Giovanni Battista Gervasi, Mara Baldacci and Marco Bertini*
R&D Department, Laboratori Baldacci SpA, Italy
Corresponding Author:
Marco Bertini
R&D Department, Laboratori Baldacci SpA
Italy
Tel: 39050313271
E-mail: bertini@baldaccilab.com
Received date: August 04, 2016; Accepted date: August 08, 2016; Published date: August 16, 2016
Citation: Gervasi GB, Baldacci M, Bertini M. Feralgine® a New Co-processed Substance to Improve Oral Iron Bioavailability, Taste and Tolerability In Iron Deficiency Patients. Arch Med. 2016, 8:4
Copyright: © 2016 Gervasi GB, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Oral Iron Bioavailability
Iron deficiency is the most common nutritional disorder worldwide and accounts for aproximately one half of anemia cases [1,2]. Iron deficiency anemia can result from inadequate iron intake, decreased iron absorption, increased iron demand and increased iron loss [3]. Oral iron therapy is the first line therapy to control iron deficiency but, unfortunately, adherence to oral iron therapy can be a barrier to treatment because of Gastro-Intestinal adverse effects such us epigastric discomfort, nausea, diarrhea and costipation [4,5]. These effects may be reduced when iron is taken with meals, but absorption may decrease by 40% [4,5]. Medication such us PPIs (Proton Pump Inhibitors) and events that induce gastric acid hyposecretion (chronic atrophic gastritis, recent gastrostomy or vagotomy) are associated with reduced absorption of dietary iron and iron tablets [4]. Diagnosis of iron deficiency anemia requires laboratory-confirmed evidence of anemia (Table 1) [5] as well as evidence of low iron stores (Ferritin levels low than 30 ng per ml) [5]. Ferrous Sulphate for oral route is still considered the gold standard for iron deficiency anemia also if a poor domiciliary compliance has been observed because of its gastro-intestinal adverse events. In order to improve patient’s compliance versus oral iron therapy a new and particular source of iron, ferrous bysglicinate chelate, has been developed and used in clinical practice [6-16]. Ferrous bysglicinate chelate consists of one molecule of ferrous iron bound to two molecules of glycine to form two heterocyclic rings (Figure 1) [6].
| Age |
Hemoglobin level (g per dl [g per L]) |
MCV (µm3 [fL]) |
| |
Mean |
Diagnostic at anemia |
Mean |
Diagnostic of microcytosis |
| 3 to 6 months |
11.5(115) |
9.5 (95) |
91(91) |
74(74) |
| 6 months to 2 years |
12.0(120) |
10 5 (105) |
78(78) |
70(70) |
| 2 to 6 years |
12.5(125) |
11.5 (115) |
81(81) |
75(75) |
| 6 to 12 years |
13.5(135) |
11.5 (115) |
86(86) |
77(77) |
| 12 to 18 years (female) |
14.0(140) |
12.0 (120) |
90(90) |
78(78) |
| 12 to 18 years (male) |
14.5(145) |
13.0 (130) |
88(88) |
78(78) |
| 20 to 59 years (white men) |
NA |
13.7 (137) |
90(90) |
80(80) |
| 60 years and older (white men) |
NA |
13.2 (132) |
90 |
80 |
| 20 years and older (white women) |
NA |
12.2 (122) |
90 |
80 |
| 20 to 59 years (black men) |
NA |
12.9 (129) |
90 |
80 |
| 60 years and cider (black men) |
NA |
12.7 (127) |
90 |
80 |
| 20 years and Oder (black women) |
NA |
11.5 (115) |
90 |
80 |
MCV: Mean Corpuscular Wolurne; NA: Not Available
Table 1: Age-related variations in hemoglobin level and MCV [17].
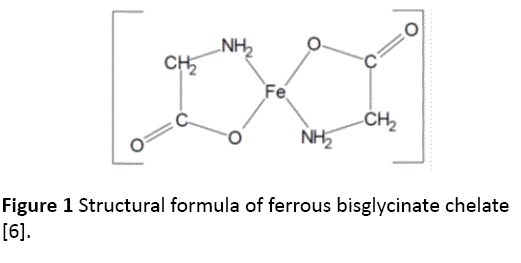
Figure 1: Structural formula of ferrous bisglycinate chelate [6].
The absorption of iron from ferrous bysglicinate chelate is regulated through the same physiological mechanisms as other inorganic forms of iron. Following oral administration, ferrous bysglicinate chelate adds to the intestinal intraluminal pool of inorganic, non-haem iron and is absorbed intact into the mucosal cells of the intestine, and is subsequently hydrolised into its iron and glycine components [6].
