Keywords
Human topoisomerase inhibitors; Acacia catechu; Terminalia chebula; Terminalia belerica; Emblica officinalis; Spondias dulcis
Introduction
Topoisomerases are ubiquitous nuclear enzymes which alter the topological state of DNA by introducing or removing super helical coils, tying or untying DNA knots and by decatenating interlocked DNA [1,2]. They have critical roles in removal of DNA super coils generated by various cellular processes like replication and transcription, unlinking of intertwined DNA duplexes, chromosome segregation, DNA condensation and genome stability [1,2]. These enzymes catalyze the breakage and the rejoining of DNA strands. Broadly, all cells contain two major forms of topoisomerase, type I which causes transient break in one DNA strand and type II which cleaves both the strands [1,2]. DNA topoisomerases of various kinds, as targets of important anticancer and antibacterial drugs, have been reviewed [3].
Over the past few years there have been considerable interest in topoisomerases since they are molecular targets of many antibiotics and chemotherapeutic agents [3]. Cancer cells generally have higher levels of topoisomerase compared to normal cells [4], which is attributed to higher rate of DNA replication and transcription for increased cellular proliferation. Compounds active against eukaryotic topoisomerases are clinically useful anticancer agents such as camptothecin (topoisomerase I inhibitor) and etoposide (topoisomerase II inhibitor). Normally, topoisomerase mediated nicking of DNA strand is transient and the nick is rapidly religated after strand rotation. However, many topoisomerase inhibitors stabilize the covalent enzyme-DNA complex, preventing DNA religation. This generates DNA lesions, initiates cell cycle arrest and ultimately induces apoptosis [5]. The other class of topoisomerase inhibitors interferes with the catalytic activity of the enzyme without trapping the covalent complex.
Although many topoisomerase-directed agents are currently in clinical use for cancer chemotherapy, their utility is limited due to severe side effects such as myelo suppression, nausea, hair loss, congestive heart failure and in some cases increased risk of secondary malignancies [6,7]. Therefore it is crucial to develop novel nontoxic topoisomerase inhibitors that can spare normal cells.
For ages plants have served as repertoires of chemicals possessing medicinal properties. Historically, many useful anticancer drugs have been developed either directly from plant compounds or by their modification [8]. Many topoisomerase inhibitors in current use have been originally isolated from plant sources and then chemically modified to improve their pharmacological properties. For example, camptothecin, a topoisomerase I inhibitor, was first identified from the tree Camptotheca acuminata [9], mostly occurring in China and Tibet. Three water soluble derivatives of camptothecin are approved for clinical use: topotecan, irinotecan (worldwide) and belotecan (in South Korea) [1,8].
The Indian medicinal plants chosen for our study are Acacia catechu, Dolichos biflorus, Emblica officinalis, Hemidesmus indicus, Spondias dulcis, Terminalia chebula and Terminalia belerica. These plants have been traditionally documented in India for their popular medicinal uses [10], compiled by organic chemists [11,12] and their antioxidant activity is well documented by another group at our institute [13-17]. The anticancer properties of Acacia catechu and Emblica officinalis on cancer cell lines were reviewed [8]. Recent studies have demonstrated the anticancer activity of T. belerica and A. catechu on human lung and breast carcinoma cells through apoptosis by altering the Bax/Bcl-2 ratio [18,19]. Previously the same group has shown that, extracts of T. chebula, T. belerica, E. officinalis cause selective growth inhibition, cell cycle deregulation and apoptosis of Ehrlich’s Ascites Carcinoma cells while lacking such effects on normal cells [20]. But the role, if any, of such extracts on topoisomerases was not addressed [13-20].
One mechanism to rationalize the anticancer properties of these plant extracts has recently been shown to be through modulating the fenton reaction-mediated damage to DNA constituents [21], but it is quite possible that other mechanisms work in tandem.
Since nuclear fragmentation was directly observed by confocal microscopy for cancerous cells [18,19], we wanted to explore if the activity of human topoisomerases is affected by the plant extracts. In this study we show that many of the plant extracts completely inhibit the activity of topoisomerase I at a concentration of 40 μg/mL and partially inhibit topoisomerase II at a concentration of 120 μg/mL.
Materials and Methods
Enzymes, DNA and chemicals
Human DNA topoisomerase I, topoisomerase II and kinetoplast DNA (kDNA) and their reaction buffers were purchased from TopoGEN (Buena Vista, CO, USA). Negatively supercoiled plasmid DNA (pBSK+) was isolated from cultures of E. coli XL-1 Blue cells using plasmid purification kit, purchased from Qiagen (Hilden, Germany). HPLC grade methanol was purchased from Merck KGaA (Darmstadt, Germany). Camptothecin, etoposide and ethidium bromide were obtained from Sigma-Aldrich (St. Louis, MO, USA). Camptothecin was dissolved in DMSO and etoposide in 50% DMSO at 10 mM concentrations. Drug solutions were kept frozen at -20°C and dilutions were made in double distilled water immediately before use.
