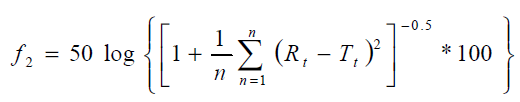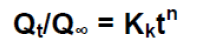Keywords
HPMC K-100M (Hydroxypropyl Methyl Cellulose), Xanthan Gum, Venlafaxine Hydrochloride, Higuchi Model And Multi Layered Matrix Sustained Release Tablets.
Introduction
Venlafaxine hydrochloride, a structurally novel antidepressant, is designated (R/S)-1-[2- (dimethylamino)-1(4- methoxyphenyl)ethyl]cyclohexanol hydrochloride or (±)-1-[1- [(dimethylamino)methyl]-pmethoxybenzyl] cyclohexanol hydrochloride.
Venlafaxine and its active metabolite, Odesmethylvenlafaxine (ODV), are potent inhibitors of neuronal serotonin and Norepinephrine reuptake inhibitor and weak inhibitors of dopamine reuptake [1, 2, 6]. Its low affinity for brain muscarinic, cholinergic, histaminergic or alpha adrenergic receptor made its free of adverse effect common to tricyclic antidepressants (anticholinergic, sedative, cardiovascular side effects)[3]. Venlafaxine and ODV exhibits linear kinetics over the dose range of 75 to 450 mg/day. The recommended daily dose of Venlafaxine hydrochloride is 75 to 450 mg/day [6]. The steady state half lives of Venlafaxine and ODV are 5±2 and 11±2 hours, respectively, necessitating the administration 2 or 3 times daily so as to maintain adequate plasma levels of drug [4, 6]. The half-life of Venlafaxine is relatively short, and, therefore, patients are directed to adhere to strict medication routine, avoiding missing a dose. Even a single missed dose can result in the withdrawal symptoms [5, 6]. In such case the formulation releasing the drug in sustained manner will aid the patient to adhere to strict medication routine by avoiding the need to take the dosage form 2 or 3 times daily. The use of sustain released formulation is associated with less nausea and dizziness [6, 7].
Xanthan Gum is a microbial desiccationresistant polymer prepared commercially by aerobic submerged fermentation from Xanthomonas campestris. The primary structure of this naturally produced cellulose derivative contains a cellulose backbone (E-D-glucose residues) and a trisaccharide side chain of E-D-mannose- E-D-glucuronic acid-F- D-mannose attached with alternate glucose residues of the main chain [8]. Xanthan gum is widely used in controlled release formulations and generally regarded as non toxic and non irritant [8]. Xanthan gum swells in gastric fluid to produce high viscous layer around tablet through which the drug can slowly diffuse. This property makes it a useful ingredient for sustained release application. Xanthan gum is having special advantages over other polymer like high viscosity at low concentration, very resistant to pH-variations, highly resistant to enzymatic degradation, absence of initial burst release, good compatibility with wide variety of ingredients and more reproducibility in drug release which makes it more suitable for above purpose [8].
The main objective of this work was to fabricate the Sustain release tablets of Venlafaxine hydrochloride using natural polymer (Xanthan gum and HPMC K-100M) and to match the releasing profile with the innovator product i.e. EFFEXOR - XR capsules (Wyeth Lederle). Potential pharmacokinetic advantages of Sustain Release Drug Delivery System of Venlafaxine Hydrochloride formulated, will include optimum peak plasma drug concentrations and smaller fluctuations between peak and throughout plasma drug concentrations, which might influence the tolerability of these medications [9]. It was further objective of the present research work to provide pharmaceutical formulation that will provide 24 hr control over symptoms of depression or Generalized Anxiety Disorder. The main advantage of Multi-layered tablets as compare to multi-particulate system is higher productivity, shorter processing time and minimum variation between batches.