The intact absorbption of ferrous bysglicinate chelate directly into the mucosal cells of the intestine represents the main differences between this iron source and all the other iron salts making ferrous bysglicinate chelate more available and with less Gastro-Intestinal adverse effects after oral administration.
A lot of clinical trials shown clinical bioequivalence between Ferrous Sulphate at high elemental iron dosage and Ferrous Bysglicinate Chelate at low dosage (ratio 4 to 1) confirming the pharmacokinetic differences between the two sources of iron after oral administration [8-11].
Nevertheless, also Ferrous Bysglicinate Chelate treatment alone results in a number of adverse events that limit, also if at a less extent that Ferrous Sulphate, the patient’s domiciliary compliance to oral iron therapy.
Also the taste of the Ferrous Bysglicinate solution, because of “the iron taste” could represent a limit for patients compliance, especially in infants and in children. In order to ameliorate even more oral Iron bioavailabilty, tolerability and taste of Ferrous Bysglicinate Chelate a new compound named “FERALGINE®” has been developed.
FERALGINE® is a “co-processed compound” between two well Known substances: Ferrous Bysglicinate Chelate and Alginic Acid, two substances defined like G.R.A.S. (Generally Recognized As Safe) by F.D.A: by using spray drying technologies we have been able to “co-processsed” the two substances in a new one patent pending compound with a very attractive and interesting profile.
Spray drying technology, the technology that has been used to develop FERALGINE®, comes of age during World War II, with the sudden need to reduce the transport weight of foods and other materials.
This tecniques enables the transformation of feed from a fluid state into dried particulate form by spraying the feed into a hot drying medium [18,19]. It is a continuous particle processing drying operation. Spray drying process mainly involves five steps 1) Concentration: feedstock is normally concentrated prior to introduction into the spray dryer; 2) Atomization: the atomization stage creates the optimum condition for evaporation to a dried product having the desired characteristic; 3) Droplet-air contact: in the chamber, atomized liquids brought into contact with hot gas, resulting in evaporation of 95% of the water contained in the droplets in a matter of a few minutes; 4) Droplet drying: moisture evaporation takes place in two stages: during the first stage, there is sufficient moisture in the drop to replace the liquid evaporated at the surface and evaporation takes place at a relatively constant rate and the second stage begins when there is no longer enough moisture to mantain saturated conditions at the droplet surface, causing a dried shell to form at the surface. Evaporation then depends on the diffusion od moisture through the shell, which is increasing in thickness; 5) Separation: cyclones, bag filter, and electrostatic precipitators may be used for the final separation stage. Wet Scrubbers are often used to purify and cool air so that it can be released to atmosphere (Figure 2) [18,19].
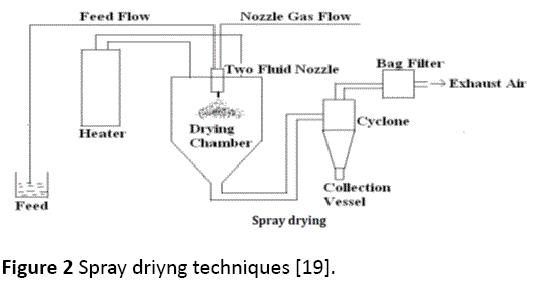
Figure 2: Spray driyng techniques [19].
Spray drying is one of the most exciting technologies for the pharmaceutical industry, being an ideal process where the end-product must comply with precise quality standards regarding particle size distribution, residual moisture content, bulk density and morphology. Alginic Acid salts have usually been used like “gastroprotector” for patients affected by GERD (Gastro Esophaegeal Reflux Disease), by gastric hyperacidity and by pyrosis [18,19]. By applying Spray Drying technology to a solution of Ferrous Bysglicinate Chelate and Alginic Acid we obtained a “new co-processed compound” in which alginic acid and Ferrous Bysglicinate Chelate are present in 1 to 1 ratio and in which every little particle of the powder has the same morphology and quantity of the two different co-processed substances. In the following picture making by stereomicroscopy Wild Heerbrugg Makroskop M420 linked to an OPTIKAM MICROSCOPY DIGITAL USB CAMERA, we could appreciated the uniformity of the content in the FERALGINE® powder (Figure 3) [20].

Figure 3: Feralgine powder (picture making by stereomicroscopy Wild Heerbrugg Makroskop M420 linked to an OPTIKAM MICROSCOPY DIGITAL USB CAMERA [20].
