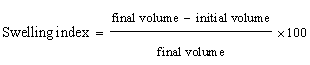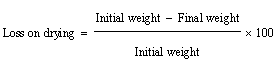Keywords
|
| Mouth dissolving tablet; Terbutaline sulphate; Coprocessed excipient; Ocimum bascilium; Superdisintegrant |
Introduction
|
| Oral solid dosage forms are most commonly preferred dosage form due to its ease of manufacturing, user friendly nature and capital interest also. It is for this reason that most drug delivery systems are currently administered in the form of tablets, capsules, powders, granules. Despite their popularity, an obvious limitation of oral solid dosage form is difficulty of swallowing. The difficulty in swallowing is called as dysphagia. Paediatric and geriatric patients are most commonly suffered due to number of reasons such as stroke, thyroid disorder, Parkinson’s disease and other neurological disorders like multiple sclerosis and cerebral palsy [1,2]. To offset the challenge of dysphagia, mouth dissolving formulation/Orally Dissolving Tablets (ODTs) has been developed. The European Pharmacopoeia describes ODTs as uncoated tablets intended to be placed in the mouth where they disperse rapidly before being swallowed as suspension and such tablets should disintegrate within 3 min [3]. FDA defines ODT as a solid dosage form which contains a medicinal substance or active ingredient which disintegrates rapidly within a matter of seconds when placed upon a tongue [4]. After coming in contact with saliva these formulations dissolve immediately and produce a suspension that can be easily swallowed by patient. |
| Numerous and significant changes in tablet manufacturing have occurred including transition from direct compression to wet and dry granulation. In wet granulation and dry granulation techniques multiple, lengthy and challenging processing steps are involved leading to higher cost and time of tablet production. The direct compression technique involves the compression of a dry blend of powders that comprises drugs and various excipients. The simplicity and low capital investment of the direct-compression process have positioned it as a preferred alternative. However, the direct compression process is highly influenced by functionality of parent excipients. Most formulations contain higher amount of excipients compared to the active drug and, as a consequence, excipients play a major role in deciding the formulation’s functionality and processability. To overcome these problems, the functionality of excipients can be improved by either developing new grades of excipients or modification of existing excipients. The development of new grade of excipient is time consuming and lengthy process and requires regulatory approval as well. Developing new grades of existing excipients or combination of existing excipients is successful alternative and that can be achieved by means of coprocessing [5]. |
| Co processing is a novel concept of processing two or more established excipients by some appropriate means to provide a synergy of functionality improvements as well as masking the undesirable properties of individual excipients. The functionality of excipients means improvement in flow properties, compressibility, and better dilution potential. These coprocessed excipients interact at sub particle level. Particle level comprises individual particle properties such as shape, size, surface area, and porosity that reflect in bulk level by improving functionality excipients [6]. |
| The new trend of using plant derived materials has evoked tremendous interest in pharmaceutical industry. Diversity of their applications such as diluents, binders, disintegrants in tablet make them as alternative to synthetic excipients. Gums and mucilage are most widely used as natural excipients in pharmaceutical manufacturing. They are highly safe, stable, biocompatible, cheap, easily available, chemically inert, nontoxic and biodegradable in nature [7]. The property of mucilage like high swelling index prompted to explore its applications as disintegrating agent in tablet manufacturing [8]. |
| The main objective of this work was to evaluate novel natural superdisintegrant as coprocessed ready to use excipient for direct compression in the development of mouth dissolving formulation. A comparative study was also made between coprocessed excipient developed from mucilage and coprocessed excipient developed from synthetic material. |
Materials and Methods
|
|
Materials
|
| Terbutaline sulphate was procured as a gift sample from Shimoga Chemicals, Sangli. Seeds of Ocimum bascilium were purchased from local vendor. Other chemicals and reagents were of AR grade and purchased from SD Fine chemicals, Mumbai. |
|
Methods
|
| Isolation of mucilage from Ocimum bascilium: The seeds were soaked in distilled water for 12 hrs and boiled for 30 min. for complete release of mucilage into the water [9]. The material was squeezed out using muslin cloth to remove the mark from the seeds. Acetone was added to precipitate the mucilage. The mucilage was separated, dried in oven at about 50-55°C and powdered. The powdered mucilage was stored in desiccator until further use. |
| Evaluation of mucilage: The mucilage was evaluated for flow properties, Swelling index, loss on drying and FTIR study [9,10]. |
| Angle of repose: A glass funnel was secured with its tip positioned at a fixed height (H) above a graph paper placed on a horizontal surface. The sample was poured through the funnel until the apex of the conical pile touched the tip of the funnel. The angle of repose was then calculated using following formula |
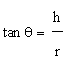 |
| Where θ is the angle of repose and r is the radius of the pile. |
| Carr’s index and Hausner’s ratio: It measures the unsettled apparent volume and the final tapped volume of the powder after tapping the material until no further volume changes occur. |
| The Carr’s index is calculated as |
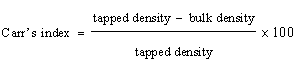 (2) (2) |
