Keywords
Breast cancer; D2-40; VEGF-C; Lymphangiogenesis; Disease free survival
Abbreviations: CI: Confidence Interval; DFS: Disease-Free Survival; ER: Estrogen Receptor; H&E: Hematoxylin/Eosin; Her-2: Human Epidermal Growth Factor Receptor 2; Igg: Immunoglobulin G; IHC: Immunohistochemistry, Immunohistochemical; I-LVD: Intratumoral Lymphatic Vessel Density; IRS: Immunoreactive Score; LN: Lymph Node; LVD: Lymphatic Vessel Density; LVI: Lymphatic Vessel Invasion; MEC: Myoepithelial Cell; OS: Overall Survival; P-LVD: Peritumoral Lymphatic Vessel Density; PR: Progesterone Receptor; VEGF-C: Vascular Endothelial Growth Factor C; VEGFR-3: Vascular Endothelial Growth Factor Receptor 3; TNM System: Tumor, Node, Metastasis System
Introduction
Normally, the epithelial layer of normal breast and noninvasive breast tissues is surrounded by a layer of myoepithelial cells (MECs), whose absence or disruption is an absolute prerequisite for invasion and metastasis of the tumor [1]. Sometimes MECs indistinguishable from subjacent myofibroblastic cells in the stroma, and they are difficult to identify in hematoxylin/eosin (H&E)-stained breast tissue sections. So, immunohistochemical staining of MECs is needed [2]. Many of MECs markers have been commonly used, including smooth muscle actin, calponin, smooth muscle myosin heavy chain, and p63, with varying sensitivity and specificity for each marker [3].
One of the most common pathways of initial tumor dissemination is via regional lymphatics, following routes of natural lymphatic drainage [4]. Lymphatic vessel invasion (LVI) is known as an independent predictor of lymph node (LN) metastases in breast cancer [5]. Diagnosis of LVI is based on the presence of tumor emboli within vascular channels that lined by a single layer of endothelial cells without presence of red blood cells. Lymphatic vessels defined as flattened channels or just open spaces that lined by a single layer of endothelial cells whose lumen sometimes contains lymphocytes. However, the identification of LVI is difficult H&E stained sections. Retraction artifacts that isolate tumor aggregates due to tissue shrinkage during fixation are sometimes confused with the true tumor emboli in lymphatic vessels [6].
Prognostic factors help in making clinical decision, selection of treatment for every individual patient and detecting patients at risk of recurrence or death [7]. Because LVI is a strong prognostic marker, D2-40 as a potential prognostic predictor for breast cancer and its effectiveness is actively investigated [6]. D2-40 plays a role in detecting tumor emboli in lymphatic vessels and to identify LVI of tumors [8]. Immunostaining with D2-40 significantly increased the accuracy of detection of lymphatic invasion compared to routine H&E staining in early breast cancer. Many studies have evaluated D2-40 expression in various malignant neoplasms. Positivity of D2-40 was helpful in detecting lymphatic tumor emboli and was associated with poorer prognosis in early colorectal cancer, esophageal squamous cell carcinoma, and small cell lung cancer [9-11].
D2-40 is one of the most specific and sensitive markers to determine tumor cells in lymphatic vessels. D2-40 is an IgG2a monoclonal antibody that was generated against an oncofetal antigen M2A, which is normally expressed in the fetal testis and re-expressed in germ cell neoplasia [12]. It is a monoclonal antibody to an Mr 40000 O-linked sialoglycoprotein that reacts with a fixation-resistant epitope on the lymphatic endothelium [13]. The D2-40 antibody has been shown to specifically recognize podoplanin, a transmembrane mucoprotein that is expressed in lymphatic endothelial cells [14] and has been shown to be a very sensitive and specific marker for lymphatic endothelium in most tissues [15] and especially in breast cancer [16]. Since tumor lymphangiogenesis promotes lymphatic metastasis, the lymphovascular density (LVD) has been shown to correlate with LN metastasis [17]. D2-40 stains the endothelium lining of lymphatic vessels, lymphangiomas, Kaposi's sarcoma and Dabska tumor, but does not stain endothelium lining of blood vessels, hemangiomas, glomus tumors, angiolipomas, pyogenic granulomas, and vascular malformations [18-20].
Vascular endothelial growth factor C (VEGF-C) is one member of VEGF family. VEGF-C is synthesized as propeptide then activated by proteolysis to form a high-affinity ligand that binds to the extracellular domain of vascular endothelial growth factor receptor-3 (VEGFR-3), which is expressed on lymphatic endothelium (LECs), and induces tyrosine phosphorylation of VEGFR-3. Thus, VEGF-C promotes lymphangiogenesis and lymphatic metastasis in tumors [21-23]. Several studies [24-25] have shown that D2-40 IHC labels glandular MECs, while using it as the marker for lymphatic endothelial cells, which triggered this study to further, explore the possibility of using D2-40 as an additional MECs marker in breast pathology and in the same time using it as a prognostic marker as it is now one of the most specific and sensitive lymphatics markers.
The aims of this study were (1) To explore the expression of D2-40 as a marker for myoepithelial cells (MECs) of the breast lesions, (2) To investigate the clinicipathological significance of VEGF-C, D2-40 expression and lymphovascular density (LVD) in breast cancer patients.
Materials and Methods
Case selection and clinical information
Paraffin-embedded blocks of breast tissue of 88 female patients were retrieved from the archives of the Department of Pathology of Suez Canal University Hospital, Ismailia and Pathology laboratory of Mansoura Oncology Center, Mansoura University. The samples included 15 with benign breast lesions (8 cases with ductal hyperplasia lesions and 7 cases with fibrocystic changes of the breast), and 73 patients who had primary invasive ductal breast carcinoma in the period between January 2010 and December 2010 at the Suez Canal University Teaching Hospital and Pathology laboratory of Mansoura Oncology Center.
Patients diagnosed with ductal carcinoma in situ and those with insufficient follow up data, pathology slides, and tissue blocks were excluded. All participants have provided written consent.
Of 73 cancer breast patients, 30 underwent breast conserving surgery, and 43 underwent a mastectomy. Patients received treatment with radiation, hormones, or chemotherapy according to their pathological reports. The mean follow-up period was 35 ± 11.026 months
IHC for D2-40, calponin and VEGF-C were performed in all cases. Benign tissues from 10 patients with mammary fibroma were used as controls.
For all cases, the original H&E-stained sections of the lesions were available for histopathology review. Regarding invasive malignant lesions slides of the primary tumors and axillary LNs were available for evaluation. The paraffin blocks were available for additional sections and immune-histochemical analysis. Special care was taken to include only specimens with sufficient amount of normal tissue surrounding the invasive tumor to evaluate peritumoral LVD.
