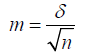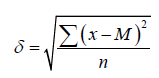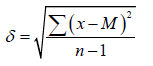Subbotin AS1* and Afanasyev NG2
1Clinical Oncology Center, State Budgetary Health Institution, Chelyabinsk, Russian Federation
2FGBOU South Ural State Medical University of the Ministry of Health of the Russian Federation, Chelyabinsk, Russian Federation
Corresponding Author:
Subbotin AS
Clinical Oncology Center, State Budgetary Health Institution
Chelyabinsk, Russian Federation.
Tel: +201112139714
E-mail: acsubbotin@yandex.ru
Received date: March 21, 2019; Accepted date: March 29, 2019; Published date: April 05, 2019
Citation: Subbotin AS, Afanasyev NG (2019) Features of the Visualization of Different Histologic Types of Hodgkin's Lymphoma p about Positron Emission Tomography Combined with Computed Tomography. Arch Can Res Vol.7 No.1:05
Keywords
Hodgkin's lymphoma; PET-CT; 18-fluorodeoxyglucose
Introduction
Lymphoma Hodgkin's considered malignant neoplasm with high curable. Complete cure is achieved in 8 0 -90% of patients with localized stages and in 60 - 7 0% of patients with common stages of the disease. Good results in the treatment of Hodgkin's lymphoma are a result of the high efficacy of modern chemotherapy and radiotherapy regimens. However, with longterm follow-up of patients treated for Hodgkin's lymphoma, it became apparent that there were a large number of longterm effects of treatment, such as the development of second tumors, complications of the cardiovascular and respiratory systems, which shorten the patient's life expectancy [1]. At the same time, the failure of the treatment is accompanied by the development of relapses of Hodgkin's lymphoma, which are more difficult to treat and in some cases are refractory to any of the applied methods of treatment [2]. The balance between the adequacy of the treatment and its safety is the basis of tactics in the fight against Hodgkin's lymphoma. An important link in the management of patients with Hodgkin's lymphoma is the primary staging of the tumor process [3-6]. A number of studies indicate the possibility of no signs of an increase in metabolic activity in tumor tissue in Hodgkin's lymphoma in 1-3% of cases before the start of treatment [7-10]. The presence or absence of an increased level of FDG accumulation in the tumor tissue at the primary staging affects the ability to assess the dynamics of the treatment. Studying the characteristics of FDG accumulation in tumor tissue in various histological types of Hodgkin's lymphoma would optimize the assessment of treatment dynamics by identifying tumor variants that are prone to lower SUVmax values during initial staging, and as a result, a more careful interpretation of the results of treatment dynamics assessment. The literature does not provide information regarding the features of the accumulation of FDG in various histological types of Hodgkin's lymphoma and analysis of the possible causes of the low level of metabolic activity of tumor tissue in this disease.
Objective
To study the characteristics of FDG accumulation in tumor tissue in various histological variants of Hodge Kin 's lymphoma at primary staging, to determine the proportion of patients with Hodgkin's lymphoma with low metabolic activity of the tumor tissue before the start of treatment.
Methods
In the PET center GBUZ CHOKOD for the period 2011-2015. 131 PET-CT studies were conducted with the aim of primary staging in patients with Hodgkin's lymphoma. All cases were verified by histological and immunohistochemical methods. Female patients prevailed (69 patients - 53%). Histological types distribution was as follows: class and Th Skye Hodgkin 123 patients (94%) nodular sclerosis - 68 patients (52%), mixed-cell variant - 35 patients (27%), lymphoid depletion - 13 patients (10%), lymphoid predominance - 7 patients (5%) ; non-classical Hodgkin's lymphoma: Hodgkin's nodular lymphoma with a lymphoid predominance (NLHLP) - 8 patients (6%). 64 patients had no common symptoms of Hodgkin's lymphoma (“B-symptoms”), 67 patients had common symptoms caused by a tumor process.
