Keywords
Hypothyroidism; Thyroxine dosage; Thyroidstimulating hormone
Introduction
Primary hypothyroidism, one of the most common endocrine diseases, is caused by a thyroid hormone deficiency due to alterations in the thyroid gland. The prevalence of hypothyroidism in the general population ranges from 3.8% to 4.6% [1-3]. A study carried out in our setting based on the prescription of thyroid hormones established its prevalence to be 8.4 cases per 1000 inhabitants, with a mean age of 60 years [4].
The clinical manifestations of hypothyroidism vary widely depending on the age of onset, duration, and the severity of the thyroid hormone deficiency. Common symptoms include fatigue, cold intolerance, weight gain, constipation, dry skin, myalgia and irregular menstrual cycle [5]. Physical examination often reveals goiter, especially in patients with iodine deficiency or autoimmune thyroiditis (Hashimoto's thyroiditis), bradycardia, hypertension, and an altered relaxation phase in deep tendon reflexes. Most patients with chronic autoimmune thyroiditis present elevated anti-thyroid peroxidase antibodies. Other associated metabolic disorders are hypercholesterolemia, macrocytic anemia, elevated creatine kinase and hyponatremia [6].
Diagnosis of hypothyroidism is based on the clinical context and the biochemical report. Measurement of thyroidstimulating hormone (TSH) is the primary test for thyroid function evaluation, as it is the most sensitive indicator of early thyroid disease. Laboratories have established reference ranges for TSH from 0.4 or 0.5 mU/L to 4.5 to 5.5 mU/L [7]. Diagnosis and treatment of hypothyroidism are often considered straightforward and are performed in primary care services, although many studies have stressed that its management may be difficult. Many patients receiving thyroid hormone therapy suffer either hormone over-replacement or under-replacement [8-10]; indeed, in a study evaluating the prevalence of under- or over-treatment of hypothyroidism in patients over 65 years of age, 41% of those receiving thyroid hormones had low TSH and 16% had elevated TSH. Patients with low weight or diabetes present poorer control, and the risk of cardiovascular or skeletal effects due to over-treatment should be taken into account [11].
After diagnosis of hypothyroidism and the initiation of treatment, TSH levels are monitored between the sixth and eighth week. The levothyroxine dose is adjusted in order to achieve a euthyroid state. The initial dose is calculated according to weight. Other factors that may influence the required dose are age, pregnancy, malabsorptive states, and drugs such as lithium or amiodarone [12-14]. Once TSH has stabilized within the normal range [15], checks are recommended every 6 or 12 months.
However, these routine checks may not in fact be necessary. The study by Pecina et al. [16] found that the best predictor of normal TSH values after 10 to 14 months was levothyroxine maintenance dose; among patients receiving a dose <75 μg/day, 91% achieved normal TSH levels, compared to 77.5% who received doses of ≥ 125 μg/day.
Inadequate hormone replacement may lead to cardiovascular disease [17], arrhythmias [18], neurocognitive alterations [19], and osteoporosis and fractures [20,21]. In this context, the aims of the study were to establish the percentage of patients in whom TSH levels remained within the normal range during a 4 year follow-up and to describe the factors associated with good TSH control in this population. We hypothesized that a higher levothyroxine maintenance dose, altered initial values of TSH and poor compliance would be associated with less stable TSH levels in future determinations.
Materials and Method
Longitudinal follow-up study of a retrospective cohort of patients from 21 primary care centers with hypothyroidism over a 5-year observation period. Data were obtained from the pharmacy drug dispensation register in the computerized medical records stored by the regional health system. Tracking the dispensation of drugs in group H03AA of the Anatomical Therapeutic Chemical Classification System [22], we identified patients with hypothyroidism who were being treated by primary care physicians and whose TSH levels were normal (0.27-5.0 MU/l) during the year 2010.
The records were reviewed to confirm the diagnosis and treatment and to identify patients who met the inclusion criteria (Figure 1). The follow-up period lasted from the first determination of TSH at the date of inclusion in 2010 until the last value recorded, death, or transfer to another health region before 31 December 2014. The research was authorized by the IDIAP Jordí Gol clinical research ethical committee, code no p15/138.