Preparation of plant extracts
Authentic parts of different plants, such as, dried fruits in cases of Terminalia chebula, Terminalia belerica and Emblica officinalis, dried seeds of Dolichos biflorus; dried roots of Hemidesmus indicus and pale catechu (rock-like) for Acacia catechu, were all purchased from a local market and finely powdered. For efficient extraction, the powder was repeatedly sieved so as to make it very fine. One hundred grams (100 g) of the powder was stirred in 500 mL of 70% (v/v) methanol in water overnight, using a magnetic stirrer. This mixture was then centrifuged at 2,850 × g for 20 min, after which the supernatant was carefully decanted. The process was repeated with the precipitated pellet. The supernatants from two rounds of extraction were mixed, concentrated using a rotary evaporator and then lyophilized. The lyophilized powders were stored at -20°C until use. For Spondias dulcis, fresh fruits purchased from local market were used for making the extract. The fruits were grinded into a smooth paste and its volume was measured. Considering this volume to be approximately equal to the 30% aqueous part in our extraction process, the paste was then mixed with a calculated amount of methanol that forms 70% of the total. Eventually, this mixture was processed using the same protocol mentioned above. Spinach (Spinacia oleracea), was a negative control in our study; fresh spinach leaves purchased from a local market were used to make the extract. The leaves were washed, grinded into a pulp, and the extraction process was same as that with Spondias dulcis.
For each of the plant extracts, the lyophilized powders were dissolved in double distilled water to obtain a stock solution of 1 mg/mL. This solution was filter sterilized using 0.22 μm syringe filter and stored at 4°C until use. From the stock solution, working solutions of 30 μg/mL, 60 μg/mL and 90 μg/mL were made. UV absorption spectra of each of the plant extracts for three different concentrations were recorded from 220 nm to 300 nm.
Isolation of active compounds from T. chebula extract
We know from available literature that, gallic acid, ellagic acid, chebulinic acid and chebulagic acid are the main polyphenolic compounds found in the fruits of T. chebula [11,12]. Earlier, these compounds of the plant extract were separated and analyzed by carrying out reverse phase HPLC using a phenyl column [22].
Our HPLC analysis was carried out on a waters chromatographic system, where 100 μl of 20 mg/mL solution (filtered) of T. chebula extract was injected into a similar polymeric reversed phase (PRP) column (150 mm × 4.1 mm and 10 μm, Hamilton, NV, USA) for separation.
The two mobile phases were - filtered, double distilled water (A) and acetonitrile (B). The gradient program was as follows: 0-4 min → isocratic with 10% B, 4-5 min → linear gradient of 10-20% B, 5-25 min → linear 20-25% B, 25-26 min → linear 25-30% B, 26-30 min → linear 30-40% B, 30-31 min → linear 40-10% B, 31-40 min → isocratic 10% B. A flow rate of 1 mL/min was maintained and the UV detection wavelength was set at 320 nm.
Ten fractions were collected which were concentrated using a speed vac concentrator (this also removes acetonitrile from the fractions) and then subjected to rechromatography under the same conditions.
The sub-fractions arising out of the second round of HPLC were evaporated to dryness, redissolved, and then analyzed by UV spectrometry from 200 nm to 400 nm. Spectrum of the third peak (λmax at 274 nm and λmin at 254 nm) from fraction eight matched with that of chebulagic acid [22]. Peak number two from fraction nine of T. chebula showed spectral similarity (λmax at 278.5 nm and λmin at 250 nm) with chebulinic acid [22].
DNA relaxation assays using topoisomerase I
Human topoisomerase I was assayed by decreased mobility in an agarose gel, of supercoiled plasmid DNA (pBSK+) after treatment with the enzyme.
The standard topoisomerase assay mixture (25 μL) contained: 10 mM Tris-HCl, pH 7.9, 1 mM EDTA, 150 mM NaCl, 0.1% BSA,0.1 mm spermidine, 5% glycerol, 0.25 μg pBSK+ and 1 unit of enzyme (one unit of topoiosmerase I activity is the amount of enzyme that can relax 0.25 μg of supercoiled plasmid DNA in 30 min at 37°C).
The control reaction contained standard assay mixture, 1 unit of topoisomerase I and 25 μm camptothecin.
The amount of camptothecin to be used in the control reaction was determined by incubating 1 unit of topoisomerase I with gradually increasing concentrations of the drug.
