Hisashi Ito*, Shigeru Fukutake and Tetsumasa Kamei
Department of Neurology, Shonan Fujisawa Tokushukai Hospital, Fujisawa, Japan
*Corresponding Author:
Dr. Hisashi Ito
Department of Neurology
Shonan Fujisawa Tokushukai Hospital
251-0041, Fujisawa, Japan
Tel: +81-466-35-1177
Fax: +81-466-35-1300
E-mail: hisashi.ito@tokushukai.jp
Received date: April 17, 2018; Accepted date: May 02, 2018, 2018; Published date: May 05, 2018
Citation: Ito H, Fukutake S, Kamei T (2018) Facial Nerve Palsy after Botulinum Toxin Therapy for Hemifacial Spasm: A Case Report. J Neurol Neurosci Vol.9 No.3:257. doi: 10.21767/2171-6625.1000257
Keywords
Hemifacial spasm; Botulinum toxin type A; Facial nerve palsy
Introduction
Botulinum toxin type A (BTX-A) is now widely used for movement disorders, pain disorders, and autonomic dysfunction. One of the characteristics of BTX-A is a minimal effect at short distances from injection site; however, local diffusion could cause paralytic effect in muscles adjacent to the target [1]. On the other hand, BTX-A was suggested to cause peripheral neuropathy [2]. In this article, we describe a case of left hemifacial spasm (HFS) followed by ipsilateral facial nerve palsy after BTX-A injection.
Case Report
A 59-year-old woman developed left HFS at the age of 55. She was treated with carbamazepine which showed no therapeutic effects. We started botulinum toxin therapy for HFS when she was 58 years old. Prior to the botulinum toxin therapy, we ruled out myasthenic syndrome with electrophysiological study. Brain MR imaging showed tortuous left vertebral artery; however, we did not observe tumor, vascular malformation, and aneurysm. BTX-A (BotoxR; Allergan, Irvine, CA) was diluted 1.25 units per 0.1ml of 0.9% saline. We injected BTX-A into orbicularis oculi, zygomaticus major, and orbicularis oris in five times. Each treatment was followed by disappearance of HFS without any adverse events. On sixth treatment, we injected 6.25 units of BTX-A into left orbicularis oculi (1.25 units per site, 3 sites), zygomaticus major (1.25 units per site, 1 site), and orbicularis oris (1.25 units per site, 1 site). There were no immediate adverse reactions after injection and facial spasm disappeared on the next day; however, five days after this treatment, she noticed the weakness of left facial muscles without preceding infectious symptoms and vaccination.
Frontal muscle and platysma, which we did not inject BTX-A, were also weak. Bell phenomenon, ciliary sign, and platysma sign were positive. We observed no rash around left ear. In electrophysiological study of left facial nerve, stimulated below ear and put pick up electrode at nasalis, the amplitude of the compound muscle action potential fell off remarkably in comparison with that of the right side (Figure 1). Brain MR imaging showed only tortuous left vertebral artery. Routine laboratory testing including antibodies of herpes simplex virus, varicella zoster virus, Epstein-Barr virus, and cytomegalovirus, did not show significant abnormalities. Antiganglioside antibodies were negative. Cerebrospinal fluid revealed neither pleocytosis nor hyperproteinorrachia. We diagnosed her as left peripheral facial nerve palsy and administered prednisolone and mecobalamin. Facial spasm relapsed after 24 weeks; however, facial nerve palsy improved without any sequelae.