Clinico-pathological data including age, grade, tumor size, staging of tumors (using the TNM system criteria from the American Joint Committee on Cancer classification [26] were retrieved from the routine Pathology Laboratory reports.
None of the patients of this study were undergone preoperative chemotherapy or radiotherapy. Follow-up data were obtained from Oncology Unit files of Suez Canal University Hospital and Oncology Unit files of Mansoura Oncology Center. All the patients received postoperative adjuvant therapy consisting of combination chemotherapy, radiotherapy and hormone treatment, and were followed up clinically for at least 60 months after surgery. Follow-up examinations included a physical examination, X-ray, ultrasound exam and CT scan. Recurrence was determined by clinical and radiological examinations or histological confirmation. Cancer recurrence was measured from the date of primary surgery until the date of first recurrence of breast cancer. Overall survival was measured from the date of the surgery until the date of death from breast cancer.
Histologic analysis of primary breast carcinoma features included the histologic type, the grade and LVI. The histologic type of primary tumor was classified based on Page et al. [27], and the College of American Pathologists recommendations. [28] Tumor grade was determined using the Nottingham modification of the Bloom and Richardson histological grading criteria. [29] The diameter of the microscopic field used for mitotic count was 0.44 mm. Revision and reassessment of estrogen receptor (ER), progesterone receptor (PR), and Her-2 receptor (Her-2) for molecular subtyping also done.
Immuno-histochemical staining
Single D2-40, calponin or VEGF-C IHC was performed on formalin-fixed, paraffin-embedded sections. The sections of 4 μm thickness were made and spread on poly-L-lysine coated slides. The paraffin sections were immersed in three changes of xylene and they were hydrated using a graded series of alcohol solutions. Antigen retrieval was routinely performed by immersing the sections in 0.01 M citrate buffer (pH 6.0) in a microwave and performing autoclaving for 15 min. The endogenous peroxidase activity was blocked with 3% hydrogen peroxide for 15 min and then the sections were incubated with primary antibody for 1 hour at room temperature. The primary antibodies were monoclonal antibodies against D2-40 (dilution 1:100; Novocastra), calponin (dilution 1:100; Novocastra) and VEGF-C (dilution 1:100; Novocastra) and staining was developed using 3′3-diaminobenzidine. The sections were counterstained for 3 min with Meyer’s hematoxylin and then they were mounted. Negative controls were obtained by omitting the primary antibodies, and as external positive control were used normal breast tissues specimens.
Quantitation of Immuno Histochemistry
Myoepithelial cell assessment
D2-40 and calponin immunohistochemistry stains the cytoplasm of MEC. The staining was scored semi-quantitatively as follows: N (no staining at all); 1 (weakly positive); 2 (moderately positive); and 3 (strongly positive). The D2-40 stain was compared to calponin stain on the serial sections in the same case.
VEGF-C assessment
Staining were semi-quantitatively assessed by combining the IHC staining intensity 0 (no staining at all); + (weakly positive); ++ (moderately positive); and +++ (strongly positive) with the percentage of tumor cells stained 0 (0%), 1 (1% to 10%), 2 (11% to 49%) or 3 (50% to 100%). The raw data were then converted to an Immunoreactive Score (IRS) by adding the scores for the staining intensity and percentage of tumor cells stained. An IRS of 0 to 2 was considered ‘-’ (negative), 3 as ‘+’, 4 to 5 as ‘++’, 6 as ‘+++’ and 7 as ‘++++’ [30]. Consensus opinions were used to assign final IRS scores to disputed cases before data analysis.
Lymphovascular density and invasion assessment
For lymphatic vessel density (LVD) assessment, intratumoral and peritumoral LVD were determined by the hotspot method with a low power magnification (40X), with sequential assessment by two investigators, as recommended by the First International Consensus on the Methodology of Lymphangiogenesis Quantification in Solid Human Tumors [31]. Intratumoral LVD (located at center of the tumor) and peritumoral LVD (located in the periphery within 2 mm of the tumor, adjacent to the invasive front) were assessed.
Slides were examined at low power magnification (40X) to identify the areas of greater concentration of stained vessels "hot spots", three areas with the highest concentration of vessels stained by D2-40 were selected in each investigated cases. Each area was evaluated with one high power (400X) field in such a way as to include the maximum number of vessels [32]. Single immunoreactive endothelial cells, or endothelial cell clusters separate from other microvessels, were counted as a vessel [33]. The highest value obtained among the three fields was reported for analysis and the mean reading of the two investigators results was calculated and reported for analysis.
Tumor lymphatic vessel invasion was identified when at least one neoplastic cell cluster was clearly visible inside a D2– 40 positive lymph vessel according to Yamauchi et al. [34]. High intratumoral and peritumoral LVD values were defined as LVD values higher than the respective median LVD values for all patients. We were blinded to all clinical and pathological data.
Statistical analysis
Statistical analysis was performed using SPSS 15.0 (SPSS, Chicago, IL, USA). The Mann -Whitney U test or analysis of variance (ANOVA) were used to compare intratumoral and peritumoral LVD values, according to the clinic-pathological variables. Multivariate analysis of risk factors for LN metastasis was performed using multivariate logistic regression analysis. Disease-free survival (DFS) curves were plotted using the Kaplan-Meier method and compared using the log-rank test. A multivariate model was generated using Cox stepwise regression analysis and used to evaluate the significance of the independent associations between the covariates and DFS and/or OS. All statistical tests were two-sided and significance was defined as p < 0.05.
Results
D2-40 and calponin expression in benign breast lesions
In benign breast lesions, D2-40 and calponin IHC labeled the MECs in their peripheral location along the breast lobules and terminal ducts in all the cases with strong intensity (Figure 1A and Table 1). The luminal epithelial cells were negative for D2-40 (Figures 1B and 1C). The identification of MECs by D2-40 was the same as calponin. Also, D2-40 stained the normal interlobular lymphatic endothelium in a characteristic linear, strong pattern (Figures ID-IF).