The concentration at which the enzyme activity was completely inhibited was used in the final positive control reaction.
In other experiments, various concentrations of the plant extracts and chebulinic and chebulagic acids were also included in the assay mixture. These concentrations were decided upon by experimentation, trying to minimize them as far as possible for reducing toxicity but at the same time producing some measurable inhibition.
The reaction was carried out at 37°C for 30 min, then stopped by adding loading dye consisting of 1% SDS, 10 mM EDTA, 0.25 μg/mL bromophenol blue and 15% glycerol.
The samples were applied to a horizontal 1% agarose gel and subjected to electrophoresis in TAE buffer (0.04 M Tris-acetate, 0.002 M EDTA, pH 8.0) at 1.5 V/cm overnight (14-16 h) at room temperature.
The gels were stained with solution of ethidium bromide in water (5 μg/mL), destained with water and then photographed under UV illumination, images inverted for greater clarity.
Pre incubation dilution assay was performed to find out whether the compounds, if incubated with the enzyme at a higher concentration briefly but later diluted to a lower concentration were inhibiting topoisomerase I.
After 5 min pre-incubation (at 37°C) of enzyme with the plant extracts and chebulagic and chebulinic acids, reaction mixture was diluted with the reaction buffer. After this dilution, supercoiled DNA was added resulting in a 10 fold dilution and relaxation assay was performed as described above.
Some further control experiments used 0.5 mm or 1.0 mm of dithiothreitol as part of the relaxation assay mixture in order to determine if it can re-activate the topoisomerase I inactivation produced by chebulagic and chebulinic acids [23].
Decatenation assays using topoisomerase II
Human topoisomerase II was assayed by decatenation of kinetoplast DNA (kDNA) and monitoring the appearance of decatenated, relaxed DNA minicircles. The standard reaction contained 0.2 μg kDNA (final volume of 20 μl), 50 mM Tris-Cl, pH 8.0, 150 mM NaCl, 10 mM MgCl2, 0.5 mm dithiothreitol, 5 mM ATP, 30 μg/mL BSA and 1 unit of enzyme (one unit of topoisomerase II is defined as the amount of enzyme required to fully decatenate 0.2 μg kDNA in 30 min at 37°C).
In the control experiment, 50 μM of etoposide (standard inhibitor) was included.
This concentration was determined by incubating 1 unit of topoisomerase II with gradually increasing concentration of the drug.
At 50 μm concentration, etoposide completely inhibited topoisomerase II activity and thus, this concentration was used in the final control reaction. In other experiments, 40 or 120 μg/mL of a plant extract was also included in the assay mixture.
Reactions were incubated for 30 min at 37°C and then terminated by adding 1% SDS, 10 mM EDTA, 0.25 μg/mL bromophenol blue and 15 % glycerol.
Decatenations were monitored by electrophoresis in 1% agarose gel at 10-12 V/cm, till the dye front had migrated 75% of the way down the gel. Staining and destaining were carried out as described above.
Reproducibility of results obtained
All the experiments were performed at least three times, with an average of 3.2 times, for validating the results.
Results
UV absorption spectra of the plant extracts
The UV spectra of the plant extracts decrease from 220 nm to 300 nm, with absorbance values at local maxima and minima using 30 μg/mL concentration shown in Table 1 below.
| Plant name |
Absorbance at λmax |
Local maxima (λmax) |
Absorbance at λmin |
Local minima (λmin) |
| A. catechu |
0.437 |
278.5 nm |
0.273 nm |
258.5 nm |
| D. biflorus |
0.068 |
274 nm |
0.061 |
255 nm |
| E. officinalis |
0.458 |
272.5 nm |
0.178 |
241.5 nm |
| H. indicus |
0.078 |
280.5 nm |
0.075 |
264.5 nm |
| S. dulcis |
0.061 |
258-274 nm |
N/A |
None |
| T. belerica |
0.992 |
272 nm |
0.723 |
245 nm |
| T. chebula |
0.507 |
274 nm |
0.346 |
247 nm |
Table 1: Summary of UV absorption spectra of the plant extracts.
