Keywords
Augmenter of Liver Regeneration (ALR); Mouse prosencephalon; Oxidative Stress (OS); Neurodegenerative diseases; Sex steroid hormones; Sexual dimorphism
Introduction
The correct functioning of neurons is based on the availability of a huge amount of ATP obtained through their enhanced mitochondrial oxidative phosphorylation (OXPHOS) and on the maintenance of normoxic conditions [1]. The oxidative stress (OS) is the result of an imbalance between production of reactive oxygen species (ROS) and efficiency of the counteracting antioxidative systems [2-4]. In the central nervous system (CNS), an excess of ROS results in a progressive, OS-related, impairment of neurons that can lead up to their death [5-7]. A condition that causes increase of ROS in CNS neurons is represented by their enhanced OXPHOS, of which ROS are a by-product [3,4]. These indications support the results of clinical studies reporting that the OS-induced damage on CNS neurons is primarily involved in the pathogenesis of several nervous disorders, including neurodegenerative diseases [8-13]. Different studies have pointedout that brain displays evident sexual dimorphism, which consists of biochemical, structural, and functional differences between female and male neurons [14,15]. The sexual dimorphism of neurons is determined by genetic, epigenetic, and environmental factors and is particularly evident in some CNS regions, such as hypothalamus, limbic lobe, olfactory lobe, amygdaloid nuclear complex and hippocampus [16-20].
An interesting aspect of the sexual dimorphism of CNS neurons concerns the efficiency of their antioxidative defenses. In fact, studies carried out both in humans and experimental animals have reported that male CNS neurons have higher levels of ROS and of markers of DNA oxidation and lipid peroxidation than female CNS neurons, mainly due to different efficiency of the antioxidative systems between female and male CNS neurons may be determined, at least partly, by the antioxidative effect of female sex steroid hormones, particularly the estrogens [21-25]. In fact, it has been observed that a loss of estrogens, howsoever caused, determines a reduction of the antioxidative defenses, associated with neuroinflammation, neuronal functional impairment and neurodegeneration [26].
It is largely accepted that the sexual dimorphism of CNS neurons differently impacts on epidemiology of some human neurodegenerative diseases: for example, the Alzheimer’s disease (AD) affects women more than men, particularly in aged subjects, when the transition to menopause, associated with a drastic drop of estrogens, increases the risk of AD [27]. On the contrary, the Parkinson’s disease (PD) affects men much more than women, suggesting that the neuroprotective effect of estrogens towards PD is more efficient than that of androgens [28-30].
In view of the pivotal participation of mitochondria in (i) the maintenance of cell normoxic conditions [31,32] (ii) the process of intrinsic apoptosis [33-36], and (iii) the modulation of metabolism of female sex steroid hormones, molecules able to produce antioxidative, antiapoptotic and mitochondrial-protective effect could play a primary role in the development and progression of neurodegenerative diseases, in particular, of those related to a sexual dimorphism of neurons [37-39]. Among the molecules having such characteristics, there is the augmenter of liver regeneration (ALR), identified by Francavilla and coworkers [40].
Starting from this hypothesis, the present study was undertaken with the aim of (i) analyzing the ALR presence in the prosencephalon (forebrain) of female and male mouse, and (ii) evaluating the existence of different expression of it between the two genders, assessing the existence of sexual dimorphic expression of ALR in CNS neurons, similarly to the muscle fibers [41].
The study was carried out on the prosencephalon of female and male adult mouse. The choice of using the prosencephalon was motivated by the following considerations: (i) the prosencephalic neurons display evident sexual dimorphism [16-18,20]; (ii) the prosencephalon is a CNS region with high concentration of ALR-expressing neurons [42]; (iii) the prosencephalic neurons, particularly those located in the isocortex, hippocampus and amygdaloid nuclear complex, are highly vulnerable to ROS [43]; (iv) the prosencephalon is one of the CNS regions most frequently and early affected by neurodegenerative diseases [44-46].
Methods
A total of 20 Mus musculus C57BL6J mice, 10 females and 10 males, 8-10 weeks old and weighing 20 to 30 g were used. The animals were housed in compliance with the guidelines promulgated by the Italian Ministry of Health (DL 116/92 and DL 111/94-B) and the Helsinki declaration concerning the care and use of experimental animals. Use of prosencephalon of mice sacrificed was part of an experimental project authorized by the Italian Ministry of Health, Minsal No. 996/2015-PR.
The animals were sacrificed by cervical dislocation. Brains of sacrificed animals were removed from cranial cavity and rinsed in saline solution for 30 min at 4°C. The prosencephala were isolated from the brains by a precollicular cut and then dissected on the midsagittal plane into their right and left halves (hemiprosencephala). Finally, the hemiprosencephala were subdivided by 2 coronary sections into 3 fragments, anterior (AH), middle (MH), and posterior (PH) (Figure 1).
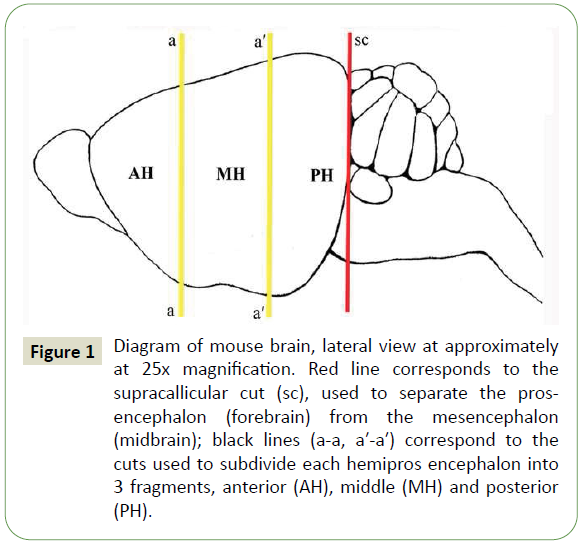
Figure 1: Diagram of mouse brain, lateral view at approximately at 25x magnification. Red line corresponds to the supracallicular cut (sc), used to separate the prosencephalon (forebrain) from the mesencephalon (midbrain); black lines (a-a, a′-a′) correspond to the cuts used to subdivide each hemipros encephalon into 3 fragments, anterior (AH), middle (MH) and posterior (PH).