Materials and Methods
Venlafaxine Hydrochloride was obtained as a gift sample from Cadila Healthcare ltd, India. Hypromellose K-100M and Xanthan gum was obtained as a gift sample from Orphic pharma, Baroda. Microcrystalline Cellulose (Avicel pH 101) and Lactose Monohydrate (Pharmatose DCL 21) were received from Colorcon Asia Pvt. Ltd, Goa. Magnesium stearate and Talc used in the project were of pharmaceutical or analytical grade procured commercially. Effexor XR capsules (Wyeth Pharmaceuticals Inc.) containing 150 mg of Venlafaxine Hydrochloride with expiry December, 2010 was used as reference product.
Preparation and evaluation of Venlafaxine Hydrochloride Tablets
Sustain release tablets of Venlafaxine hydrochloride were prepared by wet granulation technique following by secondary compression of directly compressible mass. HPMC K-100M and Xanthan gum were used as release controlling material. Avicel pH 101 and Pharmatose DCL 11 were used as diluents and compression aid. The 400 mg tablets containing 169.8 mg Venlafaxine hydrochloride were prepared by wet granulation technique. The single layered tablets were prepared using varying composition of Hypromellose, Xanthan gum and Avicel pH 101 and Pharmatose DCL 11 according to the composition in Table 1. All ingredients were mixed for 5 min using mortar and pestle. Then the drug blended powder is granulated using water as granulating agent. The wet mass was dried in tray dryer at around 60°C until loss on drying is 5%. The dried mass was passed through mesh #30 and finally blended with the extragranular fraction. The granules ready for compression were evaluated for angle of repose, Carr’s Index and Hausner ration. The tablets were prepared by compressing the lubricated blend using single punch tablet compression machine (Cadmach machinery ltd). Incase of multilayered tablet, the drug containing layer was prepared as describe above, followed by compressing the directly compressible blend (drug free barrier layer) on either one side or both side of the drug containing layer. Table 2 depicts the composition of the multi layered tablets. The drug free barrier layer consists of Xanthan gum, Pharmatose DCL 11 and magnesium stearate. The tablets were evaluated for average drug content, uniformity of weight, thickness, crushing strength, friability and in-vitro drug release characteristics. The thickness and hardness were measured on hardness tester (Dr Schleuniger Pharmatron AG, Switzerland). Friability was measured using Roche type friabilitor (Electro lab Mumbai).
| |
Batch code |
| Ingredients (mg) |
V1 |
V2 |
V3 |
Venlafaxine
hydrochloride # |
169.8 |
169.8 |
169.8 |
| HPMC K 100M |
64.9 |
45.8 |
29.8 |
| HPMC K 4M |
15.2 |
- |
- |
| Xanthan gum |
- |
25.4 |
39.8 |
| Avicel pH 101 |
- |
153.4 |
161.1 |
| Pharmatose DCL 11 |
130.3 |
- |
- |
Magnesium stearate
** |
10.1 |
5.4 |
5.3 |
| Talc ** |
10.3 |
- |
- |
| Purified water * |
Quantity sufficient |
Quantity sufficient |
Quantity sufficient |
# 169.8 mg of Venlafaxine hydrochloride is equivalent to 150 mg of Venlafaxine base.
* Purified Water is used as granulating agent
** Used as extragranular composition |
Table 1: Composition of Venlafaxine Hydrochloride Monolithic Matrix Tablets
The amount of the drug release per unit surface area of the tablet for the optimized batch was calculated and was presented in Table 3. The tablets was assumed to be cylindrical in shape; and the surface area was calculated using following equation