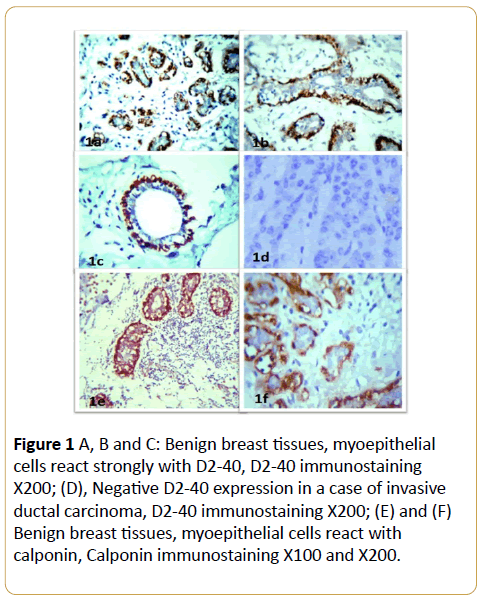
Figure 1: D2-40 expressions in invasive breast cancer. (A), (B) and (C) D2-40 positive reaction in lymphatic vessels from the normal interlobular stroma of the malignant tissue, D2-40 immunostaining X200; (D) Tumor tissue emboli inside lymphatic vessels which its wall stained with D2-40, D2-40 immunostaining X400.
D2-40 and calponin expression in invasive breast cancer
In invasive neoplastic epithelium tissue, D2-40 and calponin were negative in invasive epithelial component of the cases. D2-40 was strongly expressed in the cytoplasm of lymphatic endothelial cells (Figure 2 and Table 1). D2-40 stained the intra- and peri-tumoral microvessels in all of the cases. These intratumoral lymphatic vessels appeared linear, small and flattened. In contrast, peritumoral lymphatics were widely opened (Figures 2A-2C). D2-40 staining was helpful in identifying small lymphatic emboli and lymphatic vessels obscured by tumor cells (Figure 2D).
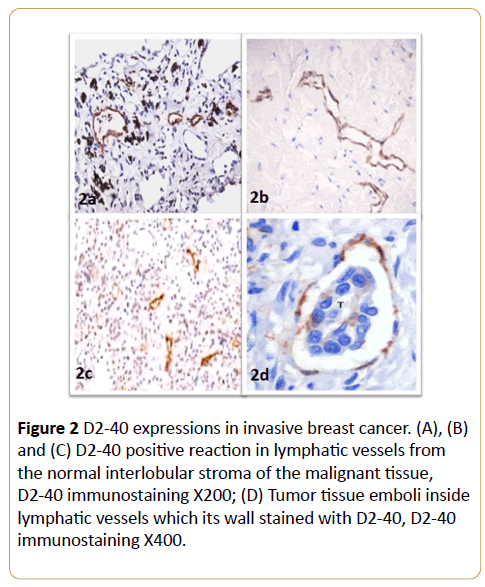
Figure 2: D2-40 expressions in invasive breast cancer. (A), (B) and (C) D2-40 positive reaction in lymphatic vessels from the normal interlobular stroma of the malignant tissue, D2-40 immunostaining X200; (D) Tumor tissue emboli inside lymphatic vessels which its wall stained with D2-40, D2-40 immunostaining X400.
| |
D2-40 |
Calponin |
| N |
1 |
2 |
3 |
N |
1 |
2 |
3 |
| Benign lesions |
0 |
2/15 |
8/15 |
5/15 |
0 |
1/15 |
5/15 |
9/15 |
| Invasive carcinoma |
73/73 |
0 |
0 |
0 |
73/73 |
0 |
0 |
0 |
N: No staining, 1: Weakly positive; 2: Moderately positive; 3: Strongly positive.
Table 1: Immunohistochemistry of D2-40 and calponin expression regarding presence of myoepithelial cells in benign and malignant breast lesions.
Table 1 Immunohistochemistry of D2-40 and calponin expression regarding presence of myoepithelial cells in benign and malignant breast lesions.
In the adjacent nonneoplastic breast epithelium, the myoepithelial cells of normal ducts and lobules also revealed positive D2-40 and calponin immunoreactivity that was moderate to strong in intensity. In addition, some stromal myofibroblasts were weakly positive for D2-40.
Clinico-pathological characteristics of breast cancer cases
The clinical and pathological characteristics of the 73 cancer patients are shown in Table 2. The age of patients ranged from 27 to 70 years with a median 52 years. About 60.2 % of patients were older than 50 years, the majority of patients were pre-menopausal at the time of cancer diagnosis (67.1%), 41.2 % of the patients had tumor size more than 5 cm. (73.9 %) had invasive duct carcinoma type, while (26.1%) were of invasive lobular type, (88.8%) of them had grade II carcinoma, (24.6%) of the patients had stage II , 41.2% of patients were in stage III while 34.2 % of them had stage IV, (79.1%) had positive axillary LN metastasis and (35.7%) of them had LVI. Most of the patients were ER, PR and Her-2 positive (73.9%, 64.3%, and 65.8% respectively).
| Patients' characteristics |
No. Of patients (%) |
| All patients |
73 (100) |
| Age (yr) |
<50 |
44 (60.2) |
| ≥ 50 |
29 (39.8) |
Age range (27 – 70) years
median age of 52 year |
| Menopausal status |
Pre-menopausal |
49 (67.1) |
| Post-menopausal |
24 (32.9) |
| Tumor Size |
T1 |
8 (10.9) |
| T2 |
10 (13.7) |
| T3 |
25(34.2) |
| T4 |
30 (41.2) |
| Histological type |
Ductal (NOS) |
54 (73.9) |
| Lobular |
19 (26.1) |
| Histological Grade |
II |
59 (88.8) |
| III |
14 (20.2) |
| TNM |
II |
18 (24.6) |
| III |
30 (41.2) |
| IV |
25(34.2) |
| LN metastasis |
Negative |
16 (21.9) |
| Positive |
67 (79.1) |
| LVI |
No |
47(64.3) |
| Yes |
26(35.7) |
| ER status |
Negative |
19 (26.1) |
| Positive |
54 (73.9) |
| PR status |
Negative |
26(35.7) |
| Positive |
47(64.3) |
| Her-2 status |
Negative |
25(34.2) |
| Positive |
48(65.8) |
ER: Estrogen Receptor; Her-2: Human Epidermal Growth Factor Receptor 2; LN: Lymph Node; LVI: Lymphatic Vessel Invasion; PR: Progesterone Receptor; TNM: Tumor, Node, Metastasis Stage.
Table 2: Patients' Clinico-pathological characteristics.
VEGF-C expression in invasive breast cancer cases
VEGF-C immunoreactivity was observed as positive cytoplasmic staining in breast cancer cells (Figure 3). VEGF-C expression was not detected in the breast cancer tissues of 10/73 patients (13.6%); 17/73 (23.3%) of the patients were ‘+’, 27/73 (35.9%) were ‘++’, 12/73 (16.4%) were ‘+++’ and 7/73 (9.6%) were ‘++++’ for VEGF-C. Weak VEGF-C immunereactivity was observed in 5/20 (25%) of the benign breast tissue samples, the remainder of the control samples did not express VEGF-C. The expression of VEGF-C was significantly higher in invasive breast cancer than the control benign breast tissues (p < 0.01; Table 3).
