Yoshinobu Ijiri1*, Junichiro Yamamoto2, Tadayuki Wako3 and Masahiro Murakami4
1Faculty of Health and Nutrition, Osaka Shoin Women’s University, Osaka 577-8550, Japan
2Kobe Gakuin University, Kobe 651-2180, Japan
3Vegetable Breeding and Genome Division, Institute of Vegetable and Floriculture Science, National Agriculture and Food Research Organization, Mie 514-2392, Japan
4Faculty of Pharmacy, Osaka Ohtani University, Osaka 584-8540, Japan
- Corresponding Author:
- Yoshinobu Ijiri
PhD, Faculty of Health and Nutrition Osaka Shoin Women’s University, Osaka 577-8550, Japan
Tel: +81-6-6723-8181 E-mail: ijiri.yoshinobu@osaka-shoin.ac.jp
Received April 28, 2016; Accepted May 09, 2016; Published May 15, 2016
Citation: Ijiri Y, Yamamoto J, Wako T, Murakami M (2016) Experimental Antithrombotic Effect of Garlic Varieties Measured by a Global In Vitro Test of Platelet Reactivity and Spontaneous Thrombolytic Activity. Int J Drug Dev & Res 8:011-017
Keywords
Cardiovascular disease; Stroke; Platelet aggregation; Thrombosis; Fibrinolysis; Garlic; Herb
Introduction
Due to leading mortality from arterial thrombotic events, mainly heart attack and stroke, prevention of arterial thrombotic diseases has a high priority in developed countries. Unsuitable life style such as inappropriate diet intake is known to increase the risk for acute thrombotic events, and a regular diet with proven antithrombotic effects might be a beneficial way to prevent the disease.
Traditional medical practice and epidemiological studies suggested that garlic may be useful for prevention and treatment of cardiovascular diseases [1-3]. Compared to the ineffective Western-style diet, clinical trials provided evidence for the reduced risk of arterial thrombosis and death from coronary heart disease in people on Mediterranean, Vegetarian and Japanese-style diets [4-12]. The use of diet for the prevention of thrombotic events was hampered by the lack of suitable test(s) for identifying and monitoring the antithrombotic effect. Earlier we found that a shear-induced platelet reactivity and spontaneous thrombolytic activity test performed from non-anticoagulated blood (Global Thrombosis Test, GTT) was useful to select antithrombotic fruits and vegetables and that observed antithrombotic effects were varied among the tested varieties [13]. The present study of testing garlic is part of our effort to find ingredient(s) of an antithrombotic diet, as garlic is a popular ingredient of cuisine of many countries.
Materials and Methods
Garlics
Fifteen accessions of garlic were cultivated in the same field of the Institute of Vegetable and Floriculture Science, National Agriculture and Food Research Organization, Mie Prefecture, Japan. The characteristics of each accession have been described in Table 1.
| JPNo* |
Accessionname |
Source |
| 169114 |
Kagawawhite |
Kagawa,Japan |
| 126972 |
Ensyuuwase(Kouchi) |
Shizuoka,Japan |
| 170540 |
Chuugokukei(Kananshoukei) |
China |
| 170613 |
Syanhaikei(Wakayama) |
China |
| 170616 |
Kateiwase(Ehime) |
Unclear,inJapan |
| 126986 |
Tottorizairai |
Tottori,Japan |
| 169111 |
Oogawarazairai |
Miyagi,Japan |
| 126995 |
Chuugokukisyuukei |
China |
| 182363 |
86196 |
Thailand |
| 182369 |
Chet’sItalianpurple |
Unclear,outsideJapan |
| 133834 |
Gravelswitch |
Unclear,outsideJapan |
| 102958 |
Namdo |
Korea |
| 133833 |
Whiteroppen |
Iwate,Japan |
| 134666 |
RAR930048 |
Kazakhstan |
| 138795 |
97KZ8 |
Kazakhstan |
Table 1: Japanese number (JP No*), accession name and source of garlic.
Preparation of garlic filtrate
Several washed garlic bulbsper each accession was crashed, blended and the obtained juice was centrifuged (3000 rpm, 15 min, 4°C). The supernatant was filtrated (pore size 0.5 μm; Schleicher and Schuell GmbH, Dassel, Germany) and the clear filtrates were stored at -80°C until use. As preliminary experiments showed significant antithrombotic effect of garlic even in x3 dilution, in the main experiments for testing, the original filtrate (x1) was diluted with saline x3; x10; and x100. Controls contained saline instead of garlic filtrate.
Animals
Male Wistar 13 week old ST rats (Japan SLC Co. Ltd., Hamamatsu, Japan) were purchased one week before the scheduled experiments. Animals were maintained in compliance with the “Guiding Principles for the Care and Use of Animals in the field of Physiological Sciences”, published by Physiological Society of Japan. The protocol was approved by the Animal Experiment Committee of Kobe Gakuin University.
