Keywords
Chronic periodontitis, Plasma, Interleukins, ELISA-test, Biomarker
Introduction
Periodontitis is a chronic inflammatory disease of the oral cavity that affects tooth-supporting tissues and alveolar bone, leading progressively to tooth loss and can affect individual’s oral health-related quality of life [1-4]. Chronic periodontitis affects nearly 50% of adult population and 60% of aged population globally, being considered the most prevalent inflammatory disease worldwide [5]. The pathogenesis of chronic periodontitis is multifactorial, resulting from a complex interaction between pathogenic microbes, the host immune responses, genetic and environmental factors [3,5,6,]. Although bacterial infection is the primary cause in triggering periodontal disease, it’s progression depends on the production of host mediators in response to bacteria and it’s metabolic products [3]. Porphyromonas gingivalis and Fusobacterium nucleatum are major culprits in periodontal destruction and are highly associated with periodontitis development [5,7].
Innate and adaptive immune reactivity control the response to periodontal pathogens [8]. While innate mechanisms include the barrier effect of an intact epithelium, salivary protection and pattern recognition receptors, adaptive immunity is characterized by specificity, memory and the ability to distinguish between self and non-self [9]. A cytokine network, regulates the cross-talk between periodontal pathogens and inflammatory process, being able to amplify or suppress tissue reactions in periodontal pathogenesis [10,11]. Cytokines, that have the role of activating T and B cells, are released when microbial antigens are recognized by the appropriate receptor, leading to cell mediated and humoral immune responses [9]. Periodontitis is characterized by an unbalanced host response to periodontopathogens, which lead to periodontal tissue breakdown and clinical signs of the disease [2,11]. The quality of the host immune-inflammatory response against bacterial challenge determines the severity and extent of the disease [11].
Interleukin (IL) IL-1β, IL-6, IL-8 and IL-10 are the main cytokines identified in chronic periodontal disease, with further influence on the activity of immune cells [12]. The interleukin (IL)-1 family of cytokines has a central role in triggering and perpetuating the immune and inflammatory responses [11]. Pro-inflammatory cytokines, such as interleukin 1β, play a key role in the initiation and regulation of immune responses in periodontium [13]. It is well documented, that interleukin 1β has been involved in the pathogenesis of inflammation-induced bone resorption [13]. Interleukin-8 is a α chemokine, member of the CXC chemokine family [12,14]. It is a relevant mediator of granulocyte accumulation by attracting PMNs in the inflammatory region, representing a host defense against periodontal bacteria [12]. It plays an important role as a chemotactic factor for neutrophils and lymphocytes [14]. It’s expression has been reported to increase in chronically inflamed periodontal tissue, as well as in gingival crevicular fluid of patients with periodontitis [14]. Interleukin-10 inhibits cytokine production by activating T-helper (Th1) clones, due to inhibitory effect on monocyte and macrophages [12]. It specifically modulates the expression of cytokine of myeloid origin, thus affecting activation and maintenance of immune response in periodontitis [12]. It is considered an anti-inflammatory cytokine that is able to inhibit the synthesis of several pro-inflammatory cytokines, such as IL-1, IL-6, IL-8 and TNF-α [2]. Higher levels of IL-10 in periodontal tissues have already been correlated with a reduction in the expression of the osteoclastogenic factor RANKL [2]. Interleukin (IL)-13 inhibits pro-inflammatory cytokines, chemokines and profibrogenic cytokines synthesis [15]. It is a pleiotropic Th2 cytokine, produced by a wide variety of different cell types and is responsible for a broad range of biology and functions [16,17]. It is responsible for inhibiting osteoclast formation by decreasing expression of other osteoclastogenic cytokines [18]. Rationale for targeting IL-13 is most robust in asthma, atopic dermatitis, allergic rhinitis, chronic obstructive pulmonary disease (COPD), cancer, inflammatory bowel disease, autoimmune disease and fibrotic disease [17]. So far there has been no correlation made between plasma expression of interleukin 13 and chronic periodontitis. Plasma cytokine levels in chronic periodontitis may be used as biomarkers of the disease, as so far it’s diagnosis relies almost exclusively on clinical parameters and dental radiography [19,20].
The aim of the study was to evaluate associations between interleukin 1β, interleukin 8, interleukin 10 and interleukin 13 in patients with chronic periodontitis, in order to determine if there is a correlation between clinical stage of the disease and plasma concentration levels of these cytokines.