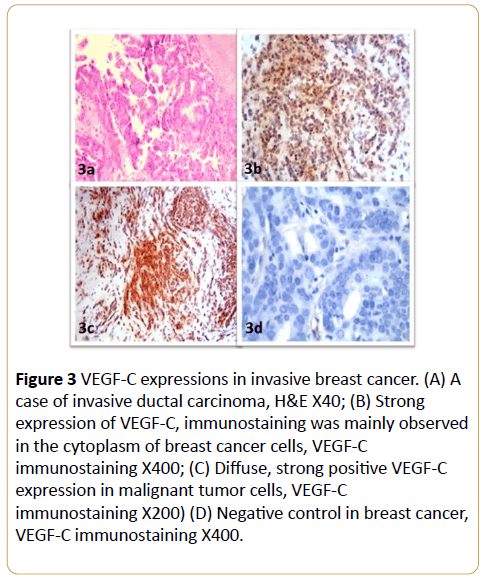
Figure 3: VEGF-C expressions in invasive breast cancer. (A) A case of invasive ductal carcinoma, H&E X40; (B) Strong expression of VEGF-C, immunostaining was mainly observed in the cytoplasm of breast cancer cells, VEGF-C immunostaining X400; (C) Diffuse, strong positive VEGF-C expression in malignant tumor cells, VEGF-C immunostaining X200) (D) Negative control in breast cancer, VEGF-C immunostaining X400.
| VEGF-C |
No. of cases |
I- LVD (mean ± SD) |
p - value |
P-LVD (mean ± SD) |
p - value |
| Negative |
10 |
5.07 ± 2.93 |
0.96 |
5.47 ± 2.47 |
0.001 |
| + |
17 |
5.72 ± 2.19 |
7.62 ± 2.65 |
| ++ |
27 |
5.61 ± 1.96 |
9.12 ± 2.86 |
| +++ |
12 |
5.43 ± 2.19 |
10.71 ± 3.11 |
| ++++ |
7 |
5.50 ± 2.42 |
11.32 ± 3.16 |
I-LVD: Intratumoral Lymphatic Vessel Density; P-LVD: Peritumoral Lymphatic Vessel Density; VEGF-C: Vascular Endothelial Growth Factor C
Table 3: Association of VEGF-C expression with P-LVD and I-LVD in breast cancer (73 cases).
D2-40 expression and characteristics of intratumoral lymphatics and peritumoral lymphatics in invasive breast cancer
In our study, we noticed that, in the invasive breast cancer specimens there was D2-40 positive reaction in all cases. The D2-40-stained lymphatic vessels had unevenly distributed pattern throughout the breast tumors tissue. The intratumoral lymphatic vessels appeared small, linear and flattened, while the peritumoral lymphatics were more frequent, dilated and tortuous. D2-40 immunostaining highlighted the presence of lymphatic invasion and presence of tumor emboli. Invasion of the carcinoma cells into the lymphatics was also observed in peritumoral tissue at the periphery of tumors (Figure 2). The peritumoral LVD (8.76 ± 3.31) was significantly higher than the intratumoral LVD (p < 0.05) (Table 3).
Relationship of intratumoral LVD and peritumoral LVD with VEGF-C expression and clinic-pathological features
Results of our work revealed a significant correlation between the expression of VEGF-C and P- LVD in invasive breast cancer (p < 0.01). While no similar relationship between VEGF-C and I-LVD was observed (Table 3). The associations of I-LVD and P-LVD with the clinico-pathological features of breast cancer patients are shown in Table 4.
| Clinico-pathological characteristics |
No. of cases |
I-LVD |
P I-LVD |
| mean ± SD |
p-value |
mean ± SD |
p-value |
| Age |
| >50 |
45 |
5.21 ± 2.03 |
0.87 |
8.43 ± 2.72 |
0.66 |
| ≤50 |
28 |
5.67 ± 1.96 |
9.32 ± 3.70 |
| Size |
| ≤5 cm |
33 |
6.23 ± 1.95 |
0.01 |
8.33 ± 3.47 |
0.24 |
| >5 cm |
40 |
4.85 ± 1.98 |
9.34 ± 3.12 |
| Grade |
| II |
55 |
5.77 ± 2.06 |
0.57 |
8.16 ± 2.88 |
0.46 |
| III |
18 |
5.34 ± 2.01 |
8.96 ± 3.41 |
| LN metastasis |
| Negative |
34 |
5.57 ± 1.93 |
0.73 |
7.58 ± 3.11 |
0.005 |
| Positive |
39 |
5.39 ± 2.15 |
9.83 ± 3.14 |
| TNM |
| I |
18 |
5.27 ± 2.09 |
0.76 |
2.15 ± 2.99 |
0.011 |
| II |
30 |
5.43 ± 2.06 |
7.81 ± 3.43 |
| III |
25 |
5.73 ± 2.03 |
9.78 ± 2.90 |
| LVI |
| Negative |
48 |
5.68 ± 2.12 |
0.39 |
8.03 ± 2.87 |
0.017 |
| Positive |
25 |
5.39 ± 1.96 |
9.18 ± 3.60 |
| ER status |
| Negative |
19 |
5.45 ± 2.03 |
0.58 |
8.97 ± 3.42 |
0.45 |
| Positive |
54 |
5.65 ± 2.04 |
8.14 ± 2.86 |
|
| PR status |
| Negative |
26 |
5.43 ± 1.98 |
0.37 |
9.15 ± 3.55 |
0.019 |
| Positive |
47 |
5.57 ± 2.09 |
8.01 ± 2.76 |
| Her-2 status |
| Negative |
25 |
5.39 ± 1.96 |
0.39 |
7.58 ± 3.11 |
0.005 |
| Positive |
48 |
6.23 ± 1.95 |
8.85 ± 3.12 |
ER: Estrogen Receptor; Her-2: Human Epidermal Growth Factor Receptor 2; I-LVD: Intratumoral Lymphatic Vessel Density; LVI: Lymphatic Vessel Invasion; P-LVD: Peritumoral Lymphatic Vessel Density; PR: Progesterone Receptor; TNM: Tumor, Node, Metastasis Stage.
Table 4: P-LVD and I-LVD association with the clinicopathological features of primary breast cancer patients.
In our study, I-LVD did not correlate with patients age, tumor grade, LN metastasis, TNM stage, LVI; ER, PR, Her-2 expression; it was only correlated significantly with tumor size (p = 0.01). While P-LVD correlated significantly with LN metastasis (p = 0.005), LVI (p = 0.017), TNM stage (p = 0.011) and Her-2 expression (p = 0.005) (Table 4).