Global Thrombosis Test (GTT)
GTT (Thromboquest Limited, London, UK) has been described in detail elsewhere [14,15]. Figure 1 shows the embodiment (a) and the principle of the technique (b). Briefly, there are flat segments along the inner wall of a conical plastic tube and when perfectly round ceramic ball bearings are placed into such a conical tube, the flat segments prevent the ball bearings from occluding the lumen. When non-anticoagulated blood is added to such tube, it flows through the narrow gaps by the ball bearing and exits in droplets into an adjacent collecting tube. The latter is transilluminated by a light emitter and a sensor opposite generates a signal whenever a blood drop interrupts the light path. In essence, the instrument detects the time interval (d; sec) between consecutive blood drops. Blood flows at 37°C by gravity through the narrow gaps formed between the upper ball bearing and the inner wall of the tube, where high shear stress activates and aggregates platelets. Platelet aggregates formed and then captured in the gaps by the lower ball bearing, arresting the blood flow. At the start, blood flow is rapid and hence (d) is small. Subsequently, the flow rate gradually decreases and hence (d) increases. When the actual (d) exceeds 15 seconds (occlusion-d), the instrument displays “Occlusion Time (OT)”, which is the time elapsed from the detection of the first drop of blood until OT. Later, the blood flow is completely arrested. Eventually, due to thrombolysis, flow is restored as indicated by the detection of the first drop of blood after complete occlusion (Lysis Time- LT). GTT can measure platelet reactivity and endogenous thrombolytic activity simultaneously. Compared to controls, increased or decreased OT indicates inhibition or enhancement of platelet reactivity, respectively. Increase or decrease of LT indicates inhibition or enhancement of endogenous (spontaneous) thrombolysis, respectively.
Four ml of blood was withdrawn from the abdominal aorta of anaesthetized rats with 21G needle into 10 ml syringe containing 4.0 ml pre-warmed (37°C) saline. The diluted native blood was inverted three times for mixing and 3.6 ml was transferred into 5 ml syringe in which 0.4 ml of garlic filtrate or saline (control) was added and the mixture was inverted three times for mixing and then transferred into a GTT test tube. Measurement started immediately after adding the blood to the test tube. The details of the measurements have been described previously [16].
Six animals were used for assessing antithrombotic and spontaneous thrombolytic activities in all samples, controls in x1 to x100 dilutions. Coefficient of variations (CV) of OT and LT measurements in GTT was 6.07% and 33.67%, respectively [17]. In humans, the intra-assay CV of OT was 10% and 6% for LT and the inter-assay CV was 8% for OT and 9% for LT [18] (Figure 1).
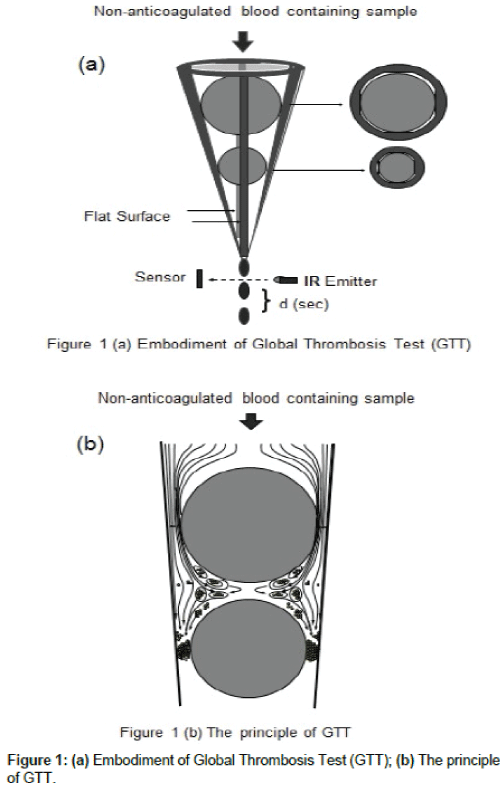
Figure 1: (a) Embodiment of Global Thrombosis Test (GTT); (b) The principle of GTT.
Statistical analysis
OT and LT were analyzed by repeated ANOVA, followed by post hoc (Dunnett). Values were expressed as percentages against saline (control) and means ± SEM. P<0.05 was considered as statistically significant. Analysis was performed by statistical package software (Unistat Light 5.6, Unistat Ltd., London, UK).