| |
Batch code |
| Ingredients (mg) |
VS1 |
VS2 |
VT1 |
VT2 |
VT3 |
VT4 |
| Drug containing layer |
Venlafaxine
hydrochloride # |
169.8 |
169.8 |
169.8 |
169.8 |
169.8 |
169.8 |
| Avicel pH 101 |
148.2 |
49.5 |
49.8 |
50.1 |
50.2 |
50.2 |
| HPMC K100M |
46.0 |
45.8 |
45.7 |
37.5 |
37.4 |
37.4 |
| Xanthan gum |
25.6 |
33.4 |
33.5 |
39.8 |
39.6 |
39.6 |
| Magnesium stearate ** |
5.2 |
5.1 |
4.9 |
4.8 |
5.2 |
5.1 |
| Purified water * |
QS |
QS |
QS |
QS |
QS |
QS |
| Drug free barrier layer |
| Xanthan gum |
34.8 |
34.6 |
67.7 |
70.3 |
68.2 |
72.4 |
| Pharmatose DCL 11 |
32.5 |
32.4 |
71.4 |
62.4 |
64.6 |
60.6 |
| Magnesium stearate |
3.5 |
3.6 |
3.3 |
3.2 |
3.2 |
3.4 |
| Talc |
- |
- |
2.4 |
2.7 |
2.4 |
2.4 |
# 169.8 mg of Venlafaxine hydrochloride is equivalent to 150 mg of Venlafaxine base.
* Purified Water is used as granulating agent
** Used as extragranular composition QS: Quantity Sufficient |
Table 2: Composition of Venlafaxine Hydrochloride Multi-Layer Matrix Tablets.
Surface area = 2Qrh + 2Qr2
Where r and h are the radius and average thickness of the tablet in centimeters. Round punch with 1.15 cm diameter were used for preparing tablets. The average thickness (n=20) of the optimized batch VT3 was 0.48 cm. Hence the average surface area of the optimized batch was 3.81 cm2 respectively.
In-vitro drug release study [10, 11]
In-vitro release study of drug was performed using the USP XXIII basket apparatus (Model: TDT 06-T, Electrolab, India) at 37°C ± 0.5°C in 900 ml of distilled water at 100 rpm. Aliquots of 5ml were withdrawn after predetermined time intervals and an equivalent amount of fresh media maintained at the same temperature was replaced. The samples were diluted with water and suitably analyzed by measuring absorbance at a wavelength of 226 nm using UV-Visible Spectrophotometer (Model: UVLadani 1700 Pharmaspec, Shimadzu, Japan). The dissolution study is performed for reference product (Effexor ® XR capsules 150mg). The dissolution experiments were conducted in triplicate.
The Radar Graph [10-12]
Shah et al. proposed that maximum difference among the drug release at particular time point to establish similarity should not be more than 10%. The dissolution data of the reference product was taken as ideal release pattern. The ideal release pattern was scored 5, on the scale of 0 to 10. Score “0” indicate -10% release difference and score “10” indicates +10% release difference. The score of optimized batch was calculated at each dissolution time point as per following formula:
Score = 5 + {(%Tt - %Rt ) / 2}
Where %Tt percentage of drug release from test batch while %Rt is percentage of drug release from reference product at the same time.
Data analysis:
The in-vitro dissolution profile comparison may be carried out using model independent method or model dependent method:
Model independent method:
The simple model independent approach uses a similarity factor (f2) to compare dissolution profile. The formulation was compared using similarity factor (f2 value) and the results are presented in Table 4. The similarity factor f2 was calculated from the mean dissolution data according to the following equation:
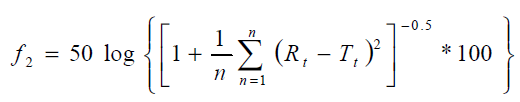
Where n, the number of time points; Rt, the reference profile at time point t and Tt, the test profile at the same time point. The value of f2 should be between 50 and 100. The f2 value of 100 suggests that the test and reference profiles are identical and as the value becomes smaller, the dissimilarity between release profiles increases [13].
Model dependent method: [13-15]
The in-vitro release data was analyzed using various kinetic models to describe the release kinetics. The various kinetic model used are zero order, first order, Higuchi equation, Hixson-Crowell equation and Korsmeymer Peppas equation. The following plots were performed:
| % drug release verses time: |
Zero order |
| Log of % cumulative drug remaining versus time: |
First order |
| Cumulative % drug release verses square root of time: |
Higuchi |
| Log cumulative % drug release verses log time: |
Korsmeymer Peppas |
| Cube root of % drug remaining in matrix versus t : |
Hixson Crowell |
To find out the mechanism of drug release data was fitted in Korsmeymer Peppas model:
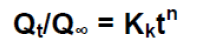
Where Qt/Q∞ is fraction of drug release at time t, Kk is a constant incorporating structural and geometric characteristic of the drug dosage form and n is the release exponent, indicative of the drug release mechanism as given in Table 5.