Materials and Methods
Study population
Study subjects were selected from the department of General and Maxillofacial Surgery, Sibiu University Hospital, Romania, from May to September 2016. A total of 88 subjects (n=88; 40 males and 48 females; age ranging from 31 to 76 years, mean age of 52.30 years) were divided into four groups, viz., control group (HG), early generalized chronic periodontitis group (EGP), moderate generalized chronic periodontitis group (MGP) and advanced generalized chronic periodontitis group (AGP). The purpose of the study was completely explained to each subject, before entering the study and informed consent was obtained from each patient. Complete medical and dental histories were taken from all subjects. None of the patients were smokers and none of them underwent any nonsurgical or surgical periodontal treatment within the past 12 months. Subjects with diabetes, osteoporosis, chronic coronary, respiratory and kidney diseases as well as pregnant women were not included in the study. Selection of the patients was made according to the clinical and radiographic criteria proposed by the 1999 International World Workshop for the Classification of Periodontal Diseases. The subjects for sampling were selected at random from individuals scheduled for a routine oral examination. All patients, included in periodontitis groups presented clinical signs of periodontal disease, such as: halitosis, gingival recession, periodontal pockets or tooth loss in advanced stages of the disease. The clinical attachment loss (CAL) was measured, classifying the severity of the disease in slight: CAL 1-2 mm, moderate: CAL 3-4 mm and severe: CAL 5 mm or more. At the screening stage, to determine the clinical periodontal status, all subjects had a clinical periodontal examination, including the measurement of pocket depth and CAL, by one examiner. The early generalized chronic periodontitis group (EGP) consisted of 21 patients, 11 females and 10 males between the ages of 36 and 62 years (mean of 51 years) that had a maximum of 2 mm CAL. The moderate chronic periodontitis group (MGP) consisted of 24 patients 10 females and 14 males between the ages of 38-71 (mean of 60 years). They had moderate alveolar bone loss and CAL of maximum 4 mm. The advanced chronic periodontitis group (AGP) consisted of 24 patients 13 females and 11 males, between the ages of 48-75 years (mean age 67 years). They had severe alveolar bone loss, CAL of ≥ 6 mm and probing depth of gingival sulcus (PD) of ≥ 4 mm, in multiple sites of all four quadrants of the mouth. The healthy group (HG) consisted of 19 patients, 9 females and 10 males, ranged in age from 31-54 years, with a mean age of 44 years, who exhibited no CAL, PD of 1-2 mm, no clinical or sulcular bleeding and no radiographic evidence of bone loss.
Collection of blood samples
Venous blood samples were collected from each subject, from the antecubital vein, in 4 ml vacutainer glass blood collection tubes for coagulation, buffered with sodium citrate 3.2%. All samples were centrifuged after clotting, to separate the serum from the cells, for 20-30 min at 2000-3000 g. The obtained blood serum was placed into sterile Eppendorf vials and kept at −40°C until being analyzed.
Biochemical analysis
The IL-1β (Boster Biological Technology Co., Ltd. Pleasanton, CA, USA), IL-8 (Boster Biological Technology Co., Ltd. Pleasanton, CA, USA), IL-10 (Boster Biological Technology Co., Ltd. Pleasanton, CA, USA) and IL-13 (MyBioSource, Inc. San Diego, CA, USA) were analyzed by enzyme-linked immunosorbent assay (ELISA), for quantification of these proteins in the blood samples. Manufacturers’ guidelines were followed for each assay and 96- well plates, precoated with appropriate antibodies, were used. The lower detection thresholds for the IL-1β, IL-8, IL-10 and IL-13 assays were 3.9 pg/ml, 15.6 pg/ml, 7.8 pg/ml and 15.6 pg/ml, respectively.
Statistical analysis
Statistical analysis was performed using SPSS, version 25.0. Comparison between interleukin concentration in different age groups and plasma interleukin testing between periodontitis groups were performed, using Fischer analysis, p<0,05 was considered to be statistically significant. Parametrical Pearson rank correlation analysis was used to analyze the correlation between plasma IL-1β, IL-8, IL-10 and IL-13, for healthy and chronic periodontitis groups and p<0.05 was considered to be statistically significant.
Results
There is an evident increase of age along with the progression of periodontal disease. Periodontal health status is more frequent among younger patients (age group 31-40 years and 41-50 years). Moderate and advanced stages of the disease are more frequent among older patients (61-70 years and over 70 years) (Figure 1).
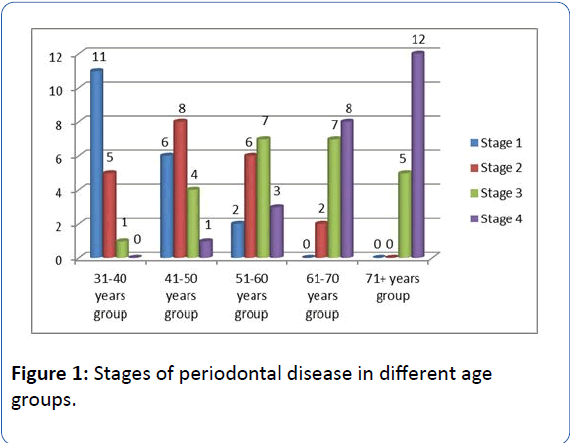
Figure 1: Stages of periodontal disease in different age groups.
The medium concentration of IL-1β increases with age and is significantly higher in older patients, the difference between age groups being statistically significant (p<0.001, Fischer test). Mean concentration of IL-8 increases with age as well as IL-1β, difference between age groups is statistically significant (p<0.001, Fischer test). Concentration of IL-10 decreases with advanced age, the difference between age groups is statistically significant (p<0.001, Fisher test). IL-13 decreases with advancing age, the average difference between age groups of this IL being statistically significant (p<0.001, Fischer test) (Figure 2).