Results
Effects of garlic accessions on platelet reactivity and spontaneous (endogenous) thrombolytic activity
The effects of garlic filtrates diluted with saline on platelet reactivity (occlusion time, OT) and endogenous thrombolytic activity (lysis time, LT) were measured and shown as relative values (%) to controls in Table 2.
| Accessionname |
Dilution |
OT(mean±SEM) |
LT(mean±SEM) |
| Kagawawhite |
x1 |
223.6 |
± |
11.9b |
|
ND |
|
| x3 |
241.8 |
± |
17.9b |
|
ND |
|
| x10 |
228.1 |
± |
20.6b |
113.2 |
± |
53.9 |
| x30 |
112.7 |
± |
3.5b |
93.1 |
± |
8 |
| x100 |
105.9 |
± |
2.8a |
77.6 |
± |
6.9a |
| Ensyuuwase |
x1 |
234.2 |
± |
13.7b |
|
ND |
|
| (Kouchi) |
x3 |
210.3 |
± |
15.8b |
|
ND |
|
| |
x10 |
134.7 |
± |
5.6b |
89.3 |
± |
16.3 |
| |
x30 |
101.5 |
± |
2.2 |
77.7 |
± |
7.6 |
| |
x100 |
101.3 |
± |
1.6 |
90.5 |
± |
11.4 |
| Chuugokukei(Kananshoukei) |
x1 |
160.5 |
± |
4.8b |
|
ND |
|
| x3 |
167.9 |
± |
9.7b |
|
ND |
|
| x10 |
149.8 |
± |
2.2b |
99 |
± |
12.5 |
| x30 |
403.2 |
± |
2.3a |
77.3 |
± |
5.9a |
| x100 |
368.6 |
± |
3.6 |
80.1 |
± |
10.0a |
| Syanhaikei(Wakayama) |
x1 |
189.6 |
± |
9.9b |
|
ND |
|
| x3 |
177.7 |
± |
12.0b |
|
ND |
|
| x10 |
178.5 |
± |
6.6b |
59.8 |
± |
38.3a |
| x30 |
103.6 |
± |
5.2 |
112.5 |
± |
22.9 |
| x100 |
94.7 |
± |
4.8 |
115.5 |
± |
27.6 |
| Kateiwase(Ehime) |
x1 |
259 |
± |
15.1b |
|
ND |
|
| x3 |
258.1 |
± |
16.4b |
|
ND |
|
| x10 |
208.2 |
± |
18.3b |
89.4 |
± |
41.8 |
| x30 |
108.6 |
± |
4.2a |
92.9 |
± |
8.5 |
| x100 |
108.6 |
± |
1.6a |
77.2 |
± |
6.6a |
| Tottorizairai |
x1 |
173.9 |
± |
9.4b |
|
ND |
|
| x3 |
173.8 |
± |
16.9b |
|
ND |
|
| x10 |
164.8 |
± |
18.3b |
37.6 |
± |
26.2a |
| x30 |
106.6 |
± |
4.2 |
83.3 |
± |
6.1 |
| x100 |
100.1 |
± |
4.1 |
90.7 |
± |
12.3 |
| Oogawarazairai |
x1 |
242.9 |
± |
13.7b |
|
ND |
|
| x3 |
219.7 |
± |
8.7b |
|
ND |
|
| x10 |
144.8 |
± |
5.1b |
102 |
± |
8.3 |
| x30 |
105.7 |
± |
4.2 |
98.9 |
± |
20.1 |
| x100 |
98.6 |
± |
4.1 |
96.1 |
± |
21.8 |
| Chuugokukisyuukei |
x1 |
185.5 |
± |
11.5b |
|
ND |
|
| x3 |
180.5 |
± |
8.9b |
|
ND |
|
| x10 |
136.9 |
± |
3.2b |
106.6 |
± |
17.8 |
| x30 |
111.7 |
± |
3.5b |
96 |
± |
10.4 |
| x100 |
106.9 |
± |
2.9a |
89.8 |
± |
5.8 |
| 86196 |
x1 |
201.8 |
± |
23.5b |
|
ND |
|
| x3 |
192.9 |
± |
24.8b |
|
ND |
|
| x10 |
175.6 |
± |
16.2b |
|
ND |
|
| x30 |
111.6 |
± |
6.2a |
99.4 |
± |
15.4 |
| x100 |
103.6 |
± |
5 |
87.9 |
± |
15.5 |
| Chet’sItalianpurple |
x1 |
223.7 |
± |
11.5b |
|
ND |
|
| x3 |
208 |
± |
8.9b |
|
ND |
|
| x10 |
131.8 |
± |
5.2b |
89.6 |
± |
9 |
| x30 |
109.6 |
± |
4.2 |
92.4 |
± |
12.9 |
| x100 |
104.3 |
± |
3.9 |
101.4 |
± |
12.7 |
| Gravelswitch |
x1 |
185.8 |
± |
11.6b |
|
ND |
|
| x3 |
167.7 |
± |
10.7b |
|
ND |
|
| x10 |
121.8 |
± |
6.4a |
99.1 |
± |
10.6 |
| x30 |
102.9 |
± |
2.5 |
82.9 |
± |
5.4 |
| x100 |
98.4 |
± |
2.4 |
98.3 |
± |
9.5 |
| Namdo |
x1 |
254.4 |
± |
15.1b |
|
ND |
|
| x3 |
252.5 |
± |
10.9b |
|
ND |
|
| x10 |
170.5 |
± |
14.4b |
120.6 |
± |
12.4 |
| x30 |
105.2 |
± |
3.4 |
97.8 |
± |
17.6 |
| x100 |