The fragments coming from the left hemiprosencephala were subjected to Western blot analysis for ALR determination; the 3 fragments from the right hemiprosencephala were subjected to morphological methods (histological and immunohistochemical techniques) for structural analyses of nervous tissues and ALR expression.
All the experiments were conducted using a commercial, polyclonal anti-ALR primary antibody (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA). This antibody was raised in rabbit against the conserved carboxy-terminal domain of the fulllength human protein, present in all the ALR isoforms. The high specificity of this anti-ALR antibody was assessed in previous experiments demonstrating the capability to recognize ALR both by immunohistochemistry and WB analysis [47].
Western blot analysis
The Western blot analysis under denaturing electrophoresis conditions was performed on the fragments from the left hemiprosencephala of all female and male mice.
The fragments were suspended in ice cold immunoprecipitation assay buffer (Pierce RIPA buffer; Thermo Scientific, Rockford, IL, USA) with anti-proteases (Complete mini protease inhibitor; Roche, Milan, Italy) and anti-phosphatases (sodium orthovanadate 2 mM; Sigma Aldrich, Milan, Italy) freshly added, homogenized and centrifuged at 14000 rpm for 15 min at 4°C. Aliquots of 50 μg of protein concentration, determined by the standard Bradford assay (Bio-Rad Laboratories, Milan, Italy), were separated in a 4-12% pre-cast polyacrylamide gels (Invitrogen, Life Technologies, Monza, Italy), and transferred onto a polyvinylidene fluoride (PVDF) 0.2 μm membrane (Bio- Rad Laboratories) with Transblot turbo (Bio-Rad Laboratories) for 12 min. A 5% non-fat dry milk in tris-buffered saline-polysorbate 20 (TBST) blocking solution was applied, and the membranes were incubated overnight at 4°C with anti-ALR primary antibody (polyclonal antibody raised in rabbit against the conserved carboxy-terminal domain of the full-length human protein, able to reveal all ALR isoforms; Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA), diluted 1:500 in TBST. Then the membranes were incubated with horseradish peroxidase-conjugated secondary antibody (Bio-Rad Laboratories, Milan, Italy), diluted 1:10000 in TBST for 60 min at room temperature (RT), and the complex detected by chemiluminescence (ECL; Thermo Scientific, Rockford, IL, USA). To detect the β-actin housekeeping protein, the membranes were washed in the restore Western blot stripping buffer (Pierce Biotechnology, Rockford, Illinois, USA), for 15 min, and reprobed with the anti-β-actin antibody, diluted 1:1000 in TBST (Santa Cruz Biotechnology). The densitometric analysis of each protein-related signal was performed using the molecular imager ChemidocTM (Bio-Rad Laboratories) and normalized against β-actin.
Morphological analyses
The fragments from the right hemiprosencephala of all female and male mice were immersed in 0.1M phosphate buffered saline at pH 7.6 (PBS) for 2 h at 4°C, and then in a saline fixative solution containing 10.0% formaldehyde in PBS for 3 h at 4°C. After fixation, the fragments were embedded in paraffin and sectioned into 5 μm coronal serial sections. From each series of coronal sections, couples of adjacent sections were selected at intervals of 300 μm: one was stained with toluidine blue technique, to evaluate the microscopic structure of the nervous tissues; one was stained with immunohistochemical techniques for ALR, to evaluate the fine distribution patterns of the ALR-containing elements in the nervous tissues. Overall, 10 toluidine blue and 10 ALR stained sections, representative of the entire fragment, were used. The immunohistochemistry for ALR was performed using the above mentioned anti-ALR antibody and the immunoreactions were revealed using the immunoenzymatic technique, streptavidinbiotinperoxidase. Briefly, the sections were deparaffinized, rehydrated, and subjected to the following procedure: incubation with 3.0% hydrogen peroxide solution in water (quenching of endogenous peroxidase activity) for 30 min at room temperature (RT); rinse in PBS 3 times for 10 min at RT; thermostatic bath in 0.1 M ethylene diamine tetracetic acid buffer at pH 9.0 (EDTA) (antigen retrieval) for 20 min at 98°C; air cooling for 20 min at RT; rinse in PBS; incubation with blocking solution containing 1.0% bovine serum albumin (BSA), 5.0% fetal calf serum (FCS) and 10.0% donkey serum, diluted 1:10 in PBS for 60 min at RT; incubation with anti-ALR antibody, diluted 1:50 in blocking solution overnight at 4°C; rinse in PBS; incubation with donkey biotinylated secondary antibody (Santa Cruz Biotechnology Inc.), diluted 1:100 in blocking solution for 60 min at RT; rinse in PBS; incubation with streptavidin-peroxidase complex solution (Vector Laboratories, Burlingame, CA, USA) for 40 min at RT; incubation with 3,3’-diaminobenzidine (DAB; Vector Laboratories) for 10 min at RT. Negative controls of the immunoreactions were performed on randomly chosen sections (control sections) from each series, by replacing the anti-ALR antibody solution with the blocking solution.
Qualitative analyses: All the selected toluidine blue- and ALRstained sections and the control sections were observed under the light photomicroscope Vanox-T (Olympus Italia SRL, Segrate, Italy) equipped with a color video camera (Spot Insight Color V3, Diagnostic Instruments Inc., Sterling Heights, MI, USA). Observations on the toluidine blue-stained sections served to recognize in the sections the regions of interest (ROI), where quantitative analyzes of ALR immunoreactivity would be conducted.