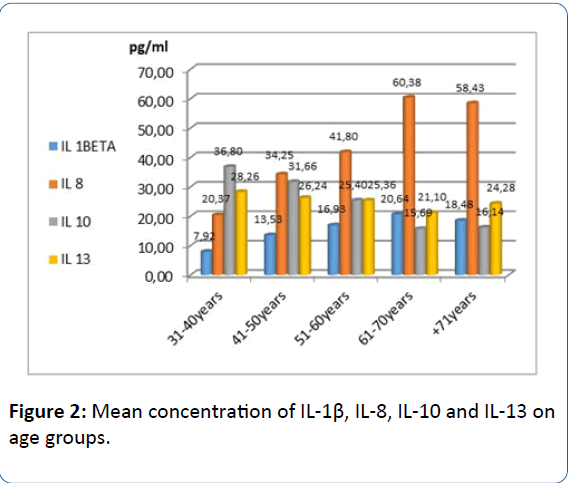
Figure 2: Mean concentration of IL-1β, IL-8, IL-10 and IL-13 on age groups.
IL-1β concentrations are significantly higher along with the progression of periodontal disease. The difference between the groups in IL-8 testing are statistically significant (p<0.001; Fischer test), this interleukin showing high concentrations in advanced stage of chronic periodontitis. IL-10 testing between the groups is statistically significant (p<0.001; Fischer test), concentrations of this interleukin in healthy group, being higher than in chronic periodontitis groups. IL-13 testing shows significant higher concentrations in the healthy group than in chronic periodontitis patients (p<0.001; Fischer test). IL concentration in different stages of the disease is statistically significant (p<0.001, Fischer test). There is an increase in IL-1β and IL-8 concentration along with advancing stages of the disease and a decrease in IL-10 and IL-13 concentrations along with progression of the disease (Figure 3).
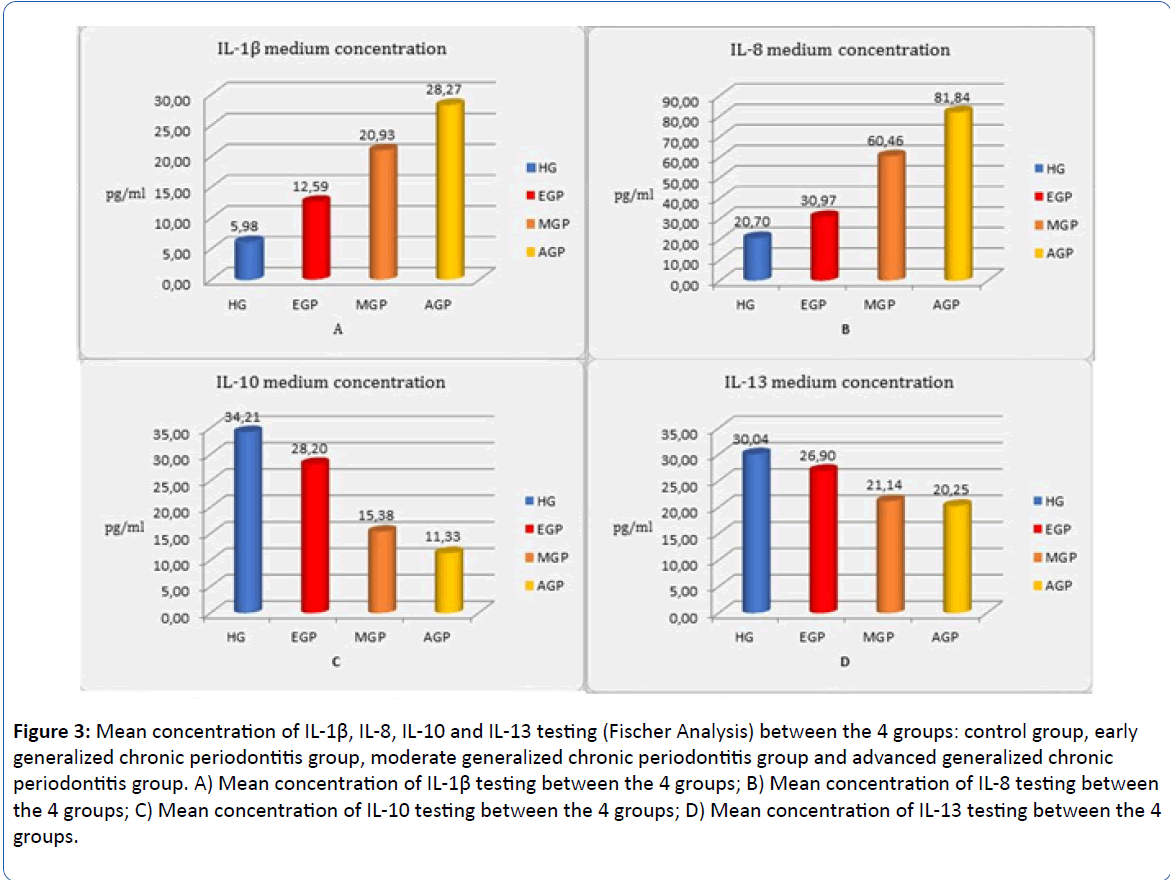
Figure 3: Mean concentration of IL-1β, IL-8, IL-10 and IL-13 testing (Fischer Analysis) between the 4 groups: control group, early generalized chronic periodontitis group, moderate generalized chronic periodontitis group and advanced generalized chronic periodontitis group. A) Mean concentration of IL-1β testing between the 4 groups; B) Mean concentration of IL-8 testing between the 4 groups; C) Mean concentration of IL-10 testing between the 4 groups; D) Mean concentration of IL-13 testing between the 4 groups.