101.4 |
± |
3.3 |
81.5 |
± |
8.6 |
| Whiteroppen |
x1 |
224.4 |
± |
15.3b |
|
ND |
|
| x3 |
178.9 |
± |
16.4b |
|
ND |
|
| x10 |
151.5 |
± |
7.4b |
89.5 |
± |
20.3 |
| x30 |
108.6 |
± |
4.7 |
98.4 |
± |
10.8 |
| x100 |
99.1 |
± |
1.9 |
83.9 |
± |
2.7 |
| RAR930048 |
x1 |
149.7 |
± |
10.0b |
|
ND |
|
| x3 |
151.3 |
± |
10.1b |
|
ND |
|
| x10 |
105.9 |
± |
2.7 |
127.9 |
± |
33.8 |
| x30 |
116.6 |
± |
6.0b |
96.9 |
± |
10.8 |
| x100 |
110.1 |
± |
2.8a |
101.4 |
± |
7.1 |
| 97KZ8 |
x1 |
203.7 |
± |
9.0b |
|
ND |
|
| x3 |
194.7 |
± |
10.4b |
|
ND |
|
| x10 |
145.5 |
± |
5.2b |
123.2 |
± |
11.6 |
| x30 |
103.1 |
± |
2.7 |
83.4 |
± |
8.9a |
| x100 |
100.1 |
± |
4.3 |
94.6 |
± |
5.4 |
| Tottorizairai |
x1 |
173.9 |
± |
9.4b |
|
ND |
|
| x3 |
173.8 |
± |
16.9b |
|
ND |
|
| x10 |
164.8 |
± |
18.3b |
37.6 |
± |
26.2a |
| x30 |
106.6 |
± |
4.2 |
83.3 |
± |
6.1 |
| x100 |
100.1 |
± |
4.1 |
90.7 |
± |
12.3 |
| Oogawarazairai |
x1 |
242.9 |
± |
13.7b |
|
ND |
|
| x3 |
219.7 |
± |
8.7b |
|
ND |
|
| x10 |
144.8 |
± |
5.1b |
102 |
± |
8.3 |
| x30 |
105.7 |
± |
4.2 |
98.9 |
± |
20.1 |
| x100 |
98.6 |
± |
4.1 |
96.1 |
± |
21.8 |
| Chuugokukisyuukei |
x1 |
185.5 |
± |
11.5b |
|
ND |
|
| x3 |
180.5 |
± |
8.9b |
|
ND |
|
| x10 |
136.9 |
± |
3.2b |
106.6 |
± |
17.8 |
| x30 |
111.7 |
± |
3.5b |
96 |
± |
10.4 |
| x100 |
106.9 |
± |
2.9a |
89.8 |
± |
5.8 |
| 86196 |
x1 |
201.8 |
± |
23.5b |
|
ND |
|
| x3 |
192.9 |
± |
24.8b |
|
ND |
|
| x10 |
175.6 |
± |
16.2b |
|
ND |
|
| x30 |
111.6 |
± |
6.2a |
99.4 |
± |
15.4 |
| x100 |
103.6 |
± |
5 |
87.9 |
± |
15.5 |
| Chet’sItalianpurple |
x1 |
223.7 |
± |
11.5b |
|
ND |
|
| x3 |
208 |
± |
8.9b |
|
ND |
|
| x10 |
131.8 |
± |
5.2b |
89.6 |
± |
9 |
| x30 |
109.6 |
± |
4.2 |
92.4 |
± |
12.9 |
| x100 |
104.3 |
± |
3.9 |
101.4 |
± |
12.7 |
| Gravelswitch |
x1 |
185.8 |
± |
11.6b |
|
ND |
|
| x3 |
167.7 |
± |
10.7b |
|
ND |
|
| x10 |
121.8 |
± |
6.4a |
99.1 |
± |
10.6 |
| x30 |
102.9 |
± |
2.5 |
82.9 |
± |
5.4 |
| x100 |
98.4 |
± |
2.4 |
98.3 |
± |
9.5 |
| Namdo |
x1 |
254.4 |
± |
15.1b |
|
ND |
|
| x3 |
252.5 |
± |
10.9b |
|
ND |
|
| x10 |
170.5 |
± |
14.4b |
120.6 |
± |
12.4 |
| x30 |
105.2 |
± |
3.4 |
97.8 |
± |
17.6 |
| x100 |
101.4 |
± |
3.3 |
81.5 |
± |
8.6 |
| Whiteroppen |
x1 |
224.4 |
± |
15.3b |
|
ND |
|
| x3 |
178.9 |
± |
16.4b |
|
ND |
|
| x10 |
151.5 |
± |
7.4b |
89.5 |
± |
20.3 |
| x30 |
108.6 |
± |
4.7 |
98.4 |
± |
10.8 |
| x100 |
99.1 |
± |
1.9 |
83.9 |
± |
2.7 |
| RAR930048 |
x1 |
149.7 |
± |
10.0b |
|
ND |
|
| x3 |
151.3 |
± |
10.1b |
|
ND |
|
| x10 |
105.9 |
± |
2.7 |
127.9 |
± |
33.8 |
| x30 |
116.6 |
± |
6.0b |
96.9 |
± |
10.8 |
| x100 |
110.1 |
± |
2.8a |
101.4 |
± |
7.1 |
| 97KZ8 |
x1 |
203.7 |
± |
9.0b |
|
ND |
|
| x3 |
194.7 |
± |
10.4b |
|
ND |
|
| x10 |
145.5 |
± |
5.2b |
123.2 |
± |
11.6 |
| x30 |
103.1 |
± |
2.7 |
83.4 |
± |
8.9a |
| x100 |
100.1 |
± |
4.3 |
94.6 |
± |
5.4 |
a: P<0.05; b: P<0.01 vs control
Table 2: Effect of fifteen garlic accessions on platelet reactivity (OT) and endogenous thrombolytic activity (LT) in vitro.