Quantitative analyses: The toluidine blue-stained sections from the female and male hemiprosencephala were scanned at the maximum available magnification (40x), using the wholeslide morphometric analysis scanning platform Aperio ScanScope CS (Leica Biosystems, Nussloch, Germany), and stored as digital high-resolution images on the workstation associated with the instrument. Digital images representative of the whole section of hemiprosencephalon were inspected with Aperio ImageScope v. 11 software (Leica Biosystems) at 25x magnification. In these images, the manual delimitation of the ROI was made using the pen tool function of the software. In AH, the ROI included the isocortex (neocortex) of the dorsolateral and medial surface of telencephalon, comprising the prefrontal, premotor and motor cortex (AH-1); the allocortex (archicortex) of ventral surface of the telencephalon (olfactory cortex; AH-2); and the anterior basal nuclei, comprising the ventral striatum (basal forebrain) and amygdaloid nuclear complex (AH-3). In the MH, the ROI included the isocortex of the dorsolateral and medial surface of telencephalon (sensory cortex) (MH-1); the allocortex of hippocampus, comprising the dentate gyrus and anterior region of the hippocampal formation (MH-2); and the posterior basal nuclei (dorsal striatum; MH3). In the PH, the ROI included the isocortex of the dorsolateral and medial surface of telencephalon (visual cortex; PH-1); the allocortex of hippocampus (posterior region of the hippocampal formation; PH-2); and the diencephalon, comprising the thalamus and hypothalamus (PH-3).
In the ALR-stained sections, the immunoreactive neuronal bodies present in each ROI were discriminated using a semiautomatic thresholding segmentation function of the software. Subsequently, 15 fields of 160 × 103 μm 2 (reference area, RA) were randomly superimposed on the ROI, and the total number of ALRimmunoreactive neuronal bodies per RA (n/RA) was calculated using a fully automated image measurement function of the software. The series of measurements, were collected separately from the fragments of female and male hemiprosencephala, were expressed as mean (M) ± standard deviation (SD).
Statistical analysis
To determine differences between the ALR distribution in the fragments of female and male prosencephala, the data obtained from both molecular biology and morphological analyses were evaluated by one-way analysis of variance (ANOVA). Statistical significance was ascribed to data when p
Results
ALR prosencephalic Western blot analysis
The ALR expression, as evaluated by Western blot analysis on the fragments from the left female and male hemiprosencephala, is illustrated in Figure 2. The analysis, carried out under denaturing electrophoresis conditions using the above mentioned anti- ALR polyclonal antibody, revealed 2 monomeric isoforms of ALR, ALR-21 and ALR-23, in both sex-derived prosencephalic tissues (Figure 2a). The higher expression of ALR-23 confirms the involvement of this isoform, identified in the mitochondrial intermembrane space [47,48], in the mitochondrial metabolism of prosencephalic neurons. The expression of both protein isoforms was significantly reduced in female-derived fragments compared to the male-derived ones (Figure 2b).
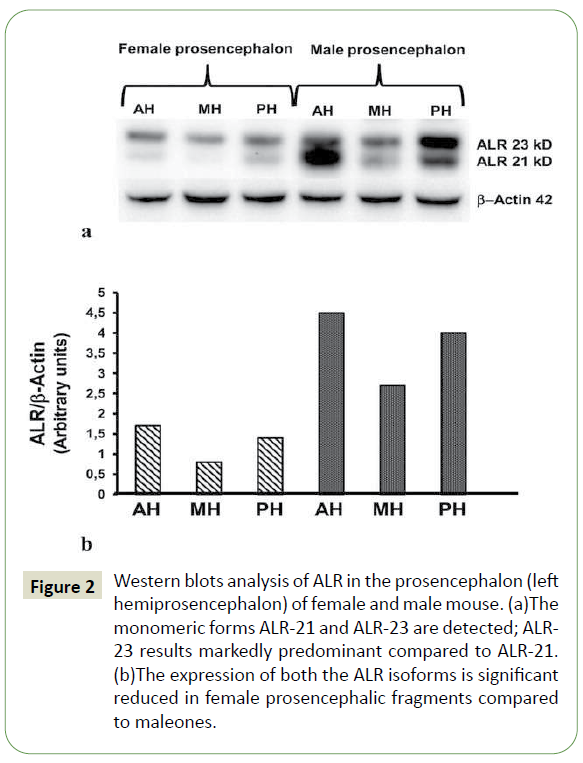
Figure 2: Western blots analysis of ALR in the prosencephalon (left hemiprosencephalon) of female and male mouse. (a)The monomeric forms ALR-21 and ALR-23 are detected; ALR- 23 results markedly predominant compared to ALR-21. (b)The expression of both the ALR isoforms is significant reduced in female prosencephalic fragments compared to maleones.
ALR Immunohistochemical analysis
Qualitative analysis: Immunoreactions for ALR stained the cytoplasm of neurons widely distributed in the female and male prosencephalon samples (right hemiprosencephala). In general, the immunoreactivity was mainly detected in neuronal bodies and large (proximal) processes (Figures 4-6). It also extended to most distal branches of processes, as suggested by the detection of immunoreactivity in the neuropil (‘background of immunoreactivity’), present in the sections despite the use of techniques that quench the non-specific background (Figures 3-5). The qualitative distribution patterns of ALR immunoreactivity in the ROI of all the examined fragments from female and male prosencephalon appeared superimposable.