Pearson rank correlation analysis shows positive statistically significant correlations (p=0.025; p<0.05; r=0.512) between IL-10 and IL-13 in healthy patients (HG). The analysis shows that interrelation between IL-10 and IL-13 is more frequent in the control group (Figure 4).
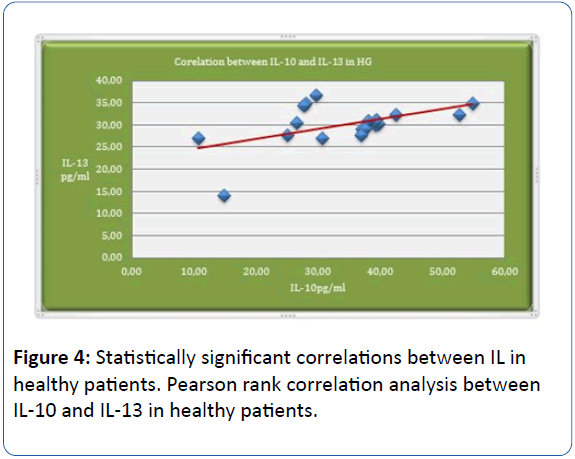
Figure 4: Statistically significant correlations between IL in healthy patients. Pearson rank correlation analysis between IL-10 and IL-13 in healthy patients.
There were no statistically significant correlations using Pearson analysis between IL1β, IL8 IL10 or IL13 in the early stage of chronic periodontitis (EGP).
Highly significant positive correlations between IL-1β and IL-8 (p<0.001, r=0.959, Pearson test) have been found in patients with moderate stages of chronic periodontitis (MGP), meaning that IL-8 concentration increases according to IL-1β along with the progression of the disease. Pearson rank correlation in moderate stage of periodontitis shows statistically significant negative correlations between IL-1β and IL-13 (p=0.034; r=-387), which indicates that increased IL-1β concentrations cause a decrease of IL-13 concentration. Pearson rank correlation in moderate stage of periodontitis shows statistically significant negative correlations between IL-8 and IL-13 (p=0.022; r=-417), which shows that increased IL 8 concentrations cause a decrease of IL-13 concentration along with the progression of the disease (Figure 5).
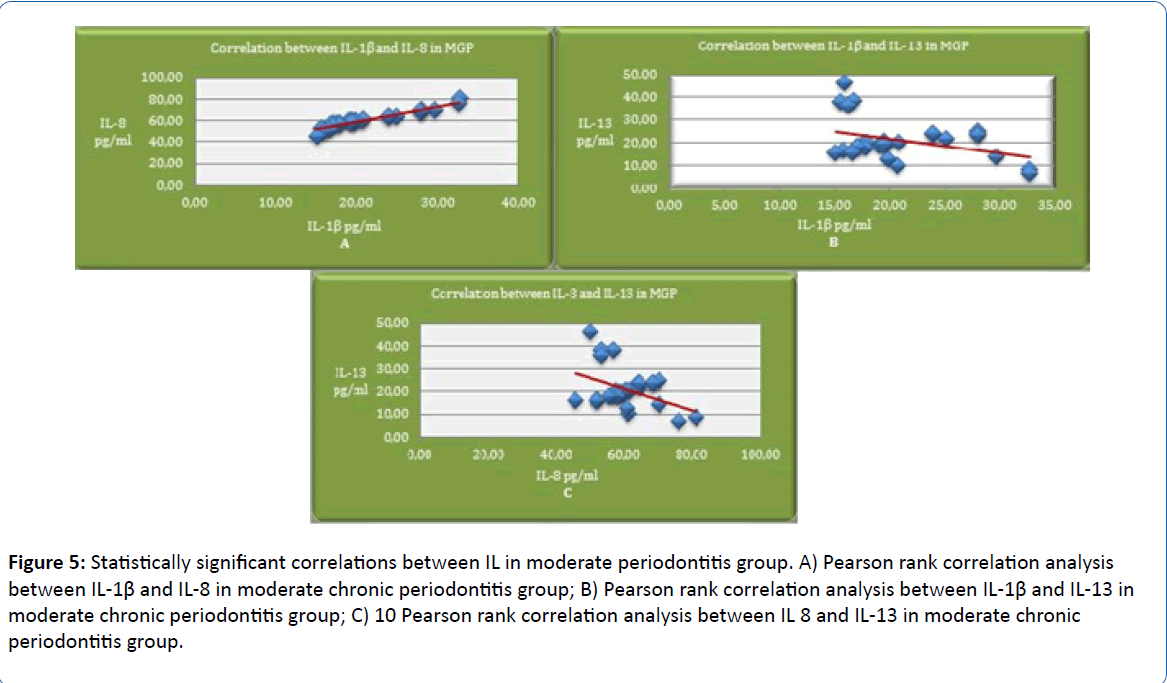
Figure 5: Statistically significant correlations between IL in moderate periodontitis group. A) Pearson rank correlation analysis between IL-1β and IL-8 in moderate chronic periodontitis group; B) Pearson rank correlation analysis between IL-1β and IL-13 in moderate chronic periodontitis group; C) 10 Pearson rank correlation analysis between IL 8 and IL-13 in moderate chronic periodontitis group.