Predictive value of VEGF-C, I-LVD and P-LVD for LN metastasis
Multivariate logistic regression analysis indicated that VEGFC expression, P-LVD and LVI were significantly associated with LN metastasis (p = 0.025, p = 0.006 and p = 0.017, respectively) while I-LVD had no predictive value for LN metastasis in breast cancer (Table 5). By using univariate survival analysis, the I-LVD demonstrated a non-significant trend towards DFS (p = 0.272; Figure 4A). While, high P-LVD was significantly associated with poorer DFS (p = 0.003; Figure 4B), the 5-year DFS rates for low P-LVD and high P-LVD were 73.3 % and 39.72% respectively (Figure 4B).
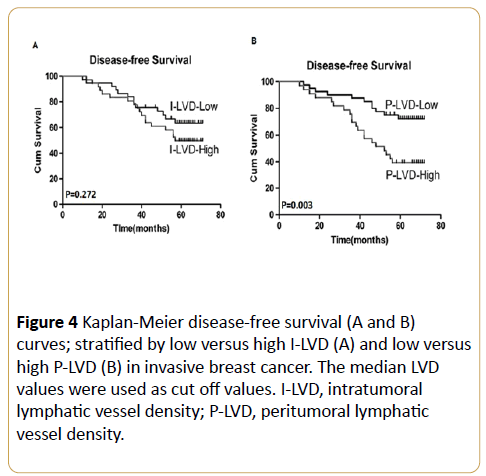
Figure 4: Kaplan-Meier disease-free survival (A and B) curves; stratified by low versus high I-LVD (A) and low versus high P-LVD (B) in invasive breast cancer. The median LVD values were used as cut off values. I-LVD, intratumoral lymphatic vessel density; P-LVD, peritumoral lymphatic vessel density.
| |
p -value |
Odds ratio |
95% CI |
| P-LVD |
0.006 |
2.235 |
1.269-4.008 |
| I-LVD |
0.178 |
0.708 |
0.434-1.156 |
| LVI |
0.017 |
4.152 |
2.755-15.363 |
| VEGF-C |
0.025 |
12.837 |
1.327-45.584 |
CI: Confidence Interval; I-LVD:Intratumoral Lymphatic Vessel Density;
LVI: Lymphatic Vessel Invasion; P-LVD:Peritumoral Lymphatic Vessel Density;
VEGFC: Vascular Endothelial Growth Factor C.
Table 5: Multivariate logistic regression analysis of the factors affecting axillary lymph node metastasis in invasive breast cancer.
Survival Analysis
The study population divided into two groups, using the median I-LVD and P-LVD values as cut-off points (6.0 and 9.0, respectively); Multivariate regression analysis indicated that PLVD was an independent prognostic factor for both OS (p <0.001) and DFS (p = 0.001).
Multivariate regression analysis indicated also that both LN metastasis and TNM stage served as independent predictors for both OS and DFS. The LN metastasis was (p = 0.037) for OS and DFS (p = 0.040). While for TNM stage, OS was (p = 0.035) and DFS (p = 0.006). There is no significant correlations were observed between I-LVD and any survival outcome (Table 5).
Discussion
Myoepithelial cells (MECs) form the basal cell layer of normal mammary duct epithelia, its identification is very important as they are retained in most benign lesions while lost in malignant lesions. As pathologists, we face daily challenges in diagnosing breast lesions based on morphologic criteria, a critical decision which determines patient's further management. Sometimes MECs recognition is very difficult in tissue sections stained with H&E as MECs are well known by their pleomorphism (in shape and size), even in normal tissue. Several MECs immunocytochemical markers are currently available for diagnostic purposes [35]. The mostly used markers, including smooth muscle actin (SMA), smooth muscle myosin heavy chain (SM-MHC), calponin and p63, are considered highly sensitive but have varying specificities. For example, SMA stains the scattered epithelial cells and reactive myofibroblasts which present in the stroma [36,37], invasive tumor cells may show focal calponin positivity, and p63 labels the malignant cells of poorly differentiated carcinoma with squamous differentiation. So, to increase the specificity and sensitivity to detect MECs, a panel of antibodies is recommended [8].
D2-40 is a mouse monoclonal antibody directed against human podoplanin, a mucin-type transmembrane protein originally reported in the lymphatic endothelial cells [38]. Also, it has occasionally been reported to stain MEC of salivary gland and breast [39,40].
D2-40 antibody is known as a very sensitive and specific marker for lymphatic endothelium in most tissues, especially breast cancer. According to “The First International Consensus on The Methodology of Lymphangiogenesis Quantification in Solid Human Tumors”, podoplanin was considered to be the most reliable available marker of lymphatic vessels [8,41].
Our study demonstrated that MECs in benign breast tissues were positive for D2-40, and as expected, no MECs were found in invasive carcinomas by D2-40. The pattern of D2-40 stain was highly correlated with those of calponin. This study showed that D2-40 immunohistochemistry reliably labels MECs in a variety of benign breast lesions, suggesting that it can be considered as an additional marker for MECs of the breast.
Also, our study confirmed that D2-40 selectively stains the endothelium of lymphatic vessels. D2-40 is not an exclusive lymphatic endothelial marker as it also stained the myoepithelial cells of normal ducts and lobules of the adjacent peritumoral parenchyma. The pattern of myoepithelial cell staining was membranous, granular and branching with a moderate to strong intensity. Myoepithelial cells surrounding the in situ ductal carcinoma foci displayed weaker residual thin/discontinuous D2-40 immunostaining. Studies have indicated that D2-40 can be useful in identifying the presence of lymphatic invasion in various malignant neoplasms by highlighting tumor emboli in lymphatics otherwise indiscernible by H&E [32].
In our study, D2-40-positive lymphatic vessels observed usually had an irregular shape and thin-walled lumen devoid of red blood cells. D2-40 immunostaining demonstrated the existence of intra- and peri-tumoral lymphatic vessels in breast carcinoma cases. Similar to previous studies, we found that most intra-tumoral lymphatics were generally small and flattened, contrasting the widely open lymphatics in peritumoral regions [42,43]. Our study confirmed that D2-40 stains the endothelium of lymphatic vessels and is useful and reliable in detecting LVI in invasive breast carcinomas.
Lymphovascular invasion as the main route of LN metastasis is defined by the presence of cancer cells in the lymphatic vessel. The presence of carcinoma cells in lymphatic vessels is a significant prognostic factor in invasive breast cancer and associated with poor survival [5,6,22,44]. So, LVI is a critical step in the process of tumor metastasis and it is an important criterion for further patient's management.