| Batches |
Average drugcontent (mg ± SD) |
Mean hardness(N ± SD) |
Friability(%) |
Similarity factor f2 |
| V1 |
98 ± 0.71 |
61.8 ± 8.2 |
0.61 |
35.1 |
| V2 |
101.8 ± 1.3 |
63.8 ± 4.6 |
0.7 |
35.8 |
| V3 |
101.2 ± 1.3 |
65.2 ± 3.8 |
0.58 |
42.4 |
| VS1 |
97.8 ± 1.3 |
60.4 ± 2.9 |
0.42 |
38.7 |
| VS2 |
98.4 ± 2.3 |
61.6 ± 1.5 |
0.58 |
40.7 |
| VT1 |
98.8 ± 1.4 |
59.6 ± 4.9 |
0.61 |
58.5 |
| VT2 |
99 ± 1.9 |
65.2 ± 1.6 |
0.54 |
74.9 |
| VT3 |
101.8 ± 1.3 |
60 ± 2.9 |
0.45 |
81.3 |
| VT4 |
100.2 ± 1.6 |
62.6 ± 2.4 |
0.52 |
78.6 |
Table 4: Physical Characterization (Average drug content, Hardness, Friability) and f2 values of formulated Batches.
| Diffusion exponent (n) |
Overall solute mechanism |
| 0.45 |
Fickian diffusion |
| 0.45 < n < 0.89 |
Non Fickian diffusion |
| 0.89 |
Case II transport |
| n > 0.89 |
Super case II transport |
Table 5: Diffusion exponent and solute release mechanism for cylindrical shape tablets
| Model |
r 2 |
| Zero order |
0.9748 |
| First order |
0.8787 |
| Higuchi |
0.9752 |
| Korsmeymer Peppas |
0.8274 |
| Hixson Crowell |
0.845 |
Table 6: The Results of Dissolution Model Fitting for Optimized Batch VT3
Results and Discussion
Xanthan gum hydrates at the faster rate as compare to HPMC K-100M, when it comes in contact with aqueous environment. The concept of adding Xanthan gum as rate controlling ingredient in addition to HPMC K-100M was to reduce the burst release of the drug observed with monolayer tablets prepared using Hypromellose only.
The reference product Effexor XR capsules (Wyeth Pharmaceuticals Inc.) contains coated pellets and the reason for performing the dissolution in distil water as a dissolution medium was that, in food and drug administration (US FDA) endorses the use of it as dissolution for the generic versions of the Venlafaxine hydrochloride. The time point’s selection was also done on the same basis covering all the time points as recommended in dissolution database of food and drug administration (US FDA).
The reference product exhibits sustain release as shown in Figure 1 and Figure 2.
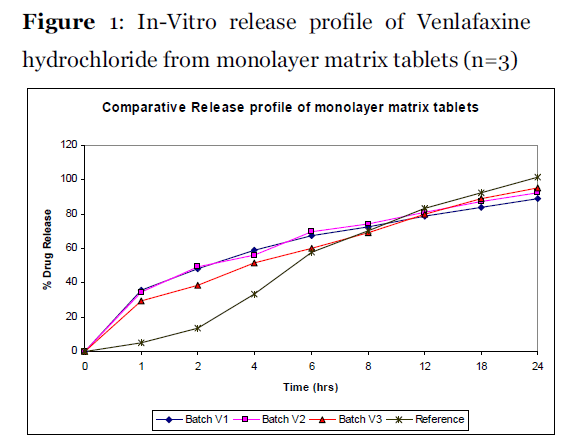