Pearson rank correlation in advanced stage of periodontitis (AGP) shows statistically significant positive correlations between IL-1β and IL-8 (p=0.001; r=0.845), which indicates that IL-8 concentration increases along with IL-1β in this stage of PD. Pearson rank correlation in advanced stage of periodontitis shows statistically significant negative correlations between IL-8 and IL-10 (p<0.001; r=-0,658), which shows that increased IL-8 concentrations cause a decrease of IL-10 concentration in this stage of the disease (Figure 6).
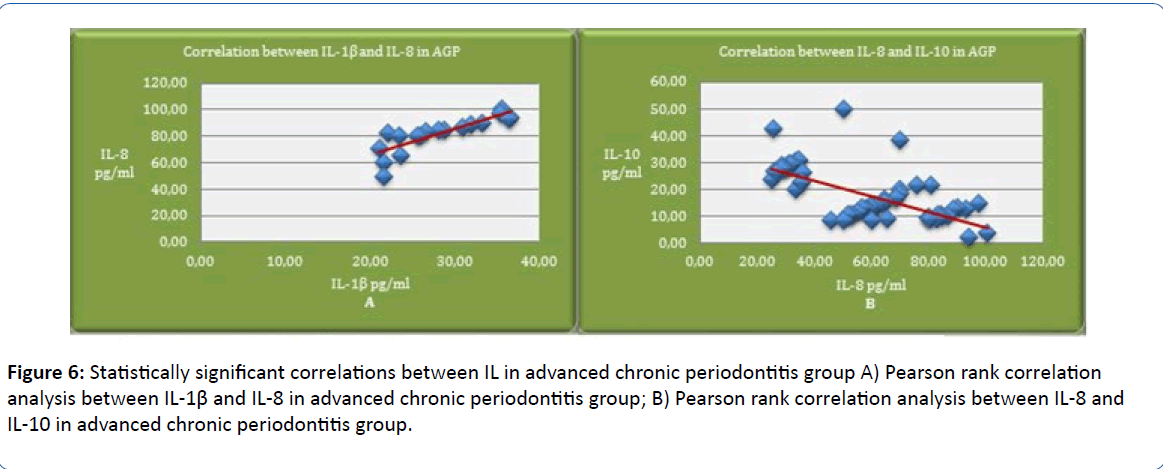
Figure 6: Statistically significant correlations between IL in advanced chronic periodontitis group A) Pearson rank correlation analysis between IL-1β and IL-8 in advanced chronic periodontitis group; B) Pearson rank correlation analysis between IL-8 and IL-10 in advanced chronic periodontitis group.
Discussion
Periodontitis is a peripheral chronic inflammatory disease, caused by an imbalance between destruction and repair of tooth supporting tissues, triggered by periodontal bacteria of the dental plaque [2,12]. The presence of a dysbiotic microbiota is not sufficient to complete the outcome of the disease in absence of a host immune response [21-23]. Irreversible tissue damage is ultimately inflicted by the immune inflammatory response, which determines the severity and extent of the disease [11,22].
Periodontal disease has been associated with increased systemic inflammatory markers such as cytokines, which play an important role in the pathogenesis and progression of the disease, by determining the strength, nature and duration of the immune response [24-28]. Cytokines in serum, plasma, GCF, and saliva have been identified as inflammatory indicators of periodontal disease and can be used as biomarkers of chronic periodontitis [29].
The main objective of our research has been to correlate clinical stage of chronic periodontal disease with plasma expression of interleukin 1β, IL-8, IL-10 and IL-13, in order to determine if a diagnosis is possible.
By assessing clinical parameters of periodontal sites in chronic periodontitis patients and controls, comparing ages, results indicate that chronic periodontitis is more frequent and evident among older patients, supporting the fact that aging causes functional alterations to innate immune cells, which can increase susceptibility to chronic diseases such as periodontitis [30]. Advanced clinical stage of periodontal disease is found to be consistent with older ages. Nevertheless, aging by itself can not necessarily cause periodontitis unless in the presence of concomitant periodontal inflammation, and increased prevalence and severity of periodontitis in old age simply reflects the cumulative effect of prolonged exposure to the periodontal microbial challenge [30].
Interleukin 1β is considered marker of acute inflammatory phase response and it’s salivary and crevicular fluid levels are strongly correlated with advanced periodontal tissue damage and periodontal infection [28,31]. Salivary levels of this cytokine appear to serve as biomarkers in periodontitis [32]. Some previous studies showed no significant differences in serum levels of IL-1 beta, between periodontitis patients and periodontally healthy controls and suggested that IL-1β detection in serum samples from periodontitis patients is useless for the detection of disease presence and/or its severity [33,34]. Other studies suggested that concentrations of IL-1beta, were, on average, significantly higher in serum samples and gingival tissue biopsies from periodontitis patients than in healthy controls [34,35]. Also IL-1β plasma levels were found to be significantly higher in aggressive periodontitis patients [36]. Our results regarding its plasma concentration, in patients with chronic periodontitis, show higher levels, compared to the healthy group. Moreover IL-1β concentration according to our analysis showed increasing levels along with progression of periodontal disease (p<0.001 Fischer test), showing that advanced stages of the disease may have an impact on IL1β plasmatic levels and may cause a systemic pro-inflammatory status [37].