All accessions prolonged OTs showing inhibition of platelet reactivity, while some accessions shortened LTs showing enhanced thrombolytic activity. Significant differences against control were summarized in Table 3.
| Variety |
Dilution |
OT |
LT |
| Kagawawhite |
x1 |
<0.01 |
ND |
| x3 |
<0.01 |
ND |
| x10 |
<0.01 |
ns |
| x30 |
<0.01 |
ns |
| x100 |
<0.05 |
<0.05 |
| Ensyuuwase(Kouchi) |
x1 |
<0.01 |
ND |
| x3 |
<0.01 |
ND |
| x10 |
<0.01 |
ns |
| x30 |
ns |
ns |
| x100 |
ns |
ns |
| Chuugokukei(Kananshoukei) |
x1 |
<0.01 |
ND |
| x3 |
<0.01 |
ND |
| x10 |
<0.01 |
ns |
| x30 |
<0.05 |
<0.05 |
| x100 |
ns |
<0.05 |
| Syanhaikei(Wakayama) |
x1 |
<0.01 |
ND |
| x3 |
<0.01 |
ND |
| x10 |
<0.01 |
<0.05 |
| x30 |
ns |
ns |
| x100 |
ns |
ns |
| Kateiwase(Ehime) |
x1 |
<0.01 |
ND |
| x3 |
<0.01 |
ND |
| x10 |
<0.01 |
ns |
| x30 |
<0.05 |
ns |
| x100 |
<0.05 |
<0.05 |
| Tottorizairai |
x1 |
<0.01 |
ND |
| x3 |
<0.01 |
ND |
| x10 |
<0.01 |
<0.05 |
| x30 |
ns |
ns |
| x100 |
ns |
ns |
| Oogawarazairai |
x1 |
<0.01 |
ND |
| x3 |
<0.01 |
ND |
| x10 |
<0.01 |
ns |
| x30 |
ns |
ns |
| x100 |
ns |
ns |
| Chuugokukisyuukei |
x1 |
<0.01 |
ND |
| x3 |
<0.01 |
ND |
| x10 |
<0.01 |
ns |
| x30 |
<0.01 |
ns |
| x100 |
<0.05 |
ns |
| 86196 |
x1 |
<0.01 |
ND |
| x3 |
<0.01 |
ND |
| x10 |
<0.01 |
ND |
| x30 |
<0.05 |
ns |
| x100 |
ns |
ns |
| Chet’sItalianpurple |
x1 |
<0.01 |
ND |
| x3 |
<0.01 |
ND |
| x10 |
<0.01 |
ns |
| x30 |
ns |
ns |
| x100 |
ns |
ns |
| Gravelswitch |
x1 |
<0.01 |
ND |
| x3 |
<0.01 |
ND |
| x10 |
<0.05 |
ns |
| x30 |
ns |
ns |
| x100 |
ns |
ns |
| Namdo |
x1 |
<0.01 |
ND |
| x3 |
<0.01 |
ND |
| x10 |
<0.01 |
ns |
| x30 |
ns |
ns |
| x100 |
ns |
ns |
| Whiteroppen |
x1 |
<0.01 |
ND |
| x3 |
<0.01 |
ND |
| x10 |
<0.01 |
ns |
| x30 |
ns |
ns |
| x100 |
ns |
ns |
| RAR930048 |
x1 |
<0.01 |
ND |
| x3 |
<0.01 |
ND |
| x10 |
ns |
ns |
| x30 |
<0.01 |
ns |
| x100 |
<0.05 |
ns |
| 97KZ8 |
x1 |
<0.01 |
ND |
| x3 |
<0.01 |
ND |
| x10 |
<0.01 |
ns |
| x30 |
ns |
<0.05 |
| x100 |
ns |
ns |
| Tottorizairai |
x1 |
<0.01 |
ND |
| x3 |
<0.01 |
ND |
| x10 |
<0.01 |
<0.05 |
| x30 |
ns |
ns |
| x100 |
ns |
ns |
| Oogawarazairai |
x1 |
<0.01 |
ND |
| x3 |
<0.01 |
ND |
| x10 |
<0.01 |
ns |
| x30 |
ns |
ns |
| x100 |
ns |
ns |
| Chuugokukisyuukei |
x1 |
<0.01 |
ND |
| x3 |
<0.01 |
ND |
| x10 |
<0.01 |
ns |
| x30 |
<0.01 |
ns |
| x100 |
<0.05 |
ns |
| 86196 |
x1 |
<0.01 |
ND |
| x3 |
<0.01 |
ND |
| x10 |
<0.01 |
ND |
| x30 |
<0.05 |
ns |
| x100 |
ns |
ns |
| Chet’sItalianpurple |