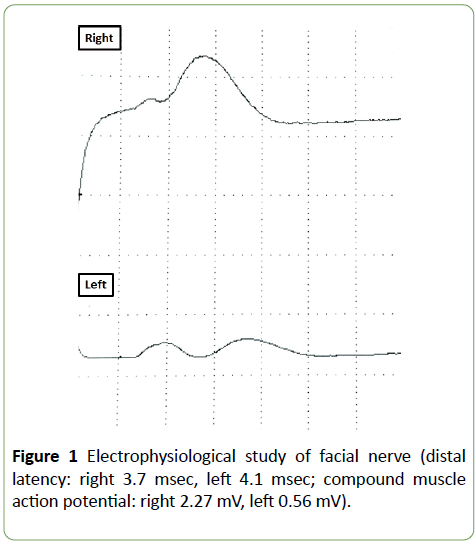
Figure 1: Electrophysiological study of facial nerve (distal latency: right 3.7 msec, left 4.1 msec; compound muscle action potential: right 2.27 mV, left 0.56 mV).
Discussion
Several cases of polyradiculoneuritis and brachial plexopathy after BTX-A injection have been reported to date [3-9] (Table 1). Compared with these cases, we had to rule out the infiltration of BTX-A because weakness occurred in muscles into which they were injected BTX-A and their adjacent muscles. Thorough neurological examination and electrophysiological study were useful for distinguishing facial nerve palsy from BTX-A related facial muscles weakness.
| Author |
Haug BA [3] |
Glanz man RL [4] |
Sampaoc, [5] |
Takenaga S [6] |
Tarsy D [7] |
Burguera JA [8] |
Onoue H [9] |
This case |
| Age, Sex |
63 y, Man |
53 y, Woman |
32 y, Man |
27 y, Man |
55 y, Man |
40 y, Man |
68 y, Man |
59 y, Woman |
| Underlying disease |
Blepharospasm |
Cervical Dystonia |
Cervical Dystonia |
Focal Dystonial In Hand |
Cervical Dystonia |
Cervical Dystonia |
Cervial Dystonia |
Hemifacial Spasm |
| Product of BTX-A |
Unapproved Toxin* |
Not Described |
Dysport |
Botox |
Not Described |
Botox |
Botox |
Botox |
| Target muscles and BTX-A dose in the final injection |
Rt/Lt Orb. Oculi (72.5U) |
Lt SM (45U)
Lt TZ (75 U) |
Rt SM (200U)
Rt/Lt posterior neck muscles (1000 U) |
Lt FDP (15 U) |
Rt SM (30U)
Rt Spl. Cap (90 U)
Rt Lev. Scap (40U) |
Rt/Lt TZ Rt/Lt Spl. Muscle (ca. 200 U, totally) |
Rt SM Lt TZ Lt Spl. Muscle (150 U, totally) |
Lt Orb. Oculi (3.75 U)
Lt Zyg. Major (1.25U)
Lt Orb. Oris (1025 U) |
| Onset of neuropathy from the final BTX-A injection |
11 Months |
2 Days |
15 Days |
4 Days |
10 Days |
6 Weeks |
10 Weeks |
5 Days |
| Type of neuropathy |
PRN |
Lt BP |
Rt/Lt BP |
Lt BP |
Lt BP |
PRN |
PRN |
Lt Facial Nerve Palsy |
| Outcome of neuropathy |
Improved Completely |
Mild Weakness |
Completely Improved |
Mild Weakness |
Mild Weakness |
Improved Completely |
Chair Bound |
Improved Completely |
Table 1: Previous reports of neuropathy developing after botulinum toxin type A injection. Rt: Right, Lt: Left, BP: Brachial Plexopathy, PRN: Polyradiculoneuritis, FDP: Flexor Digitorum Profundus, Lev. Scap: Levator Scapulae, Orb. Oculi: Orbicularis Oculi, Orb. Oris: Orbicularis Oris, SM: Sternocleidomastoid, Spl. Cap: Splenius Capitis, TZ: Trapezius, Zyg. Major: Zygomaticus Major; * From Smith Kettlewell Institute of Visual Sciences.
Some previously reported cases had too long interval from the final injection of BTX-A to the onset of neuropathy [3,8,9]. Based on three studies with valid methodology, the annual incidence of facial nerve palsy was estimated in the range of 25 to 100 per 100,000 [10-12]. The estimated incidence in Japan appeared to be similar to that in the US, based on the available literature [13]. Recognizing the long duration to the onset of neuropathy and the incidence of facial nerve palsy, it is difficult to establish whether BTX-A played some role in facial nerve palsy or the palsy occurred as a coincidence in these cases.
