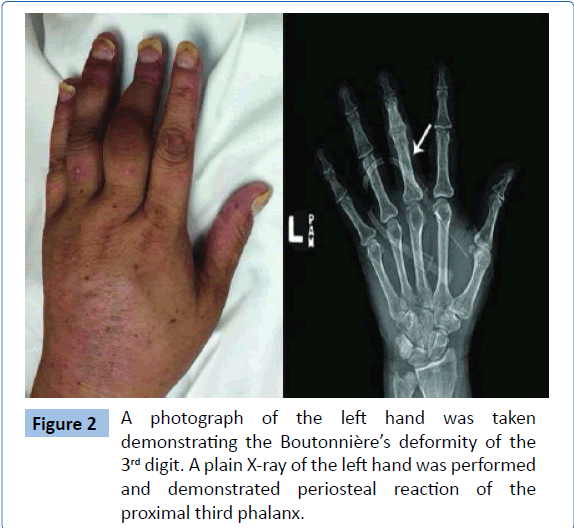
Figure 2: A photograph of the left hand was taken demonstrating the Boutonnière’s deformity of the 3rd digit. A plain X-ray of the left hand was performed and demonstrated periosteal reaction of the proximal third phalanx./p>
The skin findings and histopathology confirmed the diagnosis of psoriasis. The joint examination, fingernail findings and periosteal reaction on hand radiograph were suggestive of symmetric polyarthritis secondary to psoriatic arthritis. Previously her disease had manifested with mild rash and steroidresponsive arthritis; however, she presented with extensive skin involvement after the Introduction of HCQ. Also present on all extremities were targetoid lesions that spared the palms of the hands and soles of the feet, reminiscent of erythema multiforme. This patient carried the diagnosis of Sjogren’s syndrome, which can be associated with erythema multiforme-like skin lesions (Rowell’s syndrome). However, she had no sicca symptoms or mucosal dryness on examination making the initial diagnosis of Sjogren’s syndrome unlikely. Further, Rowell’s syndrome typically involves the palms and soles, which was not consistent with her presentation. Biopsy of a targetoid lesion was nonspecific. Overall, it was felt that the targetoid lesions were part of her psoriasis exacerbation secondary to HCQ.
Discussion
Although SADs have been effectively used to treat joint disease in rheumatoid arthritis [4] and SLE [5], they are not recommended for use in psoriatic arthritis [6]. Since the initial description in the 1950s [7], several anecdotal case reports exist in the medical literature that describe flares of psoriatic skin lesions due to SAD use. A wide-ranging incidence of SAD-exacerbated psoriasis has been presented through case series ranging from 0-100%. Several mechanisms have been speculated to support this association – (i) SADs are well-known inhibitors of the epidermal trans-glutaminase, leading to the compromise of the epidermal barrier. As a result, there is a nonspecific stimulus to epidermal proliferation that is probably sufficient to trigger psoriasis [8], (ii) SADs can enhance the accessory cell function of the epidermal antigen presenting cell in psoriatic skin [9], (iii) At pharmacological doses, SADs can induce lymphocyte proliferation and transformation [10], potentiating pathogenic cell mediated immunity [11-13], (iv) SADs modulate the Th17 axis through p38- dependent cytokine release in the presence of IL-1 cytokines [14]. Th17 cells are implicated in the pathogenesis of psoriasis as the target of IL-23 stimulation and a source of IL17A and IL-22, known promoters of keratinocytes growth and differentiation [15-17], (v) SADs have been shown to reduce plasmacytoid dendritic cell production of interferon α in vitro and in vivo [18,19], which may interfere with the cross-regulation of interferon α and tumor necrosis factor (TNF) α, and allow for pathogenic consequences of TNFα overproduction [20-23].
Gladman et al. reported exacerbation of psoriasis in under 20% of patients exposed to SAD in a case control study [1], but no defined measurements of skin disease activity were made and statistical analysis was not completed in the trial. More recently, in an attempt to clarify the conflicting evidence regarding the effect of SADs on psoriasis, a systematic review was performed [2]. At conclusion, there was a lack of high quality evidence to either support or refute the association. In summary, the available medical literature is conflicting regarding this association and prospective high quality studies are needed. Nevertheless, the best plausible explanation in our case was a drug-induced exacerbation of psoriasis, the medication in question being hydroxychloroquine.
6502
References
- Gladman DD, Blake R, Brubacher B, Farewell VT (1992) Chloroquine therapy in psoriatic arthritis. J Rheumatol 19: 1724-1726.
- Herman SM, Shin MH, Holbrook A, Rosenthal D (2006) The role of antimalarials in the exacerbation of psoriasis: a systematic review. Am J ClinDermatol 7: 249-257.