Catalytic inhibition of topoisomerase I by plant extracts
While studying the in vitro effect of the plant extracts on human topoisomerase I, we found that all the extracts from the plants chosen inhibited relaxation of plasmid DNA (Figure 1). The dose response curves for the various plants are shown in Figure 2A. However, spinach extract, chosen as a negative control, did not inhibit the action of topoisomerase I significantly, at concentrations of 40 μg/mL, 120 μg/mL and 200 μg/mL (Figure 2B). Among the seven plants, the extracts of A. catechu, E. officinalis, T. belerica, T. chebula and S. dulcis exhibited complete inhibition of relaxation activity at 40 μg/mL (Figure 1A, lanes 4, 8, 12, 14, 16 respectively; also, Figure 2A) and also at 120 μg/mL concentration (Figure 1A, lanes 5, 9, 13, 15, 17, also, Figure 2A). D. biflorus (47%, 76.3% inhibition at 40 μg/mL, 120 μg/mL) and H. indicus (33%, 36% inhibition at 40 μg/mL, 120 μg/mL) extracts showed partial and inhibition (lanes 6, 7 and 10, 11 respectively in Figure 1A). Lane 3 in the same figure shows inhibition of topoisomerase I activity by 25 μm or 8.7 μg/mL of camptothecin, the standard inhibitor [9]. The plant extracts did not unwind supercoiled DNA by themselves. In order to confirm this, supercoiled plasmid DNA was incubated with 40 μg/mL and 120 μg/mL (concentrations used in enzyme assay) of the extracts, without adding topoisomerase I, in some control experiments. No relaxation or no apparent changes in DNA conformation, due to unwinding, was observed (data not shown). Regarding changes in pH, the addition of the plant extracts to the starting buffer pH of 7.52 resulted in pH 7.50 to 7.52, increasing to 7.6 for H. indicus, D. biflorus and reducing to 7.3 for S. dulcis. In order to determine if still lower concentrations of these plant extracts were effective in inhibiting human topoisomerase I, further experiments were carried out with the five plants exhibiting the greater inhibitory effects as shown in Figure 1A. In this set of experiments, 1 μg/mL, 3 μg/mL and 9 μg/mL of extracts from A. catechu, E. Officinalis, T. Belerica, T. Chebula and S. Dulcis were used and the results shown in Figure 1B. Near complete inhibition was not observed at these lower concentrations, but extracts from S. dulcis, A. Catechu and T. Chebula show partial inhibition using these lower concentrations, particularly using 9 μg/mL. Pre incubation of the enzyme with the plant extracts for 5 min at 37°C, enhanced their inhibitory effect. One unit of topoisomerase I was pre incubated with 40 μg/mL of the plant extracts, followed by 10 fold dilution with reaction buffer before adding supercoiled DNA with a final extract concentration of 4 μg/mL. All seven plants except H. indicus showed inhibition even after this dilution (data not shown).
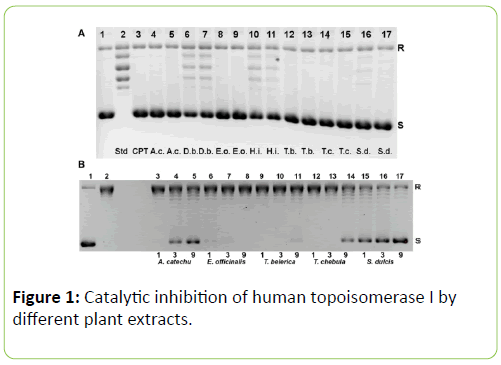
Figure 1: Catalytic inhibition of human topoisomerase I by different plant extracts.
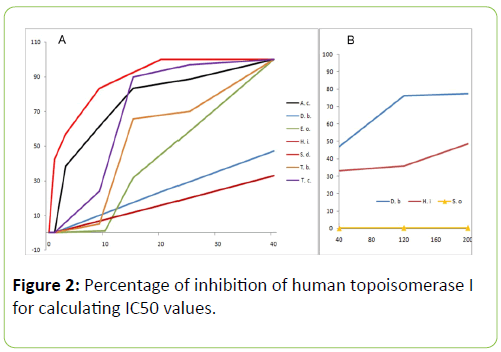
Figure 2: Percentage of inhibition of human topoisomerase I for calculating IC50 values.
Inhibition of topoisomerase I by chebulagic and chebulinic acids
Using 7.5, 15 or 30 μM of chebulinic and chebulagic acids in the relaxation assay mixture, complete inhibition of topoisomerase I activity resulted (data not shown). Hence it was reasoned that, it may be possible to inhibit human topoisomerase I using even smaller concentrations of these acids isolated from T. chebula.
Therefore a range of concentrations was used in the next round of assays for chebulagic and chebulinic acids (Figure 3A and 3B).