Plasma interleukin analysis in our study shows higher concentration levels of IL-8 in chronic periodontitis subjects, compared to the control group. The expression of IL-8 has been reported to increase in chronically inflamed periodontal tissue and gingival crevicular fluid of patients with periodontitis, contributing to PD development, through its capacity to attract and activate neutrophils [38]. Significant differences were found in salivary levels of IL-8 between periodontitis classifications [39]. Other previous studies showed increased IL-8 expression in the peripheral blood of periodontitis patients, compared to healthy ones [40]. We found that IL-8 levels increased according to stage of periodontal disease. These data suggest that dynamic changes in IL-8 plasma levels were highly associated with periodontal disease severity. Clinical parameters of the disease were positively correlated with IL-8 levels, which suggest that plasma levels of IL-8 are associated with periodontal status [41].
It’s interesting to notice that significant correlations have been found, using Pearson analysis, between IL-1β and IL-8 plasma concentrations in chronic periodontitis patients. Correlations between IL-1β and IL-8 were positive and statistically significant in the moderate (p<0.001, r=0.959, Pearson analysis) and advanced (p=0.001; r=0.845) stages of periodontal disease. This indicates that IL-8 increases according to IL-1β in these groups. It has already been shown, that IL-8 is produced by a variety of cells including monocytes, epithelial cells, endothelial cells as well as gingival fibroblasts, in response to inflammatory mediators such as interleukin 1β [38]. IL-1β has been observed to be one of the most potent inducers of IL-8 [38]. One of the novel findings in this study and maybe of most importance is that IL-1β has a stimulatory effect on IL-8 production and that significant correlation between these interleukins occurs not only in human gingival fibroblasts, as reported, but also in plasma of chronic periodontitis patients. In other words, increase of IL-8 according to IL-1β, in periodontal sites of chronic periodontitis patients, has an important systemic correspondent in plasma of these patients.
IL-10 is a multifunctional anti-inflammatory cytokine with regulatory effects in periodontal inflammation [42,43]. It has beneficial effects in limiting the excessive immune response, tissue destruction and bone resorption in periodontal disease, by inhibiting the synthesis of pro-inflammatory cytokines [42]. It has been reported that lower amounts of IL-10 may contribute to the progression of periodontal disease and that higher amounts have protecting effect on tissue destruction, by suppressing the secretion of pro-inflammatory cytokines, such as IL-1 and IL-8 [42,43]. In relation to plasma levels, we found higher concentrations of IL-10 in healthy patients compared to patients with different stages of periodontal disease and decreasing levels of this interleukin along with advanced stages of periodontitis (p<0.001, Fischer test). Other studies also found negative correlations between clinical parameters of periodontal disease and serum levels of IL-10 [44]. Also serum levels of IL-10 were found to be low in periodontitis and metabolic syndrome patients and periodontal treatment showed improvement of serum levels of this interleukin [45]. However periodontitis and non-periodontitis subjects may present a similar ability to produce this regulatory cytokine and the exact relationship amongst periodontal diseases, systemic inflammation and cytokines still remains unclear [40].
IL-13 is an immunoregulatory protein, produced mainly by activated Th2 cells and plays an important role in the maturation and differentiation of B cells [46]. IL-13 is a powerful antiinflammatory cytokine that inhibits production of proinflammatory cytokines, such as IL-1β [46]. Along with IL-10, IL-13 is one of the cytokines that inhibit osteoclast formation [18]. It’s reported to have, in association with IL-4, an inhibitory role in inflammation induced bone resorption, in diseases like periodontitis [18]. As far as we know, no other study reported plasma expression of IL-13 in chronic periodontal disease. Our results show low plasma levels of this interleukin in patients with chronic periodontitis, compared to higher levels in the control group, supporting its role in inhibiting osteoclastic cytokine activity. We found higher concentrations of IL-13 in healthy patients compared to patients with different stages of periodontal disease and decreasing levels of this interleukin along with moderate and advanced stage of periodontitis (p<0.001, Fischer test).
According to Pearson analysis in our study, there seems to be a strong correlation between anti-inflammatory cytokine IL-10 and IL-13, in the control group (p=0.025; p<0.05; r=0.512). The results suggest that IL-13 increases according to IL-10 potentiating its activity as an anti-inflammatory cytokine in the control group. Further studies are required in order to establish the exact interconnection of these interleukins in chronic diseases.
Pearson correlation analysis also showed negative statistically significant correlations between IL-1β and IL-13 (p=0.034; r=-387) and IL8 and IL13 (p=0.022; r=-417) in the moderate chronic periodontitis group and negative statistically significant correlations between IL-8 and IL-10 (p<0.001; r=-0,658) in the advanced chronic periodontitis group, indicating that an increase in pro-inflammatory cytokine levels has a negative effect on plasmatic anti-inflammatory cytokines causing an systemic pro-inflammatory status and a favorable ground for chronic periodontitis development. There were no statistically significant correlations to be found between these IL, according to Pearson analysis, in the early chronic periodontitis group.
Significant associations could be observed between PD and plasma cytokine levels, and interdependence between these interleukins is found to be much more frequent in chronic periodontitis patients. Our results suggest that elevated concentrations of these interleukins in plasma, seems to be closely associated with periodontal disease severity. The correlation between clinical periodontal disease and plasma concentrations of these cytokines, observed in our study, suggests that a causal relationship between periodontal and systemic inflammation might exist.