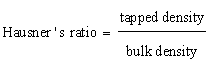 (3) (3) |
| Swelling index: A known volume of powder was placed in a graduated cylinder and consider as initial volume. Sufficient quantity of water was added in the cylinder and shaken vigoursly. It was placed for 24 hrs. Volume occupied by powder after 24 hrs was measured. It is calculated as |
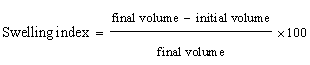 |
| Loss on drying: 1 gm of powder was placed in hot air oven at 100°C for 1 hr. Again weight of powder was measured. It is calculated as: |
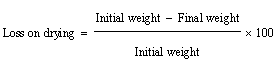 |
| FTIR study: The Fourier Transform Infra-Red (FTIR) spectroscopy study of mucilage was carried out to identify the functional group present in material. Mucilage and dried KBR were mixed in ratio 1:100 for spectroscopic analysis. Then small fraction of mixture was compressed on Automatic IR Press at pressure 10 tones to form transparent pellet. Then the IR spectrum of pellet was taken on FTIR spectrophotometer (Shimadzu 8400S, Kyoto, Japan). |
|
Preparation of coprocessed superdisintegrant
|
| A blend of Mannitol- Mucilage was added to 65 ml of isopropyl alcohol in different concentrations. The contents of the beaker were stirred on a magnetic stirrer [11]. The temperature was maintained at 65-70°C, and stirring was continued till most of isopropyl alcohol evaporated. The wet coherent mass was then granulated through sieve no. 30. The wet granules were dried in a tray dryer at 60°C for 20 minutes and stored in airtight container till further use. |
| It was evaluated for different flow properties and disintegration time. Placebo tablets were prepared containing coprocessed mucilage as superdisintegrant in different concentration and evaluated for disintegration time. A comparison was made between coprocessed mucilage and coprocessed Sodium Starch Glycolate (SSG) which was prepared using similar method described above. |
|
Preparation of mouth dissolving tablet
|
| The selected concentration of coprocessed mucilage was used in the preparation of mouth dissolving tablet. It was mixed with drug and other tablet excipients and compressed into tablet using flat-faced 6 mm punches on an eight-punch tablet machine (Karnavati Rimek). |
|
Evaluation of mouth dissolving tablets
|
| Developed tablets were evaluated for usual tablet tests such as weight variation, hardness, friability, drug content. The evaluation was carried out as described in the Pharmacopoeia [12]. Disintegration time and in vitro drug release was done by in house method. A modified method was used to determine the disintegration time and dissolution profile of the tablets simulating the conditions similar to mouth cavity or oral cavity absorption. For this purpose, a beaker was filled with 10 ml of water. The tablet was put in the beaker and the time for the tablet to completely disintegrate into fine particles was noted. |
|
Drug excipients compatibility study
|
| The drug-excipients interaction study was carried out using method descried in Cartensen and analysis done using FTIR spectrophotometer [13]. The physical interaction between Terbutaline sulphate, mucilage and SSG was studied in the hot air oven at temperature 55 ± 2°C for a period of two weeks and observed for caking, liquefaction, color change or any other incompatibility. |
|
Stability study
|
| The stability studies were carried out for the optimized formulation [14]. The samples were stored at 40 ± 2°C and 75 ± 5% RH for three month to access their stability. The protocol of stability studies was in compliance with ICH guidelines for stability testing intended for the zone IVa. After 30 days the samples were withdrawn and characterized for weight variation, hardness, disintegration time, drug content and in vitro drug release study. |
Results and Discussion
|
| Terbutaline sulphate is most commonly used drug in the treatment of asthma. Mouth dissolving formulation of Terbutaline sulphate would be useful in order to avoid swallowing problem and achieve a rapid onset of action. Requirements for a suitable tableting excipient for direct compression of ODTs are very diverse. First of all, the excipient should have good flow properties to achieve an acceptable tableting process and second one is to produce ODTs with fast disintegrating property. The flow properties may be improved by the method of coprocessing which minimizes number of excipients and improves its functionality. The mouth dissolving tablets should disintegrate in less than 30 seconds in a small liquid volume and should have sufficient strength in order to be handled during packaging and transportation [4]. In the present study, we successfully developed directly compressed tablets with sufficient hardness although exhibiting rapid disintegration. The natural mucilage has been explored as super disintegrants due to its high swelling property. Mannitol was selected to be the tablet excipient because it has favorable characteristics in developing mouth dissolving tablets, such as pleasant taste and “mouth feel,” and it is suitable for direct compression [15]. In addition, Mannitol is non-hygroscopic and may thus be used with moisture-sensitive ingredients. |
|
Characterization of mucilage
|
| The mucilage which was isolated from the seeds of Ocimum bascilium was evaluated for flow properties, Loss on drying and swelling index. Results of the evaluation study have been depicted in Table 1. The angle of repose was found to be 37.68° whereas the Carr’s index value was found to be 18.76% and Hausner’s ratio was 1.22. The results reveal that mucilage exhibited the fair flow properties. The purpose of the present study was to evaluate novel superdisintegrant. Super disintegrants are generally used for developing mouth dissolving tablets which has the requirement of faster disintegration. One of the mechanisms of super disintegrant is disintegration by swelling and the mucilage showed excellent swelling property that can be used as a super disintegrant in the preparation