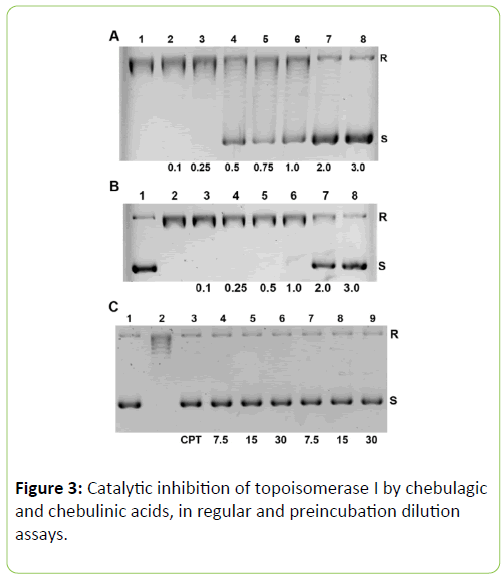
Figure 3: Catalytic inhibition of topoisomerase I by chebulagic and chebulinic acids, in regular and preincubation dilution assays.
Chebulagic acid completely inhibits human topoisomerase I at a minimal concentration of 2 μM (1.90 μg/mL) and chebulinic acid produces 72.2% inhibition at a minimal concentration of 3 μM (2.87 μg/mL) (Figure 3A and 3B).
No change in pH was observed from the buffer pH value of 7.52 upon adding the maximum concentrations of chebulagic and chebulinic acids used in our study.
Including dithiothreitol in reaction mixtures containing chebulagic and chebulinic acids could not alleviate their inhibitory effect on topoisomerase I (data not shown).
Pre-incubation dilution assays for chebulagic and chebulinic acids
Since chebulagic and chebulinic acids were found to inhibit topoisomerase I at 7.5 μM, it was reasoned that using preincubation, even smaller concentrations of these acids may inhibit the enzyme [5]. Dilution of the reaction mixtures 10-fold after preincubating at 7.5, 15 or 30 μM for 5 min. did not remove topoisomerase I inhibition, suggesting that chebulagic or chebulinic acid bound form of the enzyme remains inactive at concentrations of 0.75 μM obtained after the said dilutions during the progress of the assays (Figure 3C).
Catalytic inhibition of topoisomerase II by plant extracts
Effect of the seven plant extracts on human topoisomerase II was studied using the decatenation assay, which is highly specific for type II topoisomerases.
The plant extracts are less effective in inhibiting topoisomerase II as compared to topoisomerase I. The same concentration of the plant extracts as used in topoisomerase I assay (40 and 120 μg/mL) were used in these assays too. Using 40 μg/mL very weak inhibition or no inhibition was obtained (data not shown).
At the higher concentration (120 μg/mL) topoisomerase II activity was inhibited, though not completely, as faint bands of relaxed minicircles can be seen (Figure 4, lanes 4-10). In contrast, the known inhibitor etoposide does not allow any decatenation or the formation of faint minicircles as observed with the plant extracts (Figure 4, lane 2).

Figure 4: Human topoisomerase II assayed by decatenation of kDNA. In lanes 1, 2 and 4-10, the kinetoplast DNA (kDNA) appears as the band remaining at top (in the wells).
The percentages of inhibition of topoisomerase II by the different plant extracts at 120 μg/mL concentration is given in Table 2.
| Plant name |
Percentage of inhibition |
| A. catechu |
86.8 |
| D. biflorus |
90.8 |
| E. officinalis |
88.5 |
| H. indicus |
82.0 |
| T. belerica |
86.7 |
| T. chebula |
89.1 |
| S. dulcis |
77.8 |
Table 2: Catalytic inhibition of human topoisomerase II by 120 μg/mL of the plant extracts.
Note: the percentage is calculated as the band intensity of the kDNA band divided by its sum with the total intensity of the decatenated forms seen as minicircles shown in Figure 4.
It is likely that concentrations higher than 120 μg/mL would inhibit topoisomerase II closer to 100%, but as the toxicity limits are approaching, it was not carried out.
(A) Lane 1: control, with pBSK+ shown as the darker lower band (S) contaminated by a small amount of relaxed DNA in the upper band (R). Lane 2: pBSK+ after incubation with 1U of topoisomerase I for 30 min, showing a ladder of relaxed topoisomers. Lane 3: pBSK+ and topoisomerase I as in lane 2 with 25 μm or 8.7 μg/mL camptothecin (CPT) for 30 mins shows complete inhibition. Lanes 4-17 display results obtained with standard assay mixture plus 40 or 120 μg/mL extracts for the seven plants : A. catechu (lanes 4, 5), D. biflorus (lanes 6, 7), E. officinalis (lanes 8, 9), H. indicus (lanes 10,11), T. belerica (lanes 12,13), T. chebula (lanes 14, 15), S. dulcis (lanes 16,17): respectively. Plant name abbreviations given at the bottom. Except for D. biflorus and H. indicus which show partial inhibition, the other five plant extracts show nearly complete inhibition. (B) For the five plants showing nearly complete inhibition of topoisomerase I in part A), lower concentrations of 1, 3 or 9 μg/mL was employed here. It is seen that A. catechu (lane 5), T. chebula (lane 14) and S. dulcis (lane 17) partially inhibit at 9 μg/mL. pBSK+ before (lane 1) and after (lane 2) relaxation with 1U of enzyme are controls.