Conclusion
In conclusion we cannot rule out a lack of involvement of plasmatic IL-1β, IL-8, IL-10 and IL-13 in pathogenesis of periodontal disease. Currently, as a result of the collected data, we can foresee a possible correlation between plasma level of each of the interleukins analyzed and the progression of periodontal disease. It’s interesting to notice that correlating plasma IL-1β, IL-8, IL-10 and IL-13, we can emphasize that there might be a possible implication regarding one or several of these interleukins in the progression of periodontal disease. However further studies and correlations with other cytokines are required, in order to demonstrate that plasma levels of these cytokines may be used as indicators of chronic periodontitis progression.
Ethical Approval
This study was approved by the ethical committee of the School of Medicine, Lucian Blaga University (Protocol no: 5532/ 26.11.2015)
Conflicts of Interest
The authors declare that they have no conflicts of interest.
19455
References
- Kobayashi T, Ishida K, Yoshie H (2016) Increased expression of interleukin-6 (IL-6) gene transcript in relation to IL6 promoter hypomethylation in gingivaltissue from patients with chronic periodontitis. Arch Oral Biol69:89-94.
- Bastos JV, Queiroz-Junior CM, Caliari MV, FrancischiJN, Pacheco CM, et al. (2011) Peripheral kappa opioid receptors activation reduces alveolar bone loss in rats by modulating interleuki-6and -10. Arch Oral Biol56: 540-548.
- Khademi B, Hashemi SB, Ghaderi A,Shahres A, Mohammadianpanah M (2012) Interleukin-13 gene polymorphisms at в€’1055 C/T and +2044 G/A positions in patients with squamous cellcarcinoma of head and neck. Braz J Otorhinolaryngol78:64-68.
- He CY, GaoXY, Jiang LP (2016) The impact of smoking on levels of chronic periodontitis-associated biomarkers. Exp Mol Pathol101:110-115.
- Banjar W, Alshammari MH (2014) Genetic factors in pathogenesis of chronic periodontitis. J TaibahUnivSci9: 245-247.
- Satpathy A, Ravindra S, Thakur S, Kulkarni S, Porwal A, et al. (2015) Serum interleukin-1ОІ in subjects with abdominal obesity and periodontitis. Obes Res ClinPract 9: 513-521.
- Könönen E, Kumar PS (2015) Bacteriology of periodontal diseases. (2nd edn), Molecular Medical Microbiology pp: 957-968.
- Souto GR, Queiroz-Junior CM, Costa FO, Mesquita RA (2014) Effect of smoking on immunity in human chronic periodontitis. Immunobiology219:909-915.
- Kornman KS, Page RC, Tonetti MS (2000) The host response to the microbial challenge in periodontitis, assembling the players. Periodontol14: 33-53.
- KurşunluSF, Özturk VO, Han B, Atmaca H, Emingil G (2015) Gingival crevicular fluid interleukin-36β (-1F8), interleukin-36γ (-1F9) and interleukin-33 (-1F11) levels in different periodontal disease. Arch Oral Biol60:77-83.
- Grover HS, Kapoor S, Singh A (2016) Effect of topical simvastatin (1.2 mg) on gingival crevicular fluid interleukin-6, interleukin-8 and interleukin-10 levels in chronic periodontitis- A clinicobiochemical study. J Oral BiolCraniofac Res 6:85-92.
- Deshpande N, Deshpande A (2013)Immunoregulation in periodontal disease. Indian J Dent 4:35-37.
- Li G, Yue Y, Tian Y, Li JL, Wang M, et al. (2012) Association of matrix metalloproteinase (MMP)-1, 3, 9, interleukin (IL)-2, 8 and cyclooxygenase (COX) 2 gene polymorphisms with chronic peridontitis in a Chinese population. Cytokine60:552-560.
- Zhu C, Zhang A, Huang S, Ding G, Pan X, et al. (2010) Interleukin-13 inhibits cytokines synthesis by blocking nuclear factor-ОєB and c-Jun N-terminal kinase in human mesangial cells. J Biomed Res24:308-316.
- MertensTC, Hiemstra PS, Taube C (2016) Azithromycin differentially affects the IL-13-induced expression profile in human bronchial epithelial cells. PulmPharmacolTher39:14-20.
- May RD, Fung M (2015) Strategies targeting the IL-4/IL-13 axes in disease. Cytokine75: 89-116.
- Souza PP, Palmqvist P, Lundberg P, Lundgren I, Hanstrom L, et al. (2012) Interleukin-4 and interleukin-13 inhibit the expression of leukemia inhibitory factor and interleukin-11 in fibroblasts. Mol Immunol49:601-610.
- MenegatJS, Lira-Junior R, Siqueira MA, Brito F, Carvalho AT, et al. (2016) Cytokine expression in gingival and intestinal tissues of patients with periodontitis and inflamatory bowel disease:An exploratory study. Arch Oral Biol66:141-146.
- Mogi M, Otogoto J, Ota N, Inagaki H, Minamy M, et al. (1999) Interleukin 1B, interleukin 6, B2 macroglobulin and transforming growth factor in gingival crevicular fluid from human periodontal disease. Arch Oral Biol44:535-539.
- Sparks SP, Steffen MJ, Smith C, Jicha G, EbersoleJL, et al. (2012) Serum antibodies to periodontal pathogens are a risk factor for Alzheimer’s disease. Alzheimers Dement 8:196-203.
- Hajishengallis G (2014) Immunomicrobial pathogenesis of periodontitis: keystones, pathobionts, and host response. Trends immunol35:3-11.