- Parisi R, Symmons DP, Griffiths CE, Ashcroft DM; Identification and Management of Psoriasis and Associated ComorbidiTy (IMPACT) project team (2013) Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol 133: 377-385.
- Suarez-Almazor ME, Belseck E, Shea B, Homik J, Wells G, et al. (2000) Antimalarials for treating rheumatoid arthritis. Cochrane Database Syst Rev: CD000959.
- (1991) A randomized study of the effect of withdrawing hydroxychloroquine sulfate in systemic lupus erythematosus. The Canadian Hydroxychloroquine Study Group. N Engl J Med 324: 150-154.
- Ritchlin CT, Kavanaugh A, Gladman DD, Mease PJ, Helliwell P, et al. (2009) Treatment recommendations for psoriatic arthritis. Ann Rheum Dis 68: 1387-1394.
- ZIPRKOWSKI L, HAIM S, BANK H (1954) Atabrine in psoriasis. Acta Med Orient 13: 45-52.
- Wolf R, Schiavo AL, Lombardi ML, de Angelis F, Ruocco V (1999) The in vitro effect of hydroxychloroquine on skin morphology in psoriasis. Int J Dermatol 38: 154-157.
- Demidem A, Taylor JR, Grammer SF, Streilein JW (1992) Effects of chloroquine on antigen-presenting functions of epidermal cells from normal and psoriatic skin. J Invest Dermatol 98: 181-186.
- Schopf RE, Ockenfels HM, Schultewolter T, Morsches B (1993) Chloroquine stimulates the mitogen-driven lymphocyte proliferation in patients with psoriasis. Dermatology 187: 100-103.
- Nikaein A, Phillips C, Gilbert SC, Savino D, Silverman A, et al. (1991) Characterization of skin-infiltrating lymphocytes in patients with psoriasis. J Invest Dermatol 96: 3-9.
- Nickoloff BJ, Wrone-Smith T (1999) Injection of pre-psoriatic skin with CD4+ T cells induces psoriasis. Am J Pathol 155: 145-158.
- Wei L, Debets R, Hegmans JJ, Benner R, Prens EP (1999) IL-1 beta and IFN-gamma induce the regenerative epidermal phenotype of psoriasis in the transwell skin organ culture system. IFN-gamma up-regulates the expression of keratin 17 and keratinocyte transglutaminase via endogenous IL-1 production. J Pathol 187: 358-364.
- Said A, Bock S, Lajqi T, Müller G, Weindl G (2014) Chloroquine promotes IL-17 production by CD4+ T cells via p38-dependent IL-23 release by monocyte-derived Langerhans-like cells. J Immunol 193: 6135-6143.
- Rizzo HL, Kagami S, Ph illips KG, Kurtz SE, Jacques SL, et al. (2011) IL-23-mediated psoriasis-like epidermal hyperplasia is dependent on IL-17A. J Immunol 186: 1495-1502.
- Chiricozzi A, Guttman-Yassky E, Suarez-Farinas M (2011) Integrative responses to IL-17 and TNF-alpha in human keratinocytes account for key inflammatory pathogenic circuits in psoriasis. J Invest Dermatol 131: 677-687.
- Harper EG, Guo C, Rizzo H, Lillis JV, Kurtz SE, et al. (2009) Th17 cytokines stimulate CCL20 expression in keratinocytes in vitro and in vivo: implications for psoriasis pathogenesis. J Invest Dermatol 129: 2175-2183.
- Means TK, Latz E, Hayashi F, Murali MR, Golenbock DT, et al. (2005) Human lupus autoantibody-DNA complexes activate DCs through cooperation of CD32 and TLR9. J Clin Invest 115: 407-417.
- Sacre K, Criswell LA, McCune JM (2012) Hydroxychloroquine is associated with impaired interferon-alpha and tumor necrosis factor-alpha production by plasmacytoid dendritic cells in systemic lupus erythematosus. Arthritis Res Ther 14: R155.
- Chabot S, Williams G, Yong VW (1997) Microglial production of TNF-alpha is induced by activated T lymphocytes. Involvement of VLA-4 and inhibition by interferonbeta-1b. J Clin Invest 100: 604-612.
- Palucka AK, Blanck JP, Bennett L, Pascual V, Banchereau J (2005) Cross-regulation of TNF and IFN-alpha in autoimmune diseases. ProcNatlAcadSci U S A 102: 3372-3377.
- Cantaert T, Baeten D, Tak PP, van Baarsen LG (2010) Type I IFN and TNFα cross-regulation in immune-mediated inflammatory disease: basic concepts and clinical relevance. Arthritis Res Ther 12: 219.
- Rothuizen LE, Buclin T, Spertini F, Trinchard I, Munafo A, et al. (1999) Influence of interferon beta-1a dose frequency on PBMC cytokine secretion and biological effect markers. J Neuroimmunol 99: 131-141.