(A) Graph showing the percentage of inhibition of topoisomerase I by the seven plant extracts at different concentrations. From the graph it can be clearly seen that Spondias dulcis (red), Acacia catechu (black), Terminalia belerica (orange), Terminalia chebula (purple) and Emblica officinalis (light green) form a group where they completely inhibit topoisomerase I activity (100% inhibition) at 40 μg/mL concentration. On the other hand Dolichos biflorus (blue) and Hemidesmus indicus (maroon) constitute the second group, whose percentages of inhibition lie much below and does not reach 100% in the concentration range shown. The I.C.50 values of the different plant extracts as calculated from this graph are as follows: S. dulcis - 2.5 μg/mL, A. catechu - 6.5 μg/mL, T. chebula - 11 μg/mL, T. belerica - 13 μg/mL, E. officinalis - 22.2 μg/mL, D. biflorus - 50 μg/mL, H. indicus - 210 μg/mL. B) The two plants Dolichos biflorus (blue) and Hemidesmus indicus (maroon) showing partial inhibition in part A) were used at higher concentrations till 300 μg/mL in separate experiments, some of these results displayed here along with those from Spinacia oleracea (yellow with triangle data points) which serves as our negative control.
(A) Assays using various micromolar concentrations of chebulagic acid, are noted below lanes 2-8; standard assay without any inhibitor in lane 1. Increasing inhibition is observed with increasing concentration of chebulagic acid, (lanes 2-8), with complete inhibition occurring around 2 μM (lane 7). (B) Assays using various micromolar concentrations of chebulinic acid, noted below lanes 3-8; standard assay without any inhibitor in lane 2; substrate pBSK+ alone in lane 1. Increasing inhibition is observed with increasing concentration of chebulinic acid (lanes 3-8), with 72.2% inhibition occurring around 3 μM (lane 8). (C) Preincubation dilution assays using 7.5, 15 or 30 μM of chebulagic (lanes 4-6) and chebulinic (lanes 7-9) acids during the 5 min. incubation periods followed by a 10- fold dilution. Under these conditions, near complete inhibition occurs at 0.75 μM for both acids. Substrate pBSK+ in lane 1, standard assay in lane 2 and with 25 μM CPT in lane 3 are shown as controls.
The sample in lane 1 had only 0.2 μg kDNA. Lane 2, marked ‘+Et’ at bottom: 0.2 μg kDNA after incubation with 1U of topoisomerase II for 30 min, in the presence of 50 μM of etoposide showing complete inhibition of topoisomerase II activity. Lane 3: 0.2 μg kDNA and human topoisomerase II, as in lane 2 but lacking the inhibitor etoposide, for 30 min shows complete disappearance of the top kDNA band, i.e., decatenation and the appearance of several bands (minicircles). Lanes 4-10 display results obtained with standard assay mixture plus 120 μg/mL extracts for the seven plants, abbreviated names given at bottom : A. catechu (lane 4), D. biflorus (lane 5), E. officinalis (lane 6), H. indicus (lane 7), T. belerica (lane 8), T. chebula (lane 9) and S. dulcis (lane 10), respectively. All seven extracts show partial inhibition of human topoisomerase II, as the kDNA is partly converted to minicircles. Inhibition of topoisomerase II obtained with 40 μg/mL plant extracts was weaker (data not shown).
Discussion
Most of the topoisomerase inhibitors in current use are sourced from plants and thus there is considerable interest in identifying novel inhibitors from plant products. In continuation of the search for new topoisomerase inhibitors, we investigated 70% methanolic extracts of Acacia catechu, Dolichos biflorus, Emblica officinalis, Hemidesmus indicus, Terminalia belerica, Terminalia chebula and Spondias dulcis. Recent studies have demonstrated that some of these extracts have potential anticancer effect [18,19,21,24,25], but their effect on topoisomerases was not explored previously. The present study demonstrates the inhibitory effect of the above mentioned plant extracts on human topoisomerases I and II. Chebulagic and chebulinic acids isolated from T. chebula extract are shown to be better inhibitors of topoisomerase I than that extract as well as greater inhibitory potential than camptothecin since complete inhibition is achieved at just 2 μM concentration. Diluting preincubated mixtures of chebulagic and chebulinic acids and topoisomerase I effected complete inhibition at 0.75 μM during the assay, suggesting very strong association between topoisomerase I and these compounds. Similar results are obtained when the plant extracts are preincubated with the enzyme and nearly complete inhibition occurs at 4 μg/mL in the assay for all the plants, excepting H. indicus.