The new “Co-processed compound” obtained by spray drying technology, allow to iron powder an increased and uniform superficial area and, consequently, a quick and more extensive iron absorption together with an increase in gastrointestinal protection thanks to the more uniform alginic acid distribution in the FERALGINE® powder. In the same time, the uniform presence of alginic acid in the new co-processed compound obtained by a spray-drying technology, decreased iron taste in the final powder, increasing patient’s (but especially children) compliance to oral iron treatment. Concluding, FERALGINE®, a new patent-pending co-processed compound between Alginic Acid and Ferrous Bysglicinate Chelate, seems to be a new therapeutic option for oral iron therapy thanks to its new pharmacokinetic/pharmacodinamic properties that improve metabolism and taste of the well known Ferrous Bysglicinate Chelate.
10873
References
- World Health Organization (2001) Iron Deficiency Anaemia: Assessment, Prevention, and Control: A Guide for Programme Managers.
- Johnson-Wimbley TD, Graham DY (2011) Diagnosis and management of iron deficiency anemia in the 21st century. TherapAdvGastroentero l 4: 177-184.
- WHO Global Database on Anaemia (2008) Worldwide Prevalence of Anaemia 1993-2005.
- Ajmera AV, Shastri GS, Gajera MJ, Judge TA (2012) Suboptimal response to ferrous sulfate in iron-deficient patients taking omeprazole. Am J Ther 19: 185-189.
- Short MW, Domagalski JE (2013) Iron deficiency anemia: evaluation and management. Am Fam Physician 87: 98-104.
- Opinion of the Scientific Panel on Food Additives, Flavourings, Processing Aids and materials in Contact with Food (2006) Ferrous bisglycinate as a source of iron for use in the manufacturing of foods and in food supplements. The EFSA Journal299: 1-17.
- Ferrari P (2012) Treatment of mild non-chemotherapy-induced iron deficiency anemia in cancer patients: comparison between oral ferrous bisglycinate chelate and ferrous sulfate. Biomed Pharmacother66: 414-418.
- Bagna R (2016) Integrazionemarzialeoralenelneonatopretermine: confrontotraferrosolfato e ferrochelatobisglicinato.
- Ame C (2010) Trattamentodell’anemiasideropenica con ferrobisglicinatochelato (tecnofer) in pazienti con morboceliaco non responders a terapia con sali di ferro per os e/o e.v.
- Mazza GA (2014) Oral iron absorption test in children with celiac disease and iron deficiency anemia: new insight for a forgotten test. Dig Liver Dis 46: e114.
- Vivi G (2016) Efficacia del trattamento con ferrobisglicinatochelato in pazientipediatriciaffetti da anemia sideropenica.
- Pineda O, Ashmead HD (2001) Effectiveness of treatment of iron-deficiency anemia in infants and young children with ferrous bis-glycinate chelate. Nutrition 17: 381-384.
- Miglioranza LH, Matsuo T, Caballero-Cordoba GM, Dichi JB, Cyrino ES, et al. (2003) Effect of long-term fortification of whey drink with ferrous bisglycinate on anemia prevalence in children and adolescents from deprived areas in Londrina, Parana, Brazil. Nutrition 19: 419-421.
- Iost C, Jeppsen RB, Ashmead HD (1998) Repletinghaemoglobin in iron deficiency anaemia in young children through liquid milk fortification with bioavailable iron amino acid chelate. J Am CollNutr 17: 187-194.
- Rondinelli MB (2016) Efficacy of ferrous bisglycinate chelate for the management of preoperative anaemia in orthopaedic surgical patients: a prospective study J Blood Disord Transfus 7: 1.
- Szarfarc SC, de Cassana LMN, Fujimori E, Guerra-Shinohara EM, de Oliveira IMV (2001) Relative effectiveness of iron bis-glycinate chelate (Ferrochel) and ferrous sulphate in the control of iron deficiency in pregnant women. Arch. LatinoamNutr 51: 42-47.
- Van Vrankemn M (2010) Evaluation of microcytosis. Am Fam Physician 82: 1118.
- Patel RP, Suthar AM (2009) Spray drying technology: an overview. Indian J SciTechnol 2: 44-47.
- More SK, Wagh MP (2014) Review on spray drying technology. Int J Pharm ChemBiolSci 4: 219-225.
- Gervasi GB (2013) Feralgine© powder: stereomicroscopy study. Data on file Laboratori Baldacci s.p.a.