During St Gallen Conference, 2005, peritumoral vascular invasion, especially lymph-vascular invasion, has been included as one of the adverse prognostic factors in the guidelines and the recommendations for postoperative adjuvant systemic therapies of early breast cancer by the International Consensus Panel [45]. The presence of peritumoral vascular invasion defined an intermediate risk for patients with nodenegative breast disease. LVI is also associated with other strongest prognostic factors including tumor size, grade and regional LN involvement [46-48].
This study aimed to clarify the location of VEGF-C induced lymphangiogenesis and investigate the role of intratumoral and peritumoral lymphatic vessels in LN metastasis and the outcome of patients with breast cancer. Lymphangiogenesis is controlled by several growth factors, such as cytokines and chemokines, and all actively contribute to tumor metastasis [49]. Skobe et al. demonstrated that VEGF-C induced lymphangiogenesis and promoted metastasis and also found that overexpression of VEGF-C in breast cancer patients potently increased intratumoral lymphangiogenesis, which in turn enhanced metastasis to the regional lymph nodes and lungs [50]. Similar to previous reports [51,52], our results demonstrated that VEGF-C is expressed at significantly higher levels in breast cancer than in benign mammary lesions. We observed that P-LVD was significantly higher than I-LVD as reported by Zhao et al. [53].
Lymphovascular density (LVD) is the hallmark of lymphangiogenesis. It can be detected by D2-40, a specific marker of lymphatic endothelial cells [24]. Previous researchers of breast cancer suggested that LVD is correlated with tumor LVI and could be used as an independent parameter of disease-free survival, overall survival [5], and also as a predictor of disease relapse [54].
Debate remains regarding the role of intratumoral versus peritumoral lymphatic vessels density in human tumors. Several studies have associated I-LVD with tumor LN metastasis in head and neck squamous cell carcinoma [55,56], papillary thyroid carcinoma [58], pancreatic endocrine tumors [57], and gastric carcinoma [58].
Padera et al. in their experimental study demonstrated that the occurrence of metastatic spread in the absence of detectable intratumoral lymphatic vessels and they proposed that the functional lymphatics at the tumor margin are sufficient for the promotion of metastasis, as the tumor margins have large areas for tumor cell escape [59]. The same observation in another study in an experimental model of prostate cancer which also observed that efficient metastasis to the lymph nodes in the absence of intratumoral lymphatics [7]. Bono et al. reported that peritumoral lymphatics were more frequent than intratumoral vessels, and peritumoral lymphatic vessels density correlated with nodal metastasis [60]. Most of the available data indicate that there is a strong correlation between peritumoral lymphangiogenesis and tumor aggressiveness. Our results are in agreement with the latter studies; as we observed that the density of lymphatic vessels was usually greater at the tumor periphery than intratumorally and that a high P-LVD, not I-LVD, was associated with more aggressive behavior in the studied patients with breast carcinoma.
It is documented that both angiogenesis and lymphangiogenesis play important roles in tumor occurrence and progression and vascular endothelial growth factor (VEGF) family is the most important family of proteins involved in angiogenesis, and VEGF-C is the most important growth factor in lymphangiogenesis as it is implicated in the development of lymphatic vessels and promotion of lymphatic metastasis [55].
We also investigated the relationship between I-LVD, P-LVD and clinicipathological features in breast cancer. There was a significant correlation between P-LVD and lymphatic vessel invasion, LN metastasis, TNM stage and Her-2 expression, which indicate that VEGF-C-induced peritumoral lymphangiogenesis leads to lymphatic invasion then LN metastasis. The contradictory results of the role of intratumoral and peritumoral lymphatic vessels in tumors behavior reflect the fact that tumor lymphatic metastasis and lymphangiogenesis are very complex processes, which can differ significantly in different tumor types or in tumors at different anatomic locations [61], which still need more studies to clarify it.
We also examined the prognostic value of I-LVD and P-LVD in invasive breast carcinoma. Multivariate analysis indicated that only P-LVD was an independent predictor of axillary LN metastasis. No correlations were observed between I-LVD and patient outcome; however, increased P-LVD was associated with poorer DFS and OS. Multivariate analysis also indicated that increased P-LVD was a prognostic factor for DFS and OS in breast cancer patients.
These findings are in agreement with other studies, as the PLVD of tumors correlates with poorer outcomes in gastric [62], lung [63], colorectal [64], and prostate cancer [65]. All this evidence demonstrates that peritumoral lymphangiogenesis plays an important role in lymphatic metastasis and tumor progression and confirmed that peritumoral lymphangiogenesis is an independent predictor of LN metastasis and prognostic factor in breast carcinoma. As wellknown most solid tumors metastasize via lymphatic invasion; therefore, LN metastasis is an important prognostic factor and both LN metastasis and TNM were also prognostic factors for DFS and OS in breast cancer.
Conclusion
Our results show that D2-40 is a reliable marker that highlights MECs in benign breast lesions beside it is a useful tool for identification of LVI in breast carcinomas which reflecting a potential for lymphatic metastatic spread and possible poor prognosis. Our study also demonstrated that high expression of VEGF-C in invasive breast carcinoma may induce lymphangiogenesis in the peritumoral area and contribute to a high P-LVD, leading to increased aggressiveness, lymphatic invasion, metastatic spread and poorer outcomes. So, inhibiting the expression, interfering or blocking the function, of VEGF-C to control peritumoral lymphangiogenesis is expected to lead to the development of novel therapeutic strategies for breast cancer management; however, more investigations are needed for further characterization of the molecular mechanisms which regulate lymphangiogenesis.
18418
References
- Sternlicht MD, Barsky SH (1997) The myoepithelial defense: a host defense against cancer. Med Hypotheses 48: 37-46.
- Masood S, Sim SJ, Lu L (1992) Immunohistochemical differentiation of atypical hyperplasia vs. carcinoma in situ of the breast. Cancer Detect Prev16:225-235.
- Joshi MG, Lee AK, Pedersen CA, Schnitt S, Camus MG, et al. (1996) The role of immunocytochemical markers in the differential diagnosis of proliferative and neoplastic lesions of the breast. Mod Pathol9: 57-62.
- Cunnick GH, Jiang WG, Douglas-Jones T, Watkins G, Gomez KF, et al. (2008) Lymphangiogenesis and lymph node metastasis in breast cancer. Mol Cancer 7: 23.
- Schoppmann SF, Fenzl A, Nagy K, Unger S, Bayer G, et al. (2006) VEGF-C expressing tumor-associated macrophages in lymph node positive breast cancer: impact on lymphangiogenesis and survival. Surgery 139:839-846.
- Marinho VF, Metze K, Sanches FS, Rocha GF, Gobbi H (2008) Lymph vascular invasion in invasive mammary carcinomas identified by the endothelial lymphatic marker D2-40 is associated with other indicators of poor prognosis. BMC Cancer 8: 64.