x1 |
<0.01 |
ND |
| x3 |
<0.01 |
ND |
| x10 |
<0.01 |
ns |
| x30 |
ns |
ns |
| x100 |
ns |
ns |
| Gravelswitch |
x1 |
<0.01 |
ND |
| x3 |
<0.01 |
ND |
| x10 |
<0.05 |
ns |
| x30 |
ns |
ns |
| x100 |
ns |
ns |
| Namdo |
x1 |
<0.01 |
ND |
| x3 |
<0.01 |
ND |
| x10 |
<0.01 |
ns |
| x30 |
ns |
ns |
| x100 |
ns |
ns |
| Whiteroppen |
x1 |
<0.01 |
ND |
| x3 |
<0.01 |
ND |
| x10 |
<0.01 |
ns |
| x30 |
ns |
ns |
| x100 |
ns |
ns |
| RAR930048 |
x1 |
<0.01 |
ND |
| x3 |
<0.01 |
ND |
| x10 |
ns |
ns |
| x30 |
<0.01 |
ns |
| x100 |
<0.05 |
ns |
| 97KZ8 |
x1 |
<0.01 |
ND |
| x3 |
<0.01 |
ND |
| x10 |
<0.01 |
ns |
| x30 |
ns |
<0.05 |
| x100 |
ns |
ns |
Probability: vs control; ND: not determined; ns: not significant
Table 3: Effect of fifteen garlic accessions on platelet reactivity (OT) and endogenous thrombolytic activity (LT) in vitro.
Heat stability of antithrombotic activity
As found in our earlier animal experiments, the overall antithrombotic activity was governed by the balance between antiplatelet and thrombolytic (fibrinolytic) activities. Further, heatsensitivity of the antithrombotic effect was very important [13,16]. Heat stability of five garlic accessions with strong antiplatelet activity out of fifteen accessions were further examined after treating them at 100°C for 10 minutes. The result is shown in Table 4. Antiplatelet activity was heat stable in all accessions and increased endogenous thrombolytic activity was found in some accessions Table 4.
| Accessionname |
Heattreatment |
OT |
LT |
| Kagawawhite |
before |
176.6±8.4b |
ND |
| after |
180.7±15.4b |
62.3±40.6a |
| Chuugokukei(Kananshoukei) |
before |
185.7±16.1b |
ND |
| |
after |
179.3±11.9b |
ND |
| Kateiwase(Ehime) |
before |
150.4±3.7b |
74.3±31.1 |
| |
after |
152.7±7.8b |
85.3±68.9a |
| Chuugokukisyuukei |
before |
152.4±5.1b |
104.9±29.6 |
| |
after |
133.5±4.3b |
103.5±9.1 |
| 86196 |
before |
175.6±14.3b |
25.2±16.3b |
| |
after |
186.2±9.2b |
15.7±15.6b |
a: P<0.05; b: P<0.01 vs control
Table 4: Heat stability of platelet reactivity and thrombolytic activity.
Discussion
In establishing an antithrombotic diet, finding dietary components with potential antithrombotic effect, the choice of pathologically relevant technique(s) of measuring experimental antithrombotic effect is of crucial importance. Only such test(s) which already proved to be useful in clinical practice in monitoring the overall thrombotic status and predicting major adverse thrombotic events should be used.