The study was conducted according to standard methods. Preparation for the study included a light, carbohydrate-free dinner, the last meal no earlier than 8 hours before the study. Patients before the introduction of FDG was carried out glycemic control. FDG was administered in a volume with an activity of 200 MBq per square meter of the patient's body surface. The duration of the distribution of the radiopharmaceutical was 60- 120 minutes. Scanning began with the native phase of the MSCT study, then PET scan was performed with the scan area divided into 5-6 “beds” with a scan duration of 3 minutes each. After that, an intravenous bolus of iodine-containing monomeric non-ionic contrast medium was administered in a volume of 100-150 ml and the arterial and venous phases of MSCT were scanned. Evaluation of the metabolic activity of pathological foci was carried out by measuring the index SUVmax, as well as its comparison with the index SUVmax in the liver parenchyma.
For the purpose of statistical processing of the material, the calculation of the average error of the mean value m by the formula was applied:
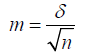
where m is the average error of the mean, δ is the standard deviation, n is the number of observations. The standard deviation was calculated by the formulas:
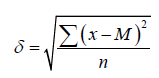
For observations in which n ≥ 30 and
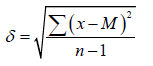
For observations in which n<30, where x is the value of the series variable, M is the arithmetic average of all variables of the series.
Results and Discussion
The following results were obtained Table 1. In 5 patients (4%) in the affected lymph nodes the accumulation of FDG was determined to be equal to or less than the accumulation of FDG by the liver parenchyma. In all cases, there was an increase in the lymph nodes of the affected groups. It should be noted that all patients with a low metabolic activity of the affected lymph nodes no symptoms of intoxication ("B symptoms"). Of these 5 patients, a complete absence of signs of increased metabolic activity in pathologically changed lymph nodes was determined in 3 patients (2%), of which 2 patients tested the mixed-cell variant of Hodgkin's lymphoma, in 1 case the Hodgkin's nodular lymphoma with lymphoid predominance. One patient with metabolically inactive lymph nodes affected by the mixed-cell variant of Hodgkin's lymphoma had HIV infection. The second patient with metabolically inactive lymph nodes with the presence of a verified biopsy material from them, confirming the presence of a mixed-cell variant of Hodgkin's lymphoma, did not have any distinctive features in the history. A patient with a metabolically inactive Nodular Hodgkin's lymphoma with lymphoid predominance noted an increase in lymph lymphomas in the course of several years.
| Histological type (N - number of patients) |
The minimum SUVmax value in the range |
The maximum value of SUVmax in the range |
Average SUVmax (± m) |
The number of patients with SUVmax in the affected structures is equal to or lower than the liver SUVmax |
| All types (N=131) |
1.4 |
40.0 |
1 4.1 ± 0.6 |
Five |
| Nodular sclerosis (N= 68) |
2.7 |
40.0 |
14.2 ± 0.9 |
One |
| Mixed cellvariant (N=35) |
1.4 |
26.0 |
13.1 ± 1.1 |
2 |
| Lymphoid depletion (N=13) |
3.2 |
22.5 |
12.5 ± 2.2 |
One |
| Lymphoid predominance (N=7) |
6.6 |
12.2 |
11.0 ± 3.4 |
0 |
| Nodular Hodgkin's lymphoma with lymphoid predominance (N=8) |
1.6 |
20.0 |
9.8 ± 2.1 |
One |
Table 1 Features of the metabolic activity of various histological variants of Hodgkin's lymphoma.
In patients without “B-symptoms,” the range of SUVmax in tumor masses ranged from 1.4 to 22.5, the average value was 10.1 ± 0.8. In patients with "B-symptoms", the range of SUVmax ranged from 5.3 to 40.0, on average - 16.3 ± 0.9.