- Wong HS, Subramaniam S, Alias Z, Taib NA, Ho GF, et al. (2015) The predictive accuracy of PREDICT: a personalized decision-making tool for Southeast Asian women with breast cancer. Medicine (Baltimore). 94: e593.
- Ren S, Abuel-Haija M, Khurana JS, Zhang X (2011) D2-40: an additional marker for myoepithelial cells of breast and the precaution in interpreting tumor lymphovascular invasion. Int J ClinExpPathol4: 175-82.
- Iwakiri S, Nagai S, Katakura H, Takenaka K, Date H, et al. (2009) D2-40-positive lymphatic vessel density is a poor prognostic factor in squamous cell carcinoma of the lung. Ann SurgOncol16:1678-1685.
- Imamura Y, Watanabe M, Nagai Y, Baba Y, Hirashima K, et al. (2012) Lymphatic vessel invasion detected by the D2-40 monoclonal antibody is an independent prognostic factor in node-negative esophageal squamous cell carcinoma. J SurgOncol105:277-283.
- Wada H, Shiozawa M, Sugano N, Morinaga S, Rino Y, et al. (2013) Lymphatic invasion identified with D2-40 immunostaining as a risk factor of nodal metastasis in T1 colorectal cancer. Int J ClinOncol18:1025-1031.
- Lau SK, Weiss LM, Chu PG (2007) D2-40 immunohistochemistry in the differential diagnosis of seminoma and embryonal carcinoma: a comparative immunohistochemical study with KIT (CD117) and CD30. Mod Pathol20:320-325.
- Dubina M, Goldenberg G (2009) Positive staining of tumor-stage Kaposi sarcoma with lymphatic marker D2-40. J Am AcadDermatol61:276-280.
- Wang Y, Sun J, Gu Y, Zhao S, Groome LJ, et al. (2011) D2-40/podoplanin expression in the human placenta. Placenta 32: 27-32.
- Evangelou E, Kyzas PA, Trikalinos TA (2005) Comparison of the diagnostic accuracy of lymphatic endothelium markers: Bayesian approach. Mod Pathol18:1490-1497.
- Mascarel I, MacGrogan G, Debled M, Sierankowski G, Brouste V, et al. (2009) D2-40 in breast cancer: Should we detect more vascular emboli? Mod Pathol22:216-222.
- Paduch R (2016) The role of lymphangiogenesis and angiogenesis in tumor metastasis. Cell Oncol (Dordr).
- Kahn HJ, Marks A (2002) A new monoclonal antibody, D2-40, for detection of lymphatic invasion in primary tumors. Lab Invest 82:1255-1257.
- Kahn HJ, Bailey D, Marks A (2002) Monoclonal antibody D2-40, a new marker of lymphatic endothelium, reacts with Kaposi's sarcoma and a subset of angiosarcomas. Mod Pathol15:434-440.
- Fukunaga M (2005) Expression of D2-40 in lymphatic endothelium of normal tissues and in vascular tumours. Histopathology 46: 396-402.
- Shibuya M (2011) Vascular Endothelial Growth Factor (VEGF) and its receptor (VEGFR) signaling in angiogenesis: A crucial target for anti- and pro-angiogenic therapies. Genes Cancer 2:1097-1105.
- Chen JC, Chang YW, Hong CC, Yu YH, Su JL (2012) The role of the VEGF-C/VEGFRs axis in tumor progression and therapy. Int J MolSci 14: 88-107.
- Yao G, He P, Chen L, Hu X, Gu F, et al. (2013) MT1-MMP in breast cancer: Induction of VEGF-C correlates with metastasis and poor prognosis. Cancer Cell Int13: 98.
- Ran S, Volk L, Hall K, Flister MJ (2010) Lymphangiogenesis and lymphatic metastasis in breast cancer. Pathophysiology 17:229-251.
- Ding M, Fu X, Tan H, Wang R, Chen Z, et al.(2012) The effect of vascular endothelial growth factor C expression in tumor-associated macrophages on lymphangiogenesis and lymphatic metastasis in breast cancer.Mol Med Rep 6:1023-1029.
- Greene FL, Page DL, Fleming ID, Fritz AG, Balch CM, et al.(2002) Morrow M: Breast. In AJCC Cancer Staging Manual 6th edition. Edited by: Greene FL, Page DL, Fleming ID, Fritz AG, Balch CM, Haller DG, Morrow M. New York: Springer 223-240.
- Page DL, Jensen RA, Simpson JF (1998) Routinely available indicators of prognosis in breast cancer. Breast Cancer Res Treat 51: 195-208.
- Fitzgibbons PL, Page DL, Weaver D, Thor AD, Allred DC, et al. (2000) Prognostic factors in breast cancer. College of American Pathologists Consensus Statement 1999. Arch Pathol Lab Med 124:966-978.
- Robbins P, Pinder S, De Klerk N, Dawkins H, Harvey J, et al. (1995) Histological grading of breast carcinomas: A study of interobserver agreement. Hum Pathol26:873-879.
- Kurahara H, Takao S, Maemura K, Shinchi H, Natsugoe S, et al. (2004) Impact of vascular endothelial growth factor-C and -D expression in human pancreatic cancer: Its relationship to lymph node metastasis. Clin Cancer Res 10:8413-8420.
- Van der Auwera I, Van den Eynden GG, Colpaert CG, Van Laere SJ, Van Dam P, et al. (2005) Tumor lymphangiogenesis in inflammatory breast carcinoma: Ahistomorphometric study. Clin Cancer Res11:7637-7642.
- Saad RS, Kordunsky L, Liu YL, Denning KL, Kandil HA, et al. (2006) Lymphatic microvessel density as prognostic marker in colorectal cancer. Mod Pathol19:1317-1323.
- Weidner N (1995) Current pathologic methods for measuring intratumoralmicrovessel density within breast carcinoma and other solid tumors. Breast Cancer Res Treat 36:169-180.
- Yamauchi C, Hasebe T, Iwasaki M, Imoto S, Wada N, et al. (2007) Accurate assessment of lymph vessel tumor emboli in invasive ductal carcinoma of the breast according to tumor areas, and their prognostic significance. Hum Pathol38:247-259.
- Kalof AN, Cooper K (2009) D2-40 immunohistochemistry--so far! AdvAnatPathol16: 62-64.
- El-Zammar OA, Haidar A (2003) Immunoreactivity of ductal cells with putative myoepithelial markers: A potential pitfall in breast carcinoma. Ann DiagnPathol7: 335.
- Lerwill MF (2004) Current practical applications of diagnostic immunohistochemistry in breast pathology. Am J SurgPathol28:1076-1091.