Figure 1: In-Vitro release profile of Venlafaxine hydrochloride from monolayer matrix tablets (n=3)
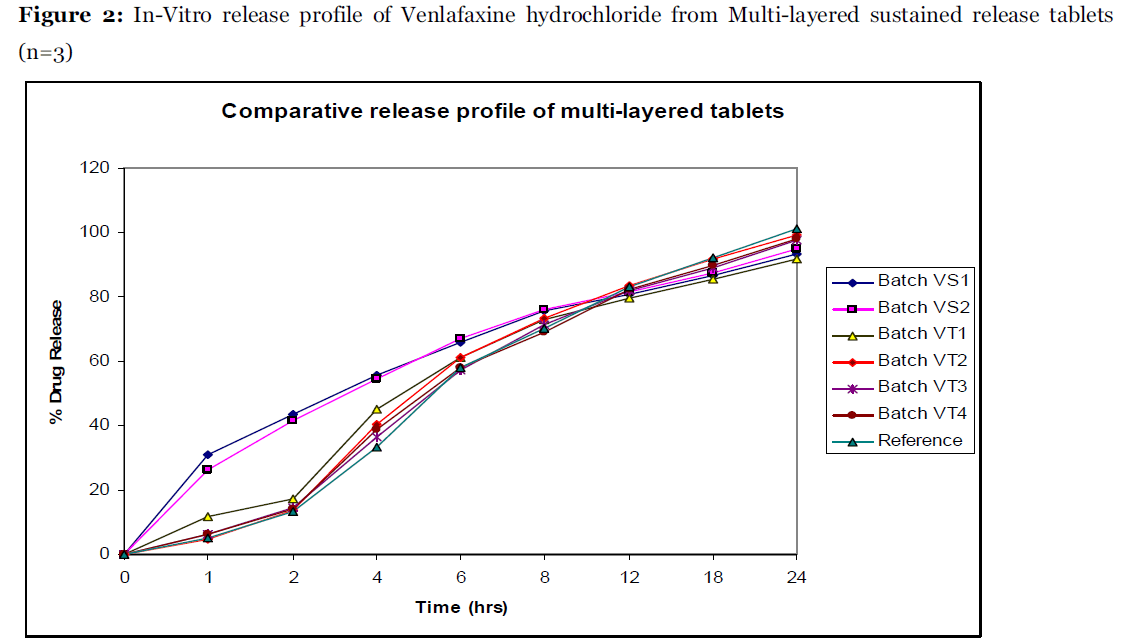
Figure 2: In-Vitro release profile of Venlafaxine hydrochloride from Multi-layered sustained release tablets (n=3).
The granules of all the batches (V1 to VT4) shows good flowability and good compressibility as the angle of repose, Carr’s index and Hausner ratio were in the range 21° - 28°, 14% - 18% and 1.17-1.25 respectively. As Table 4 indicates the friability (<1), average drug content (97.8%-101.8%) and mean hardness (59.6-65.2N) fulfills the requirement.
| |
Drug release per unit surface area (mg/cm2) |
| Time (hr) |
VT3 |
VT4 |
| 1 |
2.40 |
2.48 |
| 2 |
5.71 |
5.59 |
| 4 |
14.37 |
15.31 |
| 6 |
22.52 |
22.87 |
| 8 |
28.07 |
27.20 |
| 12 |
32.24 |
32.40 |
| 18 |
35.12 |
35.31 |
| 24 |
38.39 |
38.62 |
Table 3: Drug release profile per unit surface area for Batches with fine tunning, Batch VT3 and VT4.
The batch V1-V3 were compressed as monolayer tablets while the batch VS1-VS2 and VT1-VT4 were compressed as bi-layer and tri-layer matrix tablets. The data for in-vitro drug dissolution from monolayer matrix tablets as depicted in Figure 1 indicates an initial burst release of the drug. The high solubility of Venlafaxine hydrochloride and the lag time required for the gel formation of rate controlling ingredients might be responsible for the burst release of the drug observe for Batch V1. So in the Batch V2 and V3, rapid gelling polymer (Xanthan gum) compatible with other ingredients of the tablets was planned to be used. The batch V2 shows faster drug release until 4 hrs and reduced drug release after 8hrs. Higher content of high viscosity HPMC K100M and lesser content of fast gelling polymer Xanthan gum were concluded to be responsible for it, so it was decided to increase the content of Xanthan gum and to reduce the content of HPMC K-100M in Batch V3. As figure-1 and f2 represented in Table 4 indicates neither Batch V1-V3 shows the release profile similar to that of reference product. So it was finally conclude to go for barrier layer technology [10, 16].