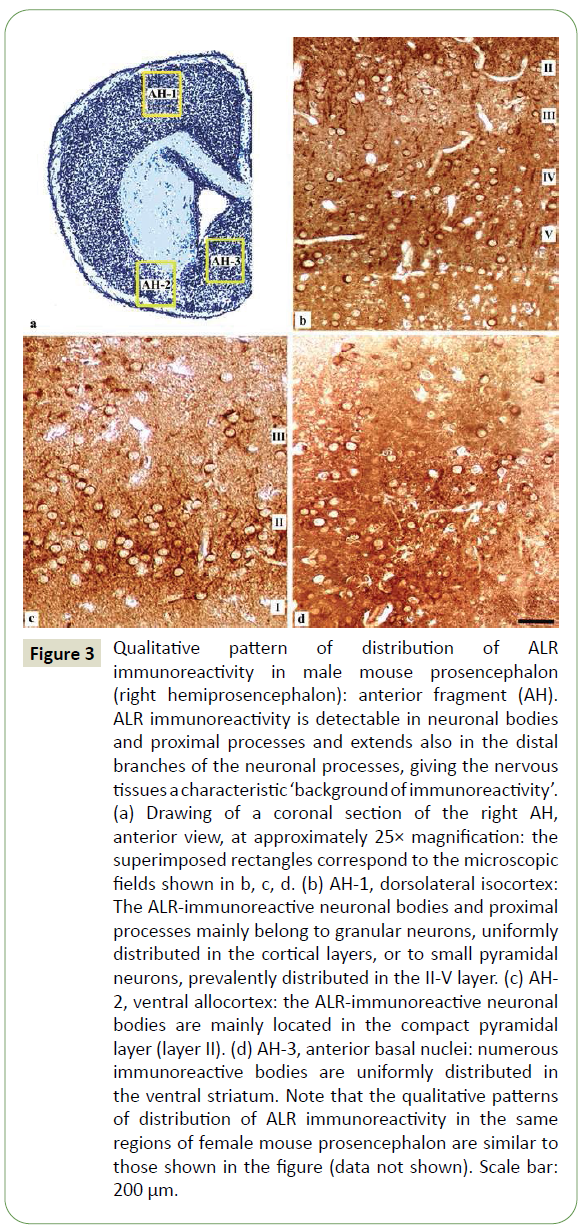
Figure 3: Qualitative pattern of distribution of ALR immunoreactivity in male mouse prosencephalon (right hemiprosencephalon): anterior fragment (AH). ALR immunoreactivity is detectable in neuronal bodies and proximal processes and extends also in the distal branches of the neuronal processes, giving the nervous tissues a characteristic ‘background of immunoreactivity’. (a) Drawing of a coronal section of the right AH, anterior view, at approximately 25× magnification: the superimposed rectangles correspond to the microscopic fields shown in b, c, d. (b) AH-1, dorsolateral isocortex: The ALR-immunoreactive neuronal bodies and proximal processes mainly belong to granular neurons, uniformly distributed in the cortical layers, or to small pyramidal neurons, prevalently distributed in the II-V layer. (c) AH- 2, ventral allocortex: the ALR-immunoreactive neuronal bodies are mainly located in the compact pyramidal layer (layer II). (d) AH-3, anterior basal nuclei: numerous immunoreactive bodies are uniformly distributed in the ventral striatum. Note that the qualitative patterns of distribution of ALR immunoreactivity in the same regions of female mouse prosencephalon are similar to those shown in the figure (data not shown). Scale bar: 200 μm.
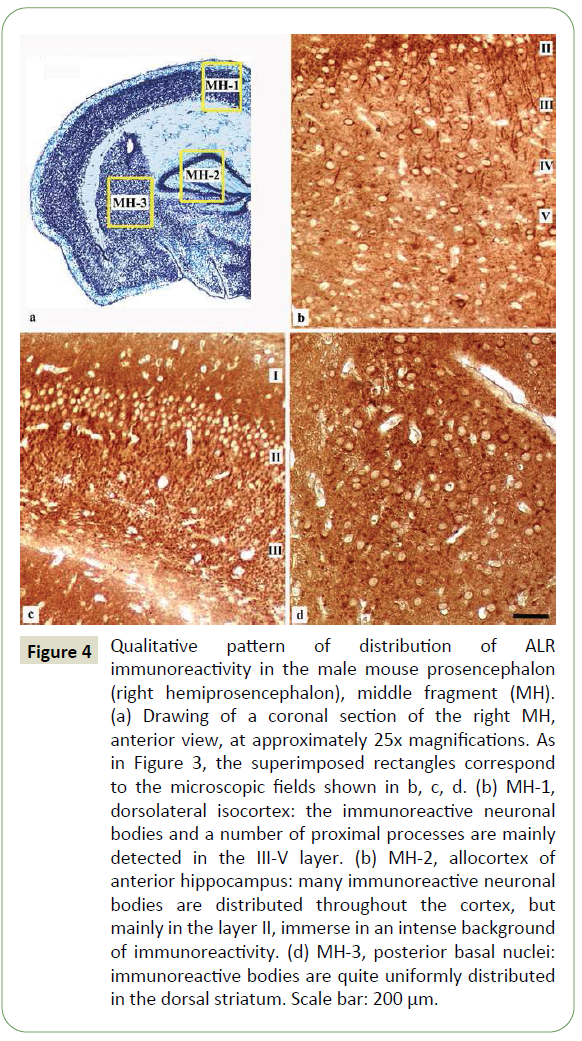
Figure 4: Qualitative pattern of distribution of ALR immunoreactivity in the male mouse prosencephalon (right hemiprosencephalon), middle fragment (MH). (a) Drawing of a coronal section of the right MH, anterior view, at approximately 25x magnifications. As in Figure 3, the superimposed rectangles correspond to the microscopic fields shown in b, c, d. (b) MH-1, dorsolateral isocortex: the immunoreactive neuronal bodies and a number of proximal processes are mainly detected in the III-V layer. (b) MH-2, allocortex of anterior hippocampus: many immunoreactive neuronal bodies are distributed throughout the cortex, but mainly in the layer II, immerse in an intense background of immunoreactivity. (d) MH-3, posterior basal nuclei: immunoreactive bodies are quite uniformly distributed in the dorsal striatum. Scale bar: 200 μm.
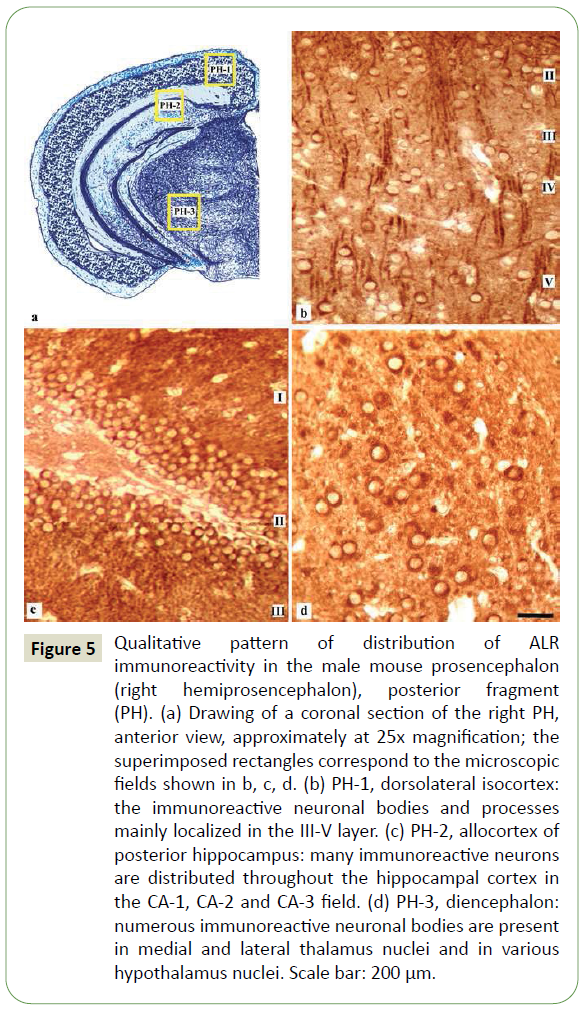
Figure 5: Qualitative pattern of distribution of ALR immunoreactivity in the male mouse prosencephalon (right hemiprosencephalon), posterior fragment (PH). (a) Drawing of a coronal section of the right PH, anterior view, approximately at 25x magnification; the superimposed rectangles correspond to the microscopic fields shown in b, c, d. (b) PH-1, dorsolateral isocortex: the immunoreactive neuronal bodies and processes mainly localized in the III-V layer. (c) PH-2, allocortex of posterior hippocampus: many immunoreactive neurons are distributed throughout the hippocampal cortex in the CA-1, CA-2 and CA-3 field. (d) PH-3, diencephalon: numerous immunoreactive neuronal bodies are present in medial and lateral thalamus nuclei and in various hypothalamus nuclei. Scale bar: 200 μm.