In most cases, Hodgkin's lymphoma is characterized by a high level of FDG accumulation, commensurate with FDG accumulation of aggressive non-Hodgkin's lymphomas. Given the small fraction of Berezovsky-Sternberg-Reed tumor cells in the tumor masses, the high accumulation of FDG cannot be explained by the active glycolysis processes in these cells. Accordingly, the hypermetabolism of the tumor tissue is most likely due to an active metabolism in the cells of the microenvironment that secrete a large amount of biologically active substances. The biochemical processes occurring in tumor tissue in Hodgkin's lymphoma are similar to active inflammatory processes, in which active utilization of FDG is also observed. Consequently, most likely a high level of FDG absorption in Hodgina lymphoma is associated with the activity of normal immunocompetent body cells that form the microenvironment for few tumor cells. This theory is confirmed by the fact that patients with the presence of common symptoms in Hodgkin's lymphoma have a higher level of metabolic activity in tumor masses in comparison with patients in whom the disease proceeds without clinically severe intoxication. All variants of classical Hodgkin's lymphoma are characterized by a commensurate high level of metabolic activity, with nonclassical Hodgkin's lymphoma, the level of metabolic activity is on average somewhat lower, which can be explained by the greater tendency of NLHLP to an indolent clinical course.
A higher average level of FDG accumulation in tumor masses in patients with the presence of "B-symptoms" can be explained by the greater activity of the tumor process.
Conclusion
For lymphomas, Hodgkin's is characteristic of a high metabolic activity of tumor masses. Classical Hodgkin's lymphoma is characterized by high metabolic activity, comparable to all four variants, the metabolic activity of non-classical Hodgkin's lymphoma is on average somewhat lower than the metabolic activity of the classical variants. In the group of patients with clinically severe intoxication, the level of metabolic activity of the tumor masses was higher than in the group of patients with Hodgkin's lymphoma without “B-symptoms”. For a more accurate assessment of the dynamics of the treatment of Hodgkin's lymphoma, it is more expedient to perform primary staging prior to initiating therapy, since in some cases the initial low accumulation of FDG in tumor masses may mimic the absence of viable tumor tissue in the affected lymph nodes when assessing the dynamics of treatment.
Conflict of Interest
Authors declare no conflicts of interest arising from writing conflicts of interest.
24297
References
- Ilyin NV, Vinogradova YN (2008) Long-term effects of radiation and combination therapy for patients with Hodgkin's lymphoma. Clinical Oncohematology 1: 131-135.
- Kurpeshev OK, Pavlov VV, Shklyaev SS (2013) The effectiveness of local hyperthermia in chemotherapy and/or radiation treatment of relapsed Hodgkinlymphoma. Siberian oncological the Magazine 4: 28-30.
- Lymphomas EAM, Granova NV (2010) Non-hodgkin's lymphomas. Cancer Radiother 3: 222-240.
- Biggi A, Gallamini A, Chauvie S (2013) International validation study for interim PET in ABVD-treated, hodgkin lymphoma. J Nucl Med 54: 683-90.
- Barrington SF, Mikhaeel NG, Kostakoglu L (2014) Number of lymphomas: International Conference on malignant lymphomas imaging working group. J Clin Oncol 32: 3048-58.
- Cheson BD, Fisher RI, Barrington SF (2014) Hodgkin and non-Hodgkin lymphoma: The Lugano classification. J Clin Oncol 32: 3059-68.
- Rufan RE, Ryazanov I, Dergounova H (2005) Combined positron emission and computed tomography (PET-CT) in oncology S-pb: ELBI-SPb. Sovmeschennaya Pozitronno-Emissionnaya p: 124.
- WeilerSagie M, Bushelev O, Eppelbaum R (2010) 18 F - FDG Avidity in Lymphoma Readdressed: A study of 766 patients. J Nucl Med 51: 25-30.
- Tsukamoto N, Kojima M, Hasegawa M (2007) The usefulness of 18Ffluorodeoxyglucose positron emission tomography (18F-FDG-PET) and a comparison of 18F-FDG-PET with 67gallium scintigraphy in the evaluation of lymphoma: Relation to histologic subtypes based on the World Health Organization classification. Cancer 110: 652-659.
- Elstrom R, Guan L, Baker G (2003) Utility of FDG-PET scanning in lymphoma. WHO Classification Blood 101: 3875-3876.