The multi layered matrix tablets overcomes the problem of nonlinearity associated with diffusion controlled matrix devices by reducing the surface area of drug containing layer exposed to dissolution medium [10]. Pharmatose DCL 11 was used in combination with Xanthan gum in drug free barrier layer to augment compressibility. The gelled particles of Xanthan gum provide the required hindrance to drug release. The figure 2 represents comparative release profile of bi-layer tablets with tri-layer tablets, the observed difference in the release rate of Batch VS2 and VT1 could be availability of limited surface area in Batch VT1 in comparison to Batch VS2. The release of the drug in the initial time points can further be controlled by increasing the percentage of polymer in barrier layer and reducing the content of soluble pore forming material in the barrier layers (Batch VT2). As figure 2 indicates incomplete drug release from batch VT1 at 24 hr, to mitigate this problem the content of high viscosity polymer (HPMC K100M) in middle layer was reduced and the content of Xanthan gum was increase. As the curved surface of the tablet is directly exposed to the dissolution media, an increase in the content of the Xanthan gum in the middle layer further aids in reducing the burst release of the drug in the initial time points. Batch VT2 shows release profile similar to reference product with f2 value 74.9. Batch VT3 and Batch VT4 were prepared to fine tune drug release. The Batch VT3 and Batch VT4 showed similarity factor of 81.3 and 78.6, respectively. Finally Batch VT3 was concluded as optimized batch with release profile similar to the reference product.
The radar diagram for Batch VT3 was represented in Figure 3. The dissolution pull times are shown on the periphery of the radar diagram. The outer surface of radar graph shows highest score (10) while the centre shows lowest score (0). Ideally all the data points shall fall on score line correspond to 5 i.e. in the middle of radar diagram. The radar graph (Figure 3) reveals that the formulated batch VT3 and reference product shows almost similar dissolution at all the time points [10].
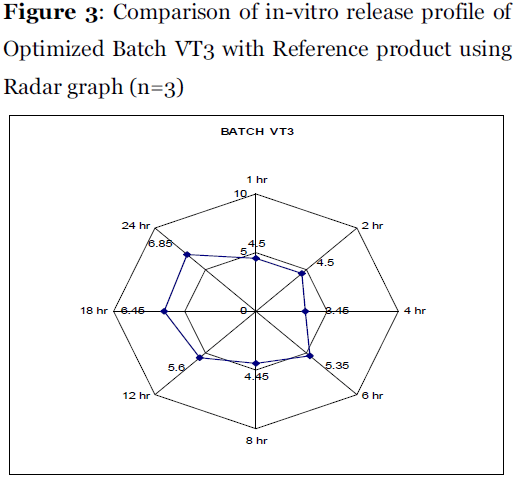
Figure 3: Comparison of in-vitro release profile of Optimized Batch VT3 with Reference product using Radar graph (n=3).
It is well known that the drug release from the sustained release tablet changes with the surface area. For batch VT3 and VT4 the complete release corresponds to 38.39 mg/cm2 and 38.62 mg/cm2 (Table 3). The percentage composition of the core tablets for the Batch VT3 and VT4 were identical however the composition of the barrier layer were different. The fine tunning in the release pattern can also be performed by focusing on the barrier layer composition.
The goodness of fit was used to determine the mechanism of drug release. The in-vitro dissolution data of the optimized batch was fitted to different mathematical model. As shown in the Table 6, the maximum r2 value obtained was for Higuchi model (0.9752), indicates the best linearity was found in Higuchi equation. This reveals that the release of drug from the matrix as square root of time dependent process based on Fickian diffusion. By incorporating the release data to Korsmeymer Peppas, the value of release exponent obtained was less than 0.45 which is beyond the limit of model. Thus it was concluded that release mechanism appears to be complex mechanism of swelling, diffusion, erosion and barrier controlled.