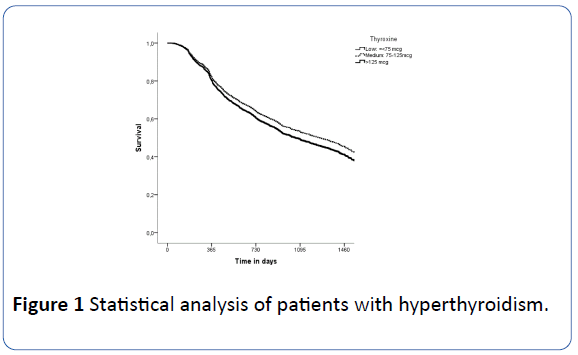
Figure 1: Statistical analysis of patients with hyperthyroidism.
The variables recorded were: age, sex, body mass index and weight at the time of inclusion, comorbidity, levothyroxine dose and compliance with treatment [23]. All baseline variables were recorded on the date of inclusion in 2010, when the patient had a TSH value considered normal. The daily dose of levothyroxine was classified as low (<75 mcg/day), medium (75-125 mcg/day) and high (>125 mcg/day). Compliance with treatment was assessed on the basis of the medication withdrawn from pharmacies; patients who withdrew more than 80% of the drug necessary for their treatment were defined as compliant [23]. The “time with normal TSH” was calculated from the date of inclusion until the day the TSH value was found to be outside the normal range. Failing that, the date of the last measurement of TSH, death, or transfer prior to 31 December 2014 was used.
Data analysis: We performed a descriptive analysis of baseline variables, expressing qualitative variables as frequencies and percentages and quantitative variables as means and standard deviation (SD). The association of treatment compliance with the baseline variables recorded was examined by the Chi square test.
The time with normal TSH was estimated using the Kaplan Meier method, both overall and by age groups. Crude and adjusted hazard ratios of TSH alteration were estimated with their 95% confidence intervals (95% CI) during follow-up by fitting Cox proportional hazards regression models. In the adjusted model, the clinically relevant and demographic variables were introduced using the Enter method.
In addition, the survival curves of the time with normal TSH in relation to thyroxine dose were estimated. The goodness of fit and the proportional hazards hypothesis of the Cox model were evaluated by Schoenfeld residual analysis. A p value <0.05 was established as statistically significant. Data management and analysis were performed using the SPSS statistical software (v17) and STATA v11-IC packages.
Results
A total of 3,170 patients were initially treated with levothyroxine. After applying the inclusion/exclusion criteria, 2,630 participants remained eligible for the study. The flowchart (Figure 1) shows the distribution of the patients according to the inclusion and exclusion, and Table 1 shows the characteristics of the patients included.
| Variable and Category |
| Sex |
Number |
Percentage (%) |
| Male |
256 |
9.7 |
| Female |
2374 |
90.3 |
| Comorbidities at baseline basal |
| CRF |
155 |
5.9 |
| Diabetes type 2 |
403 |
15.3 |
| Diabetes type 1 |
32 |
1.2 |
| IHR |
85 |
3.2 |
| Age group |
| <49 |
684 |
26 |
| 50-59 |
597 |
22.7 |
| 60-69 |
656 |
24.9 |
| >70 |
693 |
26.3 |
| Variable |
Mean |
± SD |
| Age in years |
59.6 |
± 14.7 |
| Weight in kg |
71.5 |
± 14.3 |
| BMI (kg/m2) |
28.5 |
± 5.1 |
| Thyroxine dose (mcg) |
0.07 |
± 0.01 |
SD: Standard Deviation; CRF: Chronic Renal Failure; IC: Ischemic Heart Disease.
Table 1 Baseline characteristics of the sample.
The final sample comprised 2,374 women (90.3%) and 256 men (9.7%). Mean age was 59.6 years (SD=14.7) (Table 1). The rate of compliance with treatment in the year after inclusion was 84.9% (n=2.232). Table 2 shows the compliance with treatment in relation to the variables analyzed. Older subjects maintained normalized TSH for longer.Patients receiving medium doses of levothyroxine (75-125 μg/day) had a higher rate of normal TSH during follow-up (p<0.05); this association also maintained statistical significance (p=0.04) after adjusting for the other variables analyzed (Table 3).