Despite platelets play a pivotal role in thrombosis, point-ofcare platelet function tests failed to materialize clinical expectations. Tailoring antithrombotic medication based on monitoring platelet function by these tests did not improve clinical outcome [19-21]. At present, prothrombotic status is assessed by measuring platelet aggregation to various soluble agonists (adenosine diphosphate, collagen, arachidonic acid, thrombin), and by extrapolating from the results obtained by the use of various biomarkers of coagulation and fibrinolysis [22-24]. The major shortcoming of all these tests is the use of anticoagulated blood, in which activated platelets do not generate thrombin, the most significant contributor to arterial thrombogenesis. This could be the reason why most of platelet function tests which measure platelet aggregation to various soluble agonists failed in cardiac patients guiding antithrombotic medication.
It has been shown that only those tests, which take the arterial high shear and flow conditions as well as generation of thrombin by activated platelets into account, have relevance to the patho mechanism of occlusive arterial thrombosis in vivo. We compared results obtained by commonly used platelet function tests performed from anticoagulated blood and those obtained by using shear-induced thrombosis and thrombolysis test performed from non-anticoagulated blood. Our findings showed that the commonly used platelet function tests performed at low shear rates and from anticoagulated blood did not reflect the overall thrombotic status, whilst the innovative shear-induced thrombosis tests performed from non-anticoagulated blood did [25,26]. We have also shown in animal experiments that the use of high shear stress-induced thrombosis in vitro tests using non-anticoagulated blood provided reliable assessment of the global thrombotic status in vivo [13]. In addition, GTT has been shown to be clinically useful for monitoring thrombotic status in patients on antithrombotic medication [27,28]. It was therefore reasonable to employ this technique in testing fruits and vegetables and herbal drugs for antithrombotic effects.
We assessed various fruit and vegetable varieties for antithrombotic activity with shear-induced thrombotic and thrombolytic test (GTT). Even within the same species, the experimental antithrombotic effect depended on the variety of tested fruits and vegetables. While some varieties showed significant antithrombotic effect, other varieties had the opposite, prothrombotic activity [29]. In this study all garlic accessions had antithrombotic activity, though the activities were very diverse compared other fruit and vegetable varieties. Heat treatment did not affect antithrombotic activity of garlic though it did affect in carrot varieties [16]. The relevance of testing fruits and vegetables by GTT in animal blood ex vivo to humans was shown by our finding that oral intake of an experimentally proven antithrombotic strawberry variety also ameliorated the thrombotic status in humans [30].
For the antithrombotic effect of garlic various mechanisms have been suggested [31-35]. In the present study we focused on the overall antithrombotic activity of garlic accessions, but we intend to use GTT in the future for further investigation into the mechanism of antithrombotic effect of garlic.
Conclusion
Antithrombotic activity of garlic accessions was measured in rats ex vivo by the Global Thrombosis Test (GTT). All accessions showed antithrombotic activity based mainly on inhibition of platelet reactivity. The antithrombotic activity was heat stable. Our findings suggest that daily intake of garlic as an ingredient of an antithrombotic diet may be beneficial in preventing arterial thrombotic disorders.
9484
References
- Kendler BS (1987) Garlic (Allium sativum) and onion (Allium cepa): a review of their relationship to cardiovascular disease. Prev Med 16: 670-685.
- Rahman K, Lowe GM (2006) Garlic and cardiovascular disease: a critical review. JNutr 136: 736S-740S.
- McEwen BJ (2015) The influence of herbal medicine on platelet function and coagulation: a narrative review. SeminThrombHemost 41: 300-314.
- Ulbricht TL, Southgate DA (1991) Coronary heart disease: seven dietary factors. Lancet 338: 985-992.
- De Lorgeril M, Renaud S, Mamelle N, Salen P, Martin JL, et al. (1994) Mediterranean alpha-linolenic acid-rich diet in secondary prevention of coronary heart disease. Lancet 343: 1454-1459.
- Kromhout D, Menoti A, Kesteloot H, Sans S (2002) Prevention of coronary heart disease by diet and lifestyle evidence from prospective cross-cultural, cohort and intervention studies. Circulation 105: 893-898.
- Trichopoulou A, Costacou T, Bamia C, Trichopoulos D (2003) Adherence to a mediterranean diet and survival in a greek population. N Engl J Med 348: 2599-2608.
- Key TJ, Appleby PN, Rosell MS (2006) Health effects of vegetarian and vegan diets. ProcNutrSoc 65: 35-41.
- Lichtenstein AH, Appel LJ, Brands M, Carnethon M, Daniels S, et al. (2006) Summary of American heart association diet and lifestyle recommendations revision 2006. ArteriosclerThrombVascBiol 26: 2186-2191.
- Iso H, Kobayashi M, Ishihara J, Sasaki S, Okada K, et al. (2006) Intake of fish and n3 fatty acids and risk of coronary heart disease among Japanese the Japan public health center-based (JPHC) study cohort I. Circulation 113: 195-202.
- Tada N, Maruyama C, Koba S, Tanaka H, Birou S, et al. (2011) Japanese dietary lifestyle and cardiovascular disease. J AtherosclerThromb 18: 723-734.