Conclusion
Increasing the amount of Xanthan gum could to some extend helps to control the burst release of drug at initial time points. The drug release rate was found to depend on the percentage of Xanthan gum and HPMC K100M in the core and the amount of Xanthan gum and Pharmatose DCL in the barrier layers. The multi layered matrix tablets overcome the problem of nonlinearity associated with diffusion controlled matrix devices by reducing the surface area of drug containing layer exposed to dissolution medium. Drug release kinetic of the optimized triple layered tablets best fits to Higuchi model.
Conflict of Interest: NIL
Source of Support: NONE
5658
References
- Nicholas TW, Jones L, chamberlain JC. A possible case of Venlafaxine ÃÂÃÂÃÂâÂÂÃÂâÂÂÃÂâÃÂÃÂââ¬Ã
¡ÃÂâââ¬Ã
¡ÃÂìÃÂÃÂââ¬Ã
¡ÃÂâââÂÂìÃÂ
â induced Steven Johnson syndrome. Journal of Clinical Psychiatry 2004; 65: 1431-1432.
- Haskin JT, Moyer JA, Muth EA, Sigg EB. Inhibition of nor-adrenergic neuronal activity by the novel bicyclic compounds, Wy 45030, Wy 45881. Society of Neuroscience Abstract 1984; 10: 262.
- Cusack B, Nelson A, Richelson E. Psychopharmacology 1994; 114: 559-565.
- Troy SM, Parker VD, Fruncillo RJ, Chiang ST. The pharmacokinetics of Venlafaxine when given in a twice-daily regimen. Journal of Clinical Pharmacology 1995; 35: 404-409.
- Parker G, Blennerhassett J. Withdrawal reactions associated with Venlafaxine. Australian and New Zealand journal of Psychiatry 1998; 32 (2): 291-294.
- Effexor XR, prescription guide, Cited 28 July 2010: Available from https://www.dailymed.nlm.nih.gov/dailyme d/druginfo.cfm
- Olver JS, Burrows GD, Norman TR. The treatment of depression with different formulations of Venlafaxine: a comparative analysis. Human psychopharmacology 2004; 19: 9-16.
- Rowe RC, Sheskey PJ, Weller PJ. Xanthan Gum. Handbook of Pharmaceutical Excipients, 4 th Edition. London Pharmaceutical. 2003 ; 691-693.
- Saltiel E, Ellrodt ,Gray A, Monk, John P, Langley, Mark S. A Review of its pharmacodynamic and pharmacokinetic properties, and therapeutic use in hypertension. Drugs 1988; 36: 387-428.
- Bariya SH, Gohel MC. Fabrication of triple layer Matrix Tablets of Venlafaxine Hydrochloride using Xanthan Gum. AAPS Pharm Sci Tech, Jun 2009; 10 (2): 624-630.
- Office of Generic Drugs, Drug Dissolution data base. Cited 17 July 2011. Available from https://www.accessdata.fda.gov/scripts/cder/dissolution.
- Shah VP, Tsong Y, Sathe P, Liu JP. In-vitro dissolution profile comparison statistics and analysis of the similarity factor, f2. Pharmaceutical Research 1998; 15: 889- 896
- Varshosaz J, Tavakoli N, Kheirolahi F. Use of Hydrophilic Natural Gums in Formulation of Sustained Release Matrix Tablets of Tramadol Hydrochloride. AAPS Pharm Sci Tech 2006 ; 7 (1) : E1-E7.
- Shoaib M H, Tazeen J, Hamid A, Yousuf R I. Evaluation of Drug Release Kinetics from Ibuprofen Matrix Tablets Using HPMC. Pak J Pharm Sci. 2006; 19(2): 119-124
- Thakkar V T, Shah P A, Soni T G, Parmar M Y, Gohel M C, Gandhi. Goodness of Fit Model-Dependent Approach for Release Kinetics of Levofloxacin Hemihydrates Floating Tablet. Dissolution Technologies Feb. 2009, 35-39
- Abdul S, Poddar SS. A flexible technology for modified release of drugs: multi layered tablets. Journal of Controlled Release 2004; 97: 393-405.