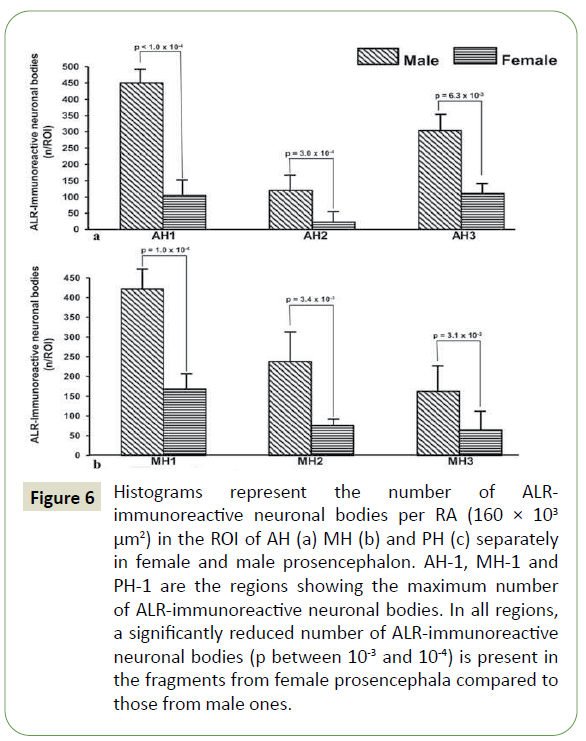
Figure 6: Histograms represent the number of ALRimmunoreactive neuronal bodies per RA (160 × 103 μm2) in the ROI of AH (a) MH (b) and PH (c) separately in female and male prosencephalon. AH-1, MH-1 and PH-1 are the regions showing the maximum number of ALR-immunoreactive neuronal bodies. In all regions, a significantly reduced number of ALR-immunoreactive neuronal bodies (p between 10-3 and 10-4) is present in the fragments from female prosencephala compared to those from male ones.
AH: In AH-1 (dorsolateral and medial isocortex), the immunoreactive neuronal bodies and processes mainly belonged to granular neurons, distributed in all cortical layers, and to small pyramidal neurons in the II-V layer (Figure 3a). In AH-2 (ventral allocortex), many neurons located in the compact pyramidal layer of the olfactory cortex (layer II) appeared immunoreactive; moreover, intense background of immunoreactivity was observed, particularly in the external molecular layer (layer I) (Figure 3b). In AH-3 (anterior basal nuclei), the immunoreactive neurons were mainly observed in the ventral striatum, particularly in the nucleus of diagonal band (of Broca) and nucleus accumbens, and in the amygdaloid nuclear complex (Figure 3c).
MH: In MH-1 (dorsolateral and medial isocortex), the sensory cortex displayed immunoreactive neuronal bodies and processes, mainly in the III-V layer (Figure 4a). In MH-2 (allocortex of anterior hippocampus), many neurons distributed throughout its thickness were detected; almost all neuronal bodies in the pyramidal layer (layer II) appeared immunoreactive, immerse in an intense background of immunoreactivity (Figure 4b). In MH-3 (posterior basal nuclei), the immunoreactive neurons were observed uniformly distributed in the dorsal striatum (putamen/ nucleus caudatus and globus pallidus) (Figure 4c).
PH: In PH-1 (dorsolateral isocortex), the distribution pattern of ALR immunoreactivity somewhat similar to that of MH-1, displaying immunoreactive neuronal bodies and processes, mainly distributed in the layer III-V (Figure 5a). In PH-2 (allocortex of posterior hippocampus), the immunoreactive neuronal bodies were detected throughout the hippocampal cortex (Figure 5b). In PH3 (diencephalon), numerous immunoreactive neuronal bodies were detected in medial and lateral thalamus nuclei and in different hypothalamus nuclei, particularly the suprachiasmatic and arcuate nucleus and the mamillary nuclear complex (Figure 5c).
Quantitative analysis: The number of ALR-immunoreactive neuronal bodies per RA identified in ROI of AH, MH and PH is reported in Figure 6, separately from the female and male hemiprosencephala. In all the examined fragments, isocortex (AH-1, MH-1 and PH-1) was the regions with the highest number of ALR-immunoreactive neuronal bodies, followed by the ventral striatum (AH-3) and the hippocampal allocortex (MH-2 and PH-2). A statistically significant reduced number of ALR-immunoreactive neuronal bodies (p between 10-3 and 10-4) was observed in all the fragments from female hemiprosencephala compared to male hemiprosencephala (Figures 6a, 6b, and 6c).