Many topoisomerase inhibitors are currently being evaluated or used for the treatment of various human cancers [26], but most of them have severe toxic side effects which limit their use. The maximum tolerated doses for A. catechu, H. indicus, T. belerica and T. chebula are compiled as 100 mg, 500 mg, 1,000 mg and 25-100 mg per kilogram of body weight, respectively [11]. In addition, D. biflorus is an edible pulse, S. dulcis fruit is eaten raw and in chutney preparations in India, thus having minimal cytotoxic effects. For this reason we explored S. dulcis fruit in lieu of S. pinnata bark [25] as both belong to the Spondias family. E. officinalis is also edible, traditionally known to be totally safe [11]. Three of the plant extracts were shown to be nontoxic to mouse spleenocytes up to 200 μg/mL [20]. The T. belerica extract was shown to have no cytotoxic effect on human fibroblast WI-38 cells till 200 μg/mL, but induced apoptosis in human breast and lung carcinoma cell lines [18]. Therefore, if some of the plant extracts studied are used in the concentration range of 10 to 40 μg/mL, cytotoxic effects to normal cells are likely to be minimal. Lower concentrations of 2 to 5 μg/mL are likely to be effective for chebulagic and chebulinic acids for inhibiting topoisomerase I and at the same time minimize cytotoxic effects [27].
The significance of topoisomerase mediated DNA breaks in tumour cell death is well known [1-3]. Our study suggests that nuclear fragmentation and subsequent apoptosis induced by the plant extracts in cancer cells is a consequence of topoisomerase inhibition. Recent studies have also demonstrated that chebulagic and chebulinic acids are potent inhibitors of vascular endothelial growth factor A mediated angiogenesis [27,28]. This property along with their topoisomerase I inhibiting ability may be helpful in combating cancer cells. Thus, chebulagic and chebulinic acids serve as natural non toxic topoisomerase I inhibitors, which may have possible chemotherapeutic potential.
Analyses of a lichen, Parmotrema reticulatum active against breast carcinoma illustrates a case where a mixture is preferable to a single compound to reduce cytotoxicity against normal cells [29]. A similar result was found regarding the antioxidant and hepatoprotective properties of Drosera burmannii extract [30]. Therefore, studies on plant crude extracts could be important due to their reduced cytotoxicity towards normal cells. More extensive studies of these plant extracts using cancer cell lines and/or tumour bearing animal models should be carried out to evaluate their anticancer potential.
Acknowledgements
The study was supported by the Department of Science and Technology, Government of India through funds for Bose Institute. In addition, I.K. was recipient of a fellowship from the Department of Biotechnology, Government of India.
18842
References
- ChampouxJ (2001) DNA topoisomerases: structure, function and mechanism. Annu Rev Biochem70: 369-413.
- Wang JC (2009) Untangling the Double Helix. 1st ed. Cold Spring Harbor Laboratory Press, New York
- Pommier Y, Leo E, Zhang H, Marchand C (2010)DNA topoisomerases and their poisoning by anticancer and antibacterial drugs. Chem Biol17: 421-433.
- Fry AM, Chresta CM, Davies SM, Walker MC, Harris AL et al. (1991)Relationship between topoisomerase II levels and chemosensitivity in human tumour cell lines. Cancer Res51: 6592-6595.
- Jain CK, Pradhan BS, Banerjee S, Mondal NB, Majumder SS et al. Sulfonoquinovosyl diacylglyceride selectively targets acute lymphoblastic leukemia cells and exerts potent anti-leukemic effects in vivo. Sci Rep5: 12082.
- Chen AY, Liu LF (1994) DNA topoisomerases: Essential enzymes and lethal targets. Annu Rev Pharmacol Toxicol.34: 191-218.
- Anderson RD, Berger NA (1994) Mutagenicity and carcinogenicity of topoisomerase interactive-agents. Mutat Res309: 109-142.
- Kintzios SE (2006) Terrestrial plant derived anticancer agents and plants used in anticancer research. Crit Rev Plant Sci 25: 79-113.
- Wall WE, Wani MC, CookeCE, Palmer KH, Mcphail AT et al. The isolation and structure of Camptothecin, a novel alkaloidal leukemia and tumor inhibitor from Camptotheca acuminate. J Am Chem Soc88: 3888-3890.