- Breiteneder-Geleff S, Soleiman A, Kowalski H, Horvat R, Amann G, et al. (1999) Angiosarcomas express mixed endothelial phenotypes of blood and lymphatic capillaries: podoplanin as a specific marker for lymphatic endothelium. Am J Pathol 154:385-394.
- De Araújo VC, Altemani A, Furuse C, Martins MT, De Araújo NS (2006) Immunoprofile of reactive salivary myoepithelial cells in intraductal areas of carcinoma ex-pleomorphic adenoma. Oral Oncol42:1011-1016.
- Kanner WA, Galgano MT, Atkins KA (2010) Podoplanin expression in basal and myoepithelial cells: utility and potential pitfalls. ApplImmunohistochemMolMorphol18:226-230.
- Van der Auwera I, Cao Y, Tille JC, Pepper MS, Jackson DG, et al. (2006) First international consensus on the methodology of lymphangiogenesis quantification in solid human tumours. Br J Cancer 95:1611-1625.
- El-Gohary YM, Metwally G, Saad RS, Robinson MJ, Mesko T, et al. (2008) Prognostic significance of intratumoral and peritumoral lymphatic density and blood vessel density in invasive breast carcinomas. Am J ClinPathol129:578-586.
- El-Gendi S, Abdel-Hadi M (2009) Lymphatic vessel density as prognostic factor in breast carcinoma: Relation to clinicopathologic parameters. J Egypt NatlCancInst21:139-149.
- Mohammed ZM, McMillan DC, Edwards J, Mallon E, Doughty JC, et al. (2013) The relationship between lymphovascular invasion and angiogenesis, hormone receptors, cell proliferation and survival in patients with primary operable invasive ductal breast cancer. BMC ClinPathol13: 31.
- Goldhirsch A, Glick JH, Gelber RD, Coates AS, Thürlimann B, et al.(2005) Panel members. Meeting highlights: International expert consensus on the primary therapy of early breast cancer 2005. Ann Oncol16:1569-1583.
- Malfettone A, Saponaro C, Paradiso A, Simone G, Mangia A (2012) Peritumoral vascular invasion and NHERF1 expression define an immunophenotype of grade 2 invasive breast cancer associated with poor prognosis. BMC Cancer 12: 106.
- Cappellani A, Di Vita M, Zanghì A, Cavallaro A, Piccolo G, et al. (2013) Prognostic factors in elderly patients with breast cancer. BMC Surg 2: S2.
- La Verde N, Biagioli E, Gerardi C, Cordovana A, Casiraghi C, et al. (2016) Role of patient and tumor characteristics in sentinel lymph node metastasis in patients with luminal early breast cancer: an observational study. SpringerPlus 5: 114.
- Arvelo F, Sojo F, Cotte C (2016) Tumour progression and metastasis.ECancerMedicalScience 10: 617.
- Skobe M, Hawighorst T, Jackson DG, Prevo R, Janes L, et al. (2001) Induction of tumor lymphangiogenesis by VEGF-C promotes breast cancer metastasis. Nat Med 7:192-198.
- Gu Y, Qi X, Guo S (2008) Lymphangiogenesis induced by VEGF-C and VEGF-D promotes metastasis and a poor outcome in breast carcinoma: a retrospective study of 61 cases. ClinExp Metastasis 25:717-725.
- Zhao YC, Ni XJ, Li Y, Dai M, Yuan ZX, et al. (2012) Peritumorallymphangiogenesis induced by vascular endothelial growth factor C and D promotes lymph node metastasis in breast cancer patients. World J SurgOncol10: 165.
- Tezuka K, Onoda N, Takashima T, Takagaki K, Ishikawa T, et al. (2007) Prognostic significance of lymphovascular invasion diagnosed by lymphatic endothelium immunostaining in breast cancer patients. Oncol Rep 17: 997-1003.
- Beasley NJ, Prevo R, Banerji S, Leek RD, Moore J, et al. (2002) Intratumorallymphangiogenesis and lymph node metastasis in head and neck cancer. Cancer Res 62:1315-1320.
- Nakamura Y, Yasuoka H, Tsujimoto M, Yang Q, Imabun S, et al. (2003) Prognostic significance of vascular endothelial growth factor D in breast carcinoma with long-term follow-up. Clin Cancer Res 9:716–721.
- Hall FT, Freeman JL, Asa SL, Jackson DG, Beasley NJ (2003) Intratumorallymphatics and lymph node metastases in papillary thyroid carcinoma. Arch Otolaryngol Head Neck Surg129:716-719.
- Sipos B, Klapper W, Kruse ML, Kalthoff H, Kerjaschki D, et al. (2004) Expression of lymphangiogenic factors and evidence of intratumorallymphangiogenesis in pancreatic endocrine tumors. Am J Pathol165:1187-1197.
- Pak KH, Jo A, Choi H J, Choi Y, Kim H, et al. (2015) The different role of intratumoral and peritumorallymphangiogenesis in gastric cancer progression and prognosis. BMC Cancer 15: 498.
- Padera TP, Kadambi A, di Tomaso E, Carreira CM, Brown EB, et al. (2002) Lymphatic metastasis in the absence of functional intratumorlymphatics. Science 296:1883-1886.
- Bono P, Wasenius VM, Heikkilä P, Lundin J, Jackson DG, et al. (2004) High LYVE-1-positive lymphatic vessel numbers are associated with poor outcome in breast cancer. Clin Cancer Res 10:7144-7149.
- Muñoz-Guerra MF, Marazuela EG, Martín-Villar E, Quintanilla M, Gamallo C (2004) Prognostic significance of intratumorallymphangiogenesis in squamous cell carcinoma of the oral cavity. Cancer 100:553-5560.
- Lee K, Park DJ, Choe G, Kim HH, Kim WH, et al. (2010) Increased intratumoral lymphatic vessel density correlates with lymph node metastasis in early gastric carcinoma. Ann Surg Oncol17: 73-80.
- Renyi-Vamos F, Tovari J, Fillinger J, Timar J, Paku S, et al. (2005) Lymphangiogenesis correlates with lymph node metastasis, prognosis, and angiogenic phenotype in human non-small cell lung cancer. Clin Cancer Res 11:7344-7353.
- Matsumoto K, Nakayama Y, Inoue Y, Minagawa N, Katsuki T, et al. (2007) Lymphatic microvessel density is an independent prognostic factor in colorectal cancer. Dis Colon Rectum 50:308-314.
- Zeng Y, Opeskin K, Horvath LG, Sutherland RL, Williams ED (2005) Lymphatic vessel density and lymph node metastasis in prostate cancer. Prostate 65:222-230.