Discussion
In the present study, the expression of ALR, an antioxidative, antiapoptotic and mitochondrialprotective factor, physiologically present in cells and highly conserved during phylogenesis [49], was analyzed in the prosencephalon of female and male mice. The results suggest that ALR-21 and ALR-23 are present at high concentrations in the prosencephalic nervous tissues. The highest expression of ALR was seen in the isocortex (AH-1, MH-1 and PH-1) and hippocampal allocortex (MH-2 and PH-2).The immunoreactivity was detected in the cytoplasm of neuronal bodies and large processes, but also in the most distal branches of processes, producing a diffuse immunoreactivity within the neuropil. This aspect, which has been indicated as ‘background of the immunoreactivity’ is a typical finding observed when substances ubiquitously distributed in neurons are tested with immunohistochemical techniques [50].
ALR expression in female prosencephalon resulted lower than in male prosencephalon, even if the qualitative patterns of distribution of ALR immunoreactivity appeared to be quite similar between the two sexes. The presence of ALR-immunoreactive prosencephalic neurons probably correlates with their ALR need at the moment of mouse sacrifice. Accordingly, the detection of ALR-immunonegative neurons does not necessarily suggest their inability to synthesize ALR, but simply could identify a neuronal population with a different functional dynamicity.
The anti-ALR antibodies used for the study, while recognizing by Western blotting both the 21 and 23 KDa ALR isoforms, did not allow a precise localization within the neuronal intracellular compartments as a diffused immunoreactivity was seen over the cytoplasm. However, since data in literature have shown that ALR-21 and ALR-23 are in the cytosol and mitochondrial intermembrane space, respectively [41,42,48], the higher expression of ALR-23 in prosencephalic tissues leads to the hypothesis that the cytoplasmic ALR immunoreactivity could be mostly related to the presence of the ALR-23. This suggests that this isoform may play a role inthe mitochondrial metabolism in the prosencephalic neurons. The results of the present study parallel those of a previous work conducted in normal striated muscular tissue from adult human female and male subjects, showing that the levels of ALR mRNA and ALR protein are significantly reduced in the female muscular tissue compared to the male one, and providing the first report on the existence of a sexual dimorphism in terms of expression of ALR [41]. Therefore, the different expression of ALR within female and male prosencephalic neurons could represent a marker of sexual dimorphism of these neurons. It is known that ALR enhances mitochondrial OXPHOS through induction of mitochondrial Transcription Factor-A (mtTFA) [51], a nuclear-derived mitochondrial DNA (mtDNA) binding protein, essential for the maintenance and expression of mtDNA [52,53]. Since mtTFA and high level of ATP are essential for maintenance and expression of mtDNA [52,53] and are reduced in neurodegenerative diseases (e.g., PD, AD, Huntington’s disease) [54,55], it might be reasonable to think that ALR is an essential factor for the correct functioning of prosencephalic neurons.
Why male prosencephalic neurons necessitate higher ALR levels than female ones? To answer this question and interpret the results of the present study, some considerations could be made. Firstly, one of the biological processes that the mitochondria are responsible for is the intrinsic apoptosis [33-35], process triggered by alteration of cellular normoxic conditions [56]. For the maintenance of normoxic conditions, several antioxidative systems are necessary, including vitamin E, superoxide dismutase (SOD), glutation (GSH), thioredoxin (TRX) and paraoxanase-1 (PON-1) [4,57-62]. Interestingly, these antioxidative systems show different activity between the 2 sexes: in experimental animals, male cells exhibit higher levels of OS markers (ROS) than female cells, as well as female cells frequently display higher levels of vitamin E, SOD, GSH and TRX than male ones [4]. The difference on the anti-oxidative capacity between the 2 sexes is further stressed by the greater anti-oxidative effect of female sex steroid hormones compared to that of male ones [4,27,37,38]. Accordingly, evidence exists that estrogens play a neuroprotective role against OS in numerous neuropathological conditions (e.g., ischemic brain injury, PD, AD, multiple sclerosis) [1,4,27,63]. Similarly, female sex steroids hormones, in particular 17β-estradiol and progesterone, have been seen to display neuroprotective effect both in vivo and in vitro, affecting the antioxidative pathway binding to the antioxidative response element (ARE) in the promoter regions of antioxidative genes [4]. On the contrary, the neuroprotective role of androgens, such as testosterone, is less evident, since they rather seem to induce neurotoxicity [28-30,37,38].
In this view, mitochondrial functioning and sex hormones activity influence each other: (i) mitochondria provide for ROS formation through OXPHOS, regulate normoxic cellular conditions through several antioxidative systems and attend biosynthesis of female sex steroid hormones through activity of mitochondrial enzymes (P450, 3β-hydroxysteroid dehydrogenase) [1,4,38,56-61,63]; (ii) female sex steroid hormones regulate, in turn, the mitochondrial functioning and act in nervous tissues as neuroprotectans in neurological diseases [37-39,63]. Because of the higher presence of female sex steroid hormones, female prosencephalic neurons appear to be more protected against OS than the corresponding male prosencephalic neurons, similarly to other female-derived cells which necessitate high ATP levels for their correct functioning [41].
This concept is even supported by the fact that, in female CNS, sex steroid hormones produced by endocrine glands are supplemented, more than in male CNS, by locally synthesized steroid hormones [38,64].
Conclusion
Moreover, the results of the present study could represent an experimental evidence of the existence of a sexual dimorphism concerning the ALR expression in prosencephalic neurons. Because of the reduced antioxidative effect of male sex steroid hormones, on male prosencephalic neurons ALR may play the protective role exerted by female steroid hormones on female prosencephalic neurons. This may represent an attempt of neuron protection against variations of normoxic conditions determined by mitochondrial metabolism. Finally, the results of the present study suggest a possible involvement of ALR in the metabolic processes consequent to alterations of normoxic conditions as it occurs in neurodegenerative disorder.
38741
References
- Singh A, Kukreti R, Saso L, Kukreti S (2019) Oxidative stress: A key modulator in neurodegenerative diseases. Molecules 24: 1583.
- Imam SZ, Karahalil B, Hogue BA, Souza-Pinto NC, Bohr VA (2006) Mitochondrial and nuclear DNA repair capacity of various brain regions in mouse is altered in an age-dependent manner. Neurobiol Aging 27: 1129-1136.
- Chiurchiù V, Orlacchio A, Maccarrone M (2016) Is modulation of oxidative stress an answer? The state of the art of redox therapeutic actions in neurodegenerative diseases. Oxid Med Cell Longev 2016: 1-11.