- Khalsa KPS, Tierra M (2010) The way of Ayurvedic Herbs : The most complete guide to Natural Healing and Health with Traditional Ayurvedic Herbalism. 1st ed. Motilal Banarsidass Publishers Private Limited, New Delhi.
- Dev SA (2006) Selection of Prime Ayurvedic Plant drugs: Ancient –Modern Concordance. 1st ed. Anamaya Publishers, New Delhi.
- Rastogi RP, Mehrotra BN (1998) Compendium of Indian Medicinal Plants, in 5 Volumes, Central Drug Research Institute, Lucknow and National Institute of Science Communication, New Delhi.
- Hazra B, Sarkar R, Biswas S, Mandal N (2010) Comparative study of the antioxidant and reactive oxygen species scavenging properties in the extracts of the fruits of Terminalia chebula, Terminalia belerica and Emblica officinalis. BMC Complement Altern Med 10: 10-20.
- Hazra B, Sarkar R, Biswas S, Mandal N (2010) The antioxidant, Iron Chelating and DNA Protective Properties of 70% Methanolic Extract of ‘Katha’ (Heartwood extract of Acacia catechu). J Complement Integr Med 7.
- Hazra B, BiswasS, Mandal N (2008) Antioxidant and free radical scavenging activity of Spondias pinnata. BMC Complement Altern Med 8: 63.
- [Mandal S, Hazra B, Sarkar R, Biswas S, Mandal N (2009) Hemidesmus indicus, an age-old plant: study of its in vitro antioxidant and free radical scavenging potentials. PhOL1: 604-617.
- Hazra B, Sarkar R, Mandal S, Biswas S, Mandal N (2009) Studies on antioxidant and antiradical activities of Dolichos biflorus seed extract. Afr J Biotechnol8: 3927-3933.
- Ghate NB, Hazra B, Sarkar R, Chaudhri D, Mandal N (2014) Alteration of Bax/Bcl-2 ratio contributes to Terminalia belerica induced apoptosis in human lung and breast carcinoma. In Vitro Cell Dev Biol Anim 50: 527-537.
- Ghate NB, Hazra B, Sarkar R, Mandal N (2014) Heartwood extract of Acacia catechu induces apoptosis in human breast carcinoma by altering Bax/Bcl-2 ratio. Pharmacogn Mag. 10: 27-33.
- Sarkar R, Mandal N (2011) In vitro cytotoxic effect of hydroalcoholic extracts of medicinal plants on Ehrlich’s Ascites Carcinoma (EAC). Int J Phytomed3: 370-380.
- Kar I, Chattopadhyaya R (2016) Effect of seven Indian plant extracts on Fenton reaction-mediated damage to DNA constituents. J Biol Struct Dyn 10: 1-15.
- MahajanAD, PaiNR (2011) Development and Validation of HPLC Method for Quantification of Phytoconstituents in Haritaki Churna. Int J ChemTech Res 3: 329-336.
- Han Q, Song J, Qiao C, Xu H (2006) Preparative Isolation of hydrolysable tannins chebulagic acid and chebulinic acid from Terminalia chebula by high-speed counter-current chromatograph. J Sep Sci29: 1653-1657.
- Kennedy S, DiCesare JC, Sheaff RJ (2011) Topoisomerase I inactivation by a novel thiol reactive naphthoquinone. Biochem Biophys Res Commun 410: 152-158.
- Ghate NB, Hazra B, Sarkar R, Mandal N (2014) In vitro anticancer activity of Spondias pinnata bark on human lung and breast carcinoma. Cytotechnology 66: 209-218.
- Li TK, Liu LF (2001) Tumor Cell Death Induced By Topoisomerase Targeting Drugs. Annu Rev Pharmacol Toxicol41: 53-77.
- Lu K, Basu S (2015) The natural compound chebulagic acid inhibits vascular endothelial growth factor A mediated regulation of endothelial cell functions. Sci R5: 9642.
- Lu K, Chakraborty D, Sarkar C, Lu T, Xie Z et al. (2012) Triphala and its Active Constituent Chebulinic Acid Are Natural Inhibitors of Vascular Endothelial Growth Factor A Mediated Angiogenesis. Plos One e43934.
- Ghate NB, Chaudhuri D, Sarkar R, Sajem AL, Panja S et al. (2013) An antioxidant extract of tropical lichen Parmotrema reticulatum, induces cell cycle arrest and apoptosis in breast carcinoma cell line MCF-7. Plos One 8: e82293.
- Ghate NB, Chaudhuri D, Das A, Panja S, Mandal N (2015) An antioxidant extract of the insectivorous plant Drosera burmannii Vahl. alleviates iron induced oxidative stress and hepatic injury in mice. Plos One e0128221.