| Variable |
Category |
Compliance |
| N |
n |
% |
p value |
| Sex |
Male |
256 |
204 |
79.70% |
0.015* |
| Female |
2374 |
2028 |
85.40% |
|
| Comorbidities at baseline basal |
CRF |
155 |
141 |
91.00% |
0.029* |
| Diabetes type 2 |
403 |
356 |
88.30% |
0.035 |
| Diabetes type 1 |
32 |
26 |
81.30% |
0.566 |
| Ischemic IHR |
85 |
82 |
96.50% |
0.002* |
| Smoking |
|
328 |
269 |
82.00% |
0.123 |
| Age group (years) |
|
<0.001* |
| |
≤ 50 |
684 |
519 |
75.90% |
|
| |
50-60 |
597 |
503 |
84.30% |
|
| |
60-70 |
656 |
585 |
89.20% |
|
| |
≤ 70 |
693 |
625 |
90.20% |
|
Table 2 Frequency (n) and percentage of treatment compliance according to variables analyzed.
| Variable |
Crude HR |
Adjusted HR |
| |
(95%CI) |
P |
(95%CI) |
P |
| Female (Ref=Male) |
1.08 |
(0.91-1.28) |
0.409 |
1.11 |
(0.88-1.40) |
0.373 |
| Compliers |
0.85 |
(0.74-0.98) |
0.027 |
0.9 |
(0.76-1.07) |
0.24 |
| Age (years) |
1 |
(0.99-1.00) |
0.065 |
0.99 |
(0.98-1.00) |
0.043* |
| Smoking |
1.11 |
(0.95-1.29) |
0.18 |
1.13 |
(0.95-1.36) |
0.172 |
| Diabetes type 2 |
1.02 |
(0.89-1.18) |
0.759 |
1.03 |
(0.88-1.21) |
0.722 |
| Weight (kg) |
1 |
(1.00-1.01) |
0.325 |
1 |
(0.99-1.01) |
0.32 |
| BMI (kg/m2) |
1.01 |
(0.99-1.02) |
0.397 |
1.02 |
(0.99-1.04) |
0.15 |
| Thyroxine dose Reference |
| Low: <75 mcg |
- |
- |
- |
- |
- |
- |
| Medium: 75-125 mcg |
0.93 |
(0.84-1.03) |
0.141 |
0.88 |
(0.78-0.99) |
0.04* |
| High: >125 mcg |
1.09 |
(0.74-1.60) |
0.665 |
0.99 |
(0.65-1.51) |
0.965 |
95%CI: 95% confidence interval; P: p value; HR=hazard ratio; *p value<0.05
Table 3: Risk of alteration of TSH during follow-up according to variables analyzed using crude hazard ratios in an adjusted Cox model.
After one year, more than 80% of patients maintained normal TSH levels regardless of thyroxine dose (i.e., TSH was normal in 80% of those taking low doses, 83% of those at medium doses and 84% of those at high doses). In the second year, 60% of the patients had normal TSH values, while in the third year 49% of those taking the low dose had normal TSH, 54% of the patients who took the medium dose and 44% of those who took the high dose. After four years, 35% of patients with medium and low doses of thyroxine had normal TSH, but only 26% of patients taking high doses. Table 4 shows the estimated median duration of normalized TSH according to age group.
| Age group |
Median estimation (days) |
95%CI |
| Lower limit |
Upper limit |
| ≤ 50 |
974 |
832 |
1116 |
| 50-60 |
1187 |
1039 |
1334 |
| 60-70 |
1197 |
995 |
1399 |
| >70 |
1246 |
1095 |
1397 |
| Overall |
1163 |
1076 |
1250 |
Table 4 Estimation of the median time of normalized TSH according to age.
Discussion
Patients receiving a medium dose of levothyroxine (75-125 mcg/day) were more likely to have normal TSH in the long term than patients with high doses (>125 mcg/day). The rate of patients with normal TSH at one year was above 80%, while at five years it was 35% among those taking a medium or low dose and 25% among those on a high dose. Older patients had normal TSH values for longer periods. The study by Okosieme et al. [24] assessed treatment with thyroid hormones in primary care and the factors associated with inadequate hormone replacement in the population. Although most levothyroxine users underwent dose adjustments after monitoring of TSH, in a large proportion (37.2%) the TSH remained altered after five years. In a study in Tayside, Scotland, between 1993 and 2001, TSH levels were unstable in 38.4% of patients taking levothyroxine after eight years of follow-up [18].