- Ruszkiewicz JA, Miranda-Vizuete A, Tinkov AA, Skalnaya MG, Skalny AV, et al. (2019) Sex-specific differences in redox homeostasis in brain norm and disease. J Mol Neurosci 67: 312-342.
- Halliwell B (2001) Role of free radicals in the neurodegenerative diseases: therapeutic implications for antioxidant treatment. Drugs Aging 18: 685-716.
- Chakrabarti S, Munshi S, Banerjee K, Thakurta IG, Sinha M, et al. (2011) Mitochondrial dysfunction during brain aging: role of oxidative stress and modulation by antioxidant supplementation. Aging Dis 2: 242-256.
- Federico A, Cardaioli E, Da Pozzo P, Formichi P, Gallus GN, et al. (2012) Mitochondria, oxidative stress and neurodegeneration. J Neurol Sci 322: 254-262.
- El-Bach’a RS, De-Lima-Filho JL, Guedes RCA (1998) Dietary antioxidant deficiency facilitates cortical spreading depression induced by photo-activated riboflavin. Nutr Neurosci 1: 205-213.
- Mandel S, Grünblatt E, Riederer P, Gerlach M, Levites Y (2003) Neuroprotective strategies in Parkinson’s disease: an update on progress. CNS Drugs 17: 729-762.
- Tian F, Tong TJ, Zhang ZY, McNutt MA, Liu XW (2009) Age-dependent down-regulation of mitochondrial 8-oxoguanine DNA glycosylase in SAM-P/8 mouse brain and its effect on brain aging. Rejuvenation Res 12: 209-215.
- Yu Z (2009) Overexpression of Tfam protects mitochondria against beta-amyloid-induced oxidative damage in SH-SY5Y cells. FEBS J 276: 3800-3809.
- Von Arnim CAF, Gola U, Biesalski HK (2010) More than the sum of its parts? Nutrition in Alzheimer’s disease. Nutrition 26: 694-700.
- Lagranha CJ, Silva TLA, Silva SCA, Braz GRF, Da Silva AI, et al. (2018) Protective effects of estrogen against cardiovascular disease mediated via oxidative stress in the brain. Life Sci. 192: 190-198.
- Cahill L (2006) Why sex matters for neuroscience. Nat Rev Neurosci 7: 477-484.
- Panzica G, Melcangi RC (2016) Structural and molecular brain sexual differences: a tool to understand sex differences in health and disease. Neurosci Biobehav Rev 67: 2-8.
- Cahill L, Uncapher M, Kilpatrick L, Alkire MT, Turner J (2004) Sex-related hemispheric lateralization of amygdala function in emotionally influenced memory: An FMRI investigation. Learn Mem 11: 261-266.
- Koutcherov Y, Paxinos G, Mai JK (2007) Organization of the human medial preoptic nucleus. J Comp Neurol 503: 392-406.
- Denley MCS, Gatford NJF, Sellers KJ, Srivastava DP (2018) Estradiol and the development of the cerebral cortex: an unexpected role? Front Neurosci 12: 245.
- Ponti G, Farinetti A, Marraudino M, Panzica G, Gotti S (2019) Postnatal genistein administration selectively abolishes sexual dimorphism in specific hypothalamic dopaminergic system in mice. Brain Res 1724: 146434.
- Yagi S, Galea LAM (2019) Sex differences in hippocampal cognition and neurogenesis. Neuropsychopharmacology 44: 200-213.
- Katalinic V, Modun D, Music I, Boban M (2005) Gender differences in antioxidant capacity of rat tissues determined by 2,2’-azinobis (3-ethylbenzothiazoline 6-sulfonate; ABTS) and ferric reducing antioxidant power (FRAP) assays. Comp Biochem Physiol Toxicol Pharmacol 140: 47- 52.
- Ngun TC, Ghahramani N, Sanchez FJ, Bocklandt S, Vilain E (2011) The genetics of sex differences in brain and behavior. Front Neuroendocrinol 32: 227-246.
- Candeias E, Duarte AI, Sebastiao I, Fernandes MA, Placido AI, et al. (2017) Middle-aged diabetic females and males present distinct susceptibility to Alzheimer disease-like pathology. Mol Neurobiol 54: 6471-6489.
- McEwen BS, Milner TA (2017) Understanding the broad influence of sex hormones and sex differences in the brain. J Neurosci Res 95: 24-39.
- Silva TL, Braz GR, Silva SC, Pedroza AA, Freitas CD, et al. (2018) Serotonin transporter inhibition during neonatal period induces sex‐dependent effects on mitochondrial bioenergetics in the rat brainstem. Eur J Neurosci 48: 1620-1634.
- Gandy S (2003) Estrogen and neurodegeneration. Neurochem Res 28: 1003-1008.
- Mosconi L, Berti V, Guyara-Quinn C, McHugh P, Petrongolo G, et al. (2017) Perimenopause and emergence of an Alzheimer’s bioenergetic phenotype in brain and periphery. PloS one 12: e0185926.
- Dluzen D, Mc Dermott J (2000) Gender differences in neurotoxicity of the nigrostriatal dopaminergic system: Implications for Parkinson’s disease. J Gend Specif Med 3: 36-42.
- Saunders-Pullman R (2003) Estrogens and Parkinson disease. Endocrine 21: 81-87.
- Jurado-Coronel JC, Cabezas R, Avila Rodriguez MF, Echeverria V, Garcia-Segura, et al. (2018) Sex differences in Parkinson’s disease: Features on clinical symptoms, treatment outcome, sexual hormones and genetics. Front Neuroendocrinol 50: 18-30.
- Pero RW, Anderson MW, Doyle GA, Anna CH, Romagna F (1990) Oxidative stress induces DNA damage and inhibits the repair of DNA lesions induced by N-acetoxy-2-acetylaminofluorene in human peripheral mononuclear leukocytes. Cancer Res 50: 4619-4625.
- Briehl MM, Tome ME, Wilkinson ST, Jaramillo MC, Lee K (2014) Mitochondria and redox homoeostasis as chemotherapeutic targets. Biochem Soc Trans 42: 939-944.
- Danial NN, Korsmeyer SJ (2004) Cell death: Critical control points. Cell 116: 205-219.