- Braosi AP, de Souza CM, Luczyszyn SM, DirschnabelAJ, Claudino M, et al. (2012) Analysis of IL1 gene polymorphism and transcript levels in periodontal and chronic kidney disease. Cytokine60:76-82.
- Loos BG (2005) Systemic markers of inflammation in periodontitis. J Periodontol76: 2106-2115.
- EbersoleJL, MacHenRL, Steffen MJ, Willmann DE (1997) Systemic acute phase reactants, C-reactive protein and haptoglobin, in adult periodontitis. ClinExpImmunol107: 347-352.
- Shi D, Meng H, Xu L, Zhang L, Chen Z, et al. (2008) Systemic inflammation markers in patients with aggressive periodontitis: a pilot study. J Periodontol79:2340-2346.
- Ali J,Pramod K, Tahir MA, Ansari SH (2011) Autoimmune responses in periodontal diseases. Autoimmun Rev10:426-431.
- Marques CP, Victor EC, Franco MM, FernandesJM, Maor Y, et al. (2016) Salivary levels of inflammatory cytokines and their association to peridontal disease in systemic lupus erythematosus patients. A case-control study. Cytokine85:165-170.
- Lin PH, Yeh SK, Hoang WC, Chen HY, Chen CH,et al. (2015) Research performance of biomarkers from biofluids in periodontal disease publications. J Dent Sci10:61-67.
- Hajishengallis G (2014) Aging and its impact on innate immunity and inflammation: Implications for periodontitis. J Oral Biosci56:30-37.
- Rathnayake N, Akerman S, Klinge B, Lundegen N, Jansson H,et al. (2013) Salivary biomarkers of oral health. J ClinPeriodontol40: 140-147.
- Miller CS, King CPJr, Langub MC, Kryscio RJ, Thomas MV (2006) Salivary biomarkers of existing periodontal disease: A cross-sectional study. J Am Dent Assoc137:322-329.
- Orozco A,Gemmel E, Bikel M, Seymour GJ (2006) Interleukin-1beta, interleukin-12 and interleukin-18 levels in gingival fluid and serum of patients with gingivitis and periodontitis. Oral MicrobiolImmunol21:256-260.
- GГіrska R, Gregorek H, Kowalski J, Leskus-Perendyk A, Syczewska M, et al. (2003) Relationship between clinical parameters and cytokine profiles in inflamed gingival tissue and serum samples from patients with chronic periodontitis. J ClinPeriodontol30:1046-1052.
- Gümüş P, Nizam N, Nalbantsoy A, Özcaka Ö, Budunelli N (2014) Saliva and serum levels of pentraxin-3 and interleukin-1 in generalized aggressive or chronic periodontitis. J Periodontol85:40-46.
- Sun XJ, MengHX, CengZB (2008) Levels of interleukin-1beta and tumor necrosis factor-alpha in patients with aggressive periodontitis. Beijing Da XueXueBao40: 24-27.
- Fiorini T, Vianna P, Weidlich P, Musskopf ML, Moreira CH, et al. (2012) Relationship between cytokine levels in serum and gingival crevicular fluid (GCF) in pregnant women. Cytokine58:34-39.
- Yucel-Lindberg T, Brunius G (2006) Epidermal growth factor synergistically enhances interleukin-8 production in human gingival fibroblasts stimulated with interleukin-1ОІ. Arch Oral Biol51:892-898.
- Tamaki N, Yoshino F, Fukui M, Hayashida H, Yoshida A, et al. (2015) Relationship among salivary antioxidant activity, cytokines, and periodontitis: the Nagasaki Island study. J ClinPeriodonta79: 2136-2142.
- Gonçalves TO, Costa DJ, Brodskyn CI, Duarte PM, Cesar NetoJB, et al. (2010) Release of cytokines by stimulated peripheral blood mononuclear cells in chronic periodontitis. Arch Oral Biol55:975-980.
- Lagdive SS, MarawarPP, Byakod G, Lagdive S (2013) Evaluation and comparison of interleuk-8 (IL-8) level in gingival crevicular fluid in health and severity of periodontal disease: a clinico-biochemical study. Indian J Dent Res24:188-192.
- Luo Y, Gong Y, Yu Y (2013) Interleukin-10 gene promoter polymorphisms are associated with cyclosporin A-induced gingival overgrowth in renal transplant patients. Arch Oral Biol58:1199-1207.
- Bozkurt FY, AyZY, Berkel E, Tepe E, Akkus S (2006) Anti-inflammatory cytokines in gingival crevicular fluid in patients with periodontitis and rheumatoid arthritis: A preliminary report. Cytokine35: 180-185.
- Gümüş P, Nizam N, Lappin DF, Buduneli N (2014) Saliva and serum levels of B-cell activating factors and tumor necrosis factor-α in patients withperiodontitis. J Periodontol85: 270-280.
- Chauhan A, Yadav SS,Dwivedi P, Lal N , Usman K, et al. (2016) Correlation of serum and salivary cytokines level with clinical parameters in metabolic syndrome with periodontitis. J Clin Lab Anal 30:649-655.
- Narożna B, HoffmannA , Sobkowiak P, Schoneich N, Breborowicz A, et al. (2016) Polymorphisms in the interleukin 4, interleukin 4 receptor and interleukin 13 genes and allergic phenotype: A case control study. Adv Med Sci61:40-45.