Among the factors we associated with TSH stability was thyroxine dose. Pecina et al. [16] considered that annual monitoring of TSH would be justified in patients receiving doses higher than 125 mcg/day, whereas in patients with doses lower than this figure it should be reviewed every two years. However, in our study, only 60-65% had controlled TSH at two years, and so annual monitoring should be maintained.
In our study, older patients presented higher TSH stability although we did not know whether this group had undergone more controls than the younger patients. In a study of patients with a mean age of 50.9 years which assessed the number of changes in thyroid treatment dose over a mean period of 16.2 years (range: 10-30), Viswanath et al. [25] reported that patients older than 60 years had more dose changes than patients below this age.
Two studies by Jonklaas et al. [26] and Devdhar et al. [27] suggest that the levothyroxine dose required by pre- and postmenopausal women disappear are the same if they are assessed using ideal weight, rather than using current body weight. These authors attributed the alterations that occur during aging to the change in weight.
Compliance with treatment was not associated with TSH stability. It is possible that, immediately prior to analyses, patients with generally poor compliance may temporarily adhere to their treatment schedule, and thus present normal TSH values. Nor did we find an association between diabetes mellitus and TSH values, although the study by Okosieme et al. [24] unexpectedly found an inverse association between diabetes status and low TSH levels. The relationship between thyroid function and diabetes is complex and is expressed through multiple metabolic and cellular pathways [28]. In any case, the impact of diabetes on levothyroxine requirements in patients with hypothyroidism is unclear. Flynn et al. [18] showed that diabetic patients tended to be less over-treated than nondiabetic patients, but the relationship between diabetes and levothyroxine requirement needs to be clarified in prospective studies.
Our study may have potential limitations. The use of clinical records from a population database entails an implicit reporting bias. To minimize this limitation, we selected only patients who were treated for hypothyroidism and for whom TSH measurements were available. In addition, the time with normal TSH values may be over-estimated in patients with only a few measurements or with long intervals between measurements. As for the evaluation of treatment compliance, the analysis was based on pharmacy drug dispensations, which is an indirect record of patients’ consumption. Finally, we could not analyze the etiology of hypothyroidism since in most cases this information was not present in the clinical history; nor did we include the presence of antibodies as a variable, which would have allowed us to determine whether the hypothyroidism had an autoimmune origin.
Conclusions
One-fifth of the patients with hypothyroidism had inadequate thyroid hormone replacement and therefore had an increased risk of complications. TSH stability was associated with medium levothyroxine dose and advanced patient age. Only between 26-35% of patients had good control after four years of followup.
Health professionals should focus on the variables that may alter TSH values, such as compliance, interactions with other medications or problems with intestinal absorption [29]. Finally, even when TSH levels are routinely checked, thyroid hormone replacement in patients with hypothyroidism is suboptimal in a proportion of the population. After the first year of our study, 20% of the patients had poor TSH values, a finding that makes annual controls mandatory.
Future studies should evaluate the factors associated with TSH instability, in order to focus more closely on this population and to help design health education interventions that favor compliance with treatment and thus increase the percentages of patients with good hormonal replacement.
Acknowledgement
The translation of this article was funded by a grant from the IDIAP Jordi Gol.
18989
References
- Hollowell JG, Staehling NW, Flanders WD, Hannon WH, Gunter EW, et al. (2002) Serum TSH, T(4), and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab 87: 489-499.
- Vanderpump MP, Tunbridge WM, French JM, Appleton D, Bates D, et al. (1995) The incidence of thyroid disorders in the community: a twenty-year follow-up of the Whickham Survey. Clin Endocrinol (Oxf) 43: 55-68.
- Leese GP, Flynn RV, Jung RT, Macdonald TM, Murphy MJ, et al. (2008) Increasing prevalence and incidence of thyroid disease in Tayside, Scotland: The Thyroid Epidemiology Audit and Research Study (TEARS).Clin Endocrinol 68: 311–316.
- Serna Arnáiz MC, Galván Santiago L, Gascó Eguiluz E, Manrique Manrique M, Foix Oña MM, et al. (2003) Estimating the prevalence of hypothyroidism in Lleida from the prescription of thyroid hormones. Rev Esp Salud Publica 77: 405-410.
- Brent GA, Davies TF (2011) Hypothyroidism and thyroiditis. Williams Textbook of Endocrinology. (12th edn), Saunders Elsevier, Philadelphia, pp: 406-439.