On the other hand, the other cases and our case developed neuropathy within almost 2 weeks after BTX-A injection [4-7]. BTX-A binds to GT1b and GQ1b with high affinity and induces the clinical features similar to Guillain-Barré syndrome [2,14]. Therefore, BTX-A might contribute to the onset of neuropathy in the latter cases.
Conclusion
To our knowledge, this is the first case of ipsilateral facial nerve palsy following botulinum toxin therapy for HFS. Acausal relationship between BTX-A and neuropathy following BTX-A injection has not been established firmly; however,we have to keep in mind that neuropathy could occur after botulinum toxin therapy.
We reported ipsilateral facial nerve palsy after botulinum toxin therapy for HFS. Detailed neurological examination and electrophysiological study are helpful for a proper diagnosis.
Acknowledgement
We thank Dr. Mitchell F Brin (University of California, Irvine, US) for his advice to this case.
Conflicts of Interest
There are no conflicts.
22879
References
- Pirazzini M, Rossetto O, Eleopra R, Montecucco C (2017) Botulinum neurotoxins: Biology, pharmacology, and toxicology. Pharmacol Rev 69: 200-235.
- Willison HJ, Kennedy PG (1993) Gangliosides and bacterial toxins in Guillain-Barré syndrome. J Neuroimmunol 46: 105-112.
- Haug BA, Dressler D, Prange HW (1990) Polyradiculoneuritis following botulinum toxin therapy. J Neurol 237: 62-63.
- Glanzman RL, Gelb DJ, Drury I, Bromberg MB, Truong DD (1990) Brachial plexopathy after botulinum toxin injections. Neurology 40: 1143.
- Sampaio C, Castro-Caldas A, Sales-Luis ML, Alves M, Pinto L, et al. (1993) Brachial plexopathy after botulinum toxin administration for cervical dystonia. J Neurol Neurosurg Psychiatry 56: 220.
- Takenaga S, Arimura K, Osame M (1994) Brachial plexopathy after botulinum toxin injections. Neurol Med 40: 222-223.
- Tarsy D (1997) Brachial plexus neuropathy after botulinum toxin injection. Neurology 49: 1176-1177.
- Burguera JA, Villaroya T, López-Alemany M (2000) Polyradiculoneuritis after botulinum toxin therapy for cervical dystonia. Clin Neuropharmacol 23: 226-228.
- Onoue H, Matsunobu A, Nagaishi A, Yukitake M, Kuroda Y (2004) A case report of acute polyradiculoneuritis developing after multiple injections of botulinum toxin for cervical dystonia. Clinca Neurol 44: 20-24.
- Savettieri G, Salemi G, Rocca WA, Meneghini F, Santangelo R, et al. (1996) Incidence and lifetime prevalence of Bell’s palsy in two Sicilian municipalities. Sicilian Neuro-Epidermiologic Study (SNES) Group. Acta Neurol Scand 94: 71-75.
- Peitersen E (2002) Bell’s palsy: The spontaneous course of 2500 peripheral facial nerve palsies of different etiologies. Acta Otolaryngol Suppl 4-30.
- Katusic SK, Beard CM, Wiederholt WC, Bergstralh EJ, Kurland LT (1986) Incidence, clinical features, and prognosis in Bell’s palsy, Rochester, Minnesota, USA during 1968-1982. Ann Neurol 20: 622-627.
- Yanagihara N (1988) Incidence of Bell’s palsy. Ann Otol Rhinol Laryngol Suppl 137: 3-4.
- Kitamura M, Iwamori M, Nagai Y (1980) Interaction between Clostridium botulinum neurotoxin and gangliosides. Biochim Biophys Acta 628: 328-335.