- Hoffman R, Gerber M (2013) Evaluating and adapting the mediterranean diet for non-mediterranean populations: A critical appraisal. Nutrition Reviews 71: 573-584.
- Yamamoto J, Tamura Y, Ijiri Y, Iwasaki M, Murakami M, et al. (2015) Evaluation of antithrombotic effect: importance of testing components and methodologies. Drug DiscovTher 9: 258-266.
- Yamamoto J, Yamashita T, Ikarugi H, Taka T, Hashimoto M, et al. (2003) Görög Thrombosis Test: a global in-vitro test of platelet function and thrombolysis. Blood Coagul Fibrinolysis 14: 31-39.
- Yamamoto J, Inoue N, Otsui K, Ishii H, Gorog DA (2014) Global Thrombosis Test (GTT) can detect major determinants of haemostasis including platelet reactivity, endogenous fibrinolytic and thrombin generating potential. Thromb Res 133: 919-926.
- Yamamoto J, Naemura A, Ijiri Y, Ogawa K, Suzuki T, et al. (2008) The antithrombotic effects of carrot filtrates in rats and mice. Blood Coagul Fibrinolysis 19: 785-792.
- Yamamoto J, Naemura A, Ura M, Ijiri Y, Yamashita T, et al. (2006) Testing various fruits for anti-thrombotic effect: I. Mulberries.Platelets17:555-564.
- Farag M, Niespialowska-Steuden M, Okafor O, Artman B, Srinivascan M, et al. (2016) Relative effects of different non-vitamin K antagonist oral anticoagulants on global thrombotic status in atrial fibrillation. Platelets.
- Gorog DA, Fuster V (2013) Platelet function tests in clinical cardiology: unfulfilled expectations. J Am CollCardiol 61: 2115-2129.
- Okafor ON, Gorog DA (2015) Endogenous fibrinolysis: an important mediator of thrombus formation and cardiovascular risk. J Am CollCardiol 65: 1683-1699.
- Gorog DA, Jeong YH (2015) Platelet function tests: why they fail to guide personalized antithrombotic medication. J Am Heart Assoc 4: e002094.
- Paniccia R, Priora R, Liotta AA, Abbate R (2015) Platelet function tests: a comparative review. Vasc Health Risk Manag 11: 133-148.
- Rosenson RS, Brewer HB, Rader DJ (2014) Lipoproteins as biomarkers and therapeutic targets in the setting of acute coronary syndrome. Circ Res 114: 1880-1889.
- Lowe G, Rumley A (2014) The relevance of coagulation in cardiovascular disease: what do the biomarkers tell us? ThrombHaemost 112: 860-867.
- Gorog DA, Kovacs IB (1995) Thrombotic status analyser. Measurement of platelet-rich thrombus formation and lysis in native blood. ThrombHaemost 73: 514-520.
- Nakajima S, Noguchi T, Taka T, Ueda T, Kaizu K, et al. (2000) A global platelet test of thrombosis and thrombolysis detects a prothrombotic state in some patients with non-insulin dependent diabetes and in some patients with stroke. Platelets 11: 459-466.
- Taomoto K, Ohnishi H, Kuga Y, Nakashima K, Ichioka T, et al. (2010) Platelet function and spontaneous thrombolytic activity of patients with cerebral infarction assessed by the global thrombosis test. PathophysiolHaemostThromb 37: 43-48.
- Otsui K, Gorog DA, Yamamoto J, Yoshioka T, Iwata S, et al. (2015) Global Thrombosis Test - a possible monitoring system for the effects and safety of dabigatran.Thromb J 13: 39.
- Hyodo K, Horii I, Nishino M, Giddings JC, Yamamoto J (2011) The antithrombotic effects of onion filtrates in rats and mice. Health 3: 319-325.
- Naemura A, Ohira H, Ikeda M, Koshikawa K, Ishii H, et al (2006) An experimentally antithrombotic strawberry variety is also effective in humans. PathophysiolHaemostThromb 35: 398-404.
- Ariga T, Oshiba S, Tamada T (1981) Platelet aggregation inhibitor in garlic. Lancet 1: 150-151.
- Banerjee SK, Maulik SK (2002) Effect of garlic on cardiovascular disorders: A review. Nutr J 1:4.
- Iciek M, Kwiecien I, Wlodek L (2009) Biological properties of garlic and garlic-derived organosulfur compounds. Environ Mol Mutagen 50: 247-265.
- Khatua TN, Adela R, Banerjee SK (2013) Garlic and cardioprotection insights into the molecular mechanisms. Can J PhysiolPharmacol 91: 448-458.
- Seki T, Hosono T (2015) Prevention of cardiovascular diseases by garlic-derived sulfur compounds. J NutrSciVitaminol 61: S83-S85.