- Emerson CH (2009) Circulating thyroid stimulating hormones: Why, when, and what to measure. Thyroid 19: 1-3.
- Somwaru LL, Arnold, AM, Joshi N, Fried LP, Cappola AA (2009) High frequency of and factors associated with thyroid hormone over-replacement and Under-replacement in men and women aged 65 and over. J Clin Endocrinol Metab 94: 1342-1345.
- Dayan CM, Panicker V (2013) Hypothyroidism and depression. Eur Thyroid J 2: 168-179.
- Laurberg P, Andersen S, Bulow Pedersen I, Carle A (2005) Hypothyroidism in the elderly: Pathophysiology, diagnosis and treatment. Drugs Aging 22: 23-38.
- Jonklaas J, Bianco A, Bauer A, Burman K, Cappola A, et al. (2014) Guidelines for the treatment of hypothyroidism: Prepared by the american thyroid association task force on thyroid hormone replacement. Thyroid 24: 1670-1751.
- Singh N, Singh PN, Hershman JM (2000) Effect of calcium carbonate on the absorption of levothyroxine. JAMA 283: 2822-2825.
- Mersebach H, Rasmussen AK, Kirkegaard L, Feldt-Rasmussen U (1999) Intestinal adsorption of levothyroxine by antacids and laxatives: Case stories and in vitro experiments. Pharmacol Toxicol 84: 107-109.
- Mandel SJ, Larsen PR, Seely EW, Brent GA (1990) Increased need for thyroxine during pregnancy in women with primary hypothyroidism. N Engl J Med 323: 91-96.
- Garber JR, Cobin RH, Gharib H, Hennessey JV, Klein I, et al. (2012) Clinical practice guidelines for hypothyroidism in adults: Cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Endocr Pract 18: 988-1028.
- Pecina J, Bernard M, Furst J, Rohrer J (2012) Hypothyroidism management: is an annual check of TSH level always necessary? J Fam Pract 61: E1-E5.
- Biondi B, Cooper DS (2008) The clinical significance of subclinical thyroid dysfunction. Endocr Rev 29: 76-131.
- Flynn RW, Bonellie SR, Jung RT, MacDonald TM, Morris AD, et al. (2010) Serum thyroid-stimulating hormone concentration and morbidity from cardiovascular disease and fractures in patients on long-term thyroxine therapy. J Clin Endocrinol Metab 95: 186-193.
- Dugbartey AT (1998) Neurocognitive aspects of hypothyroidism. Arch Intern Med 158: 1413-1418.
- Stall GM, Harris S, Sokoll LJ, Dawson-Hughes B (1990) Accelerated bone loss in hypothyroid patients overtreated with L-thyroxine. Ann Intern Med 113: 265-269.
- Paul TL, Kerrigan J, Kelly AM, Braverman LE, Baran DT (1988) Long-term L-thyroxine therapy is associated with decreased hip bone density in premenopausal women. JAMA 259: 3137-3141.
- Nor Aripin KN, Sammons HM, Choonara I (2010) Published pediatric randomized drug trials in developing countries, 1996-2002. Paediatr Drugs 12: 99-103.
- Basterra Gabarró M (1999) Therapeutic compliance. Pharmaceutical Care España 1: 97-106.
- Okosieme OE, Belludi G, Spittle K, Kadiyala R, Richards J (2011) Adequacy of thyroid hormone replacement in a general population. QJM 104: 395-401.
- Viswanath AK, Avenell A, Philip S, Acharya SH, Maclennan G, et al. (2007) Is annual surveillance of all treated hypothyroid patients necessary? BMC Endocr Disord 7: 4.
- Jonklaas J (2010) Sex and age differences in levothyroxine dosage requirement. Endocr Pract 16: 71-79.
- Devdhar M, Drooger R, Pehlivanova M, Singh G, Jonklaas J (2011) Levothyroxine replacement doses are affected by gender and weight, but not age. Thyroid 21: 821-827.
- Kadiyala R, Peter R, Okosieme OE (2010) Thyroid dysfunction in patients with diabetes: Clinical implications and screening strategies. Int J Clin Pract 64: 1130-1139.
- Morris JC (2009) How do you approach the problem of TSH elevation in a patient on high-dose thyroid hormone replacement? Clin Endocrinol 70: 671-673.