- Kroemer G, Galluzzi L, Brenner C (2007) Mitochondrial membrane permeabilization in cell death. Physiol Rev 87: 99-163.
- Taylor RC, Cullen SP, Martin SJ (2008) Apoptosis: controlled demolition at the cellular level. Nat Rev Mol Cell Biol 9: 231-241.
- Wang DB, Kinoshita C, Kinoshita Y, Morrison RS (2014) p53 and mitochondrial function in neurons. Biochim Biophys Acta 1842: 1186-1197.
- Velarde MC (2014) Mitochondrial and sex steroid hormone crosstalk during aging. Longev Healthspan 3: 2.
- Gaignard P, Liere P, Therond P, Schumacher M, Slama A, et al. (2017) Role of sex hormones on brain mitochondrial function, with special reference to aging and neurodegenerative diseases. Front Aging Neurosci 9: 406-423.
- Gaignard P, Fréchou M, Liere P, Therond P, Schumacher M, et al. (2018) Sex differences in brain mitochondrial metabolism: Influence of endogenous steroids and stroke. J Neuroendocrinol 30: e12497.
- Francavilla A, Hagiya M, Porter KA, Polimeno L, Ihara I, et al. (1994) Augmenter of liver regeneration: Its place in the universe of hepatic growth factors. Hepatology 20: 747-757.
- Polimeno L, Pesetti B, Giorgio F, Moretti B, Resta L, et al. (2009) Expression and localization of augmenter of liver regeneration in human muscle tissue. Int J Exp Pathol 90: 423-430.
- Tury A, Mairet-Coello G, Lisowsky T, Griffond B, Fellmann D (2005) Expression of the sulfhydryl oxidase ALR (augmenter of liver regeneration) in adult rat brain. Brain Res 1048: 87-97.
- Wang X, Michaelis EK (2010) Selective neuronal vulnerability to oxidative stress in the brain. Front Aging Neurosci 2: 12.
- Arriagada PV, Growdon JH, Hedley-Whyte ET, Hyman BT (1992) Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer’s disease. Neurology 42: 631-639.
- Mufson EJ, Mahady L, Waters D, Counts SE, Perez SE (2015) Hippocampal plasticity during the progression of Alzheimer’s disease. Neuroscience 309: 51-67.
- Jaroudi W, Garami J, Garrido S, Hornberger M, Keri S, et al. (2017) Factors underlying cognitive decline in old age and Alzheimer’s disease: The role of the hippocampus. Rev Neurosci 28: 705-714.
- Polimeno L, Pesetti B, De Santis F, Resta L, Rossi R, et al. (2012) Decreased expression of the augmenter of liver regeneration results in increased apoptosis andoxidative damage in human-derived glioma cells. Cell Death Dis 5: 3.
- Polimeno L, Rossi R, Mastrodonato M, Montagnani M, Piscitelli D (2013) Augmenter of liver regeneration, a protective factor against ROS-induced oxidative damage in muscle tissue of mitochondrial myopathy affected patients. Int J Biochem Cell Biol 45: 2410-2419.
- Polimeno L, Lisowsky T, Francavilla A (1999) From yeast to man - from mitochondria to liver regeneration: A new essential gene family. Ital J Gastroenterol Hepatol 31: 494-500.
- Benagiano V, Lorusso L, Flace P, Girolamo F, Rizzi A, et al. (2011) VAMP-2, SNAP-25A/B and syntaxin-1 in glutamatergic and GABAergic synapses of the rat cerebellar cortex. BMC Neurosci 12: 118.
- Polimeno L, Capuano F, Marangi LC, Margiotta M, Lisowsky T, et al. (2000). The augmenter of liver regeneration induces mitochondrial gene expression in rat liver and enhances oxidative phosphorylation capacity of liver mitochondria. Dig Liver Dis 32: 510-517.
- Li PA, Hou X, Hao S (2017) Mitochondrial biogenesis in neurodegeneration. J Neurosci Res 95: 2025-2029.
- Kang I, Chu CT, Kaufman BA (2018) The mitochondrial transcription factor TFAM in neurodegeneration: Emerging evidence and mechanisms. FEBS Lett 592: 793-811.
- Xu S, Zhong M, Zhang L, Wang Y, Zhou Z (2009) Decreased antioxidant enzyme activity and increased mitochondrial DNA damage in cellular models of Machado Joseph disease. J Neurosci Res 87: 1884-1891.
- Oka S, Leon J, Sakumi K, Ide T, Kang D, et al. (2016) Human mitochondrial transcriptional factor A breaks the mitochondria-mediated vicious cycle in Alzheimer’s disease. Sci Rep 6: 37889.
- Hengartner MO (2000) The biochemistry of apoptosis. Nature 407: 770-776.
- Liu M, Kelley MH, Herson PS, Hurn PD (2010) Neuroprotection of sex steroids. Minerva Endocrinol 35: 127-143.
- Giordano G, Tait L, Furlong CE, Cole TB, Kavanagh TJ (2013) Gender differences in brain susceptibility to oxidative stress are mediated by levels of paraoxonase-2 expression. Free Radic Biol Med 58: 98-108.
- Quillinan N, Deng G, Grewal H, Herson PS (2014). Androgens and stroke: Good, bad or indifferent? Exp Neurol 259: 10-15.
- Marmol F, Rodriguez CA, Sanchez J, Chamizo VD (2015) Anti-oxidative effects produced by environmental enrichment in the hippocampus and cerebral cortex of male and female rats. Brain Res 1613: 120-129.
- Al-Gubory KH, Garrel C (2016) Sex-specific divergence of antioxidant pathways in fetal brain, liver, and skeletal muscles. Free Radic Res 50: 366-373.
- Ruszkiewicz JA, Bowman AB, Farina M, Rocha JBT, Aschner M (2016) Sex- and structure-specific differences in antioxidant responses to methyl-mercury during early development. Neurotoxicology 56: 118-126.
- Chainy GBN, Sahoo DK (2020) Hormones and oxidative stress: An overview. Free Radic Res 54: 1-26.
- Baloyannis SJ, Costa V, Michmizos D (2004) Mitochondrial alterations in Alzheimer’s disease. Am J Alzheimer’s Dis Other Dement 19: 89-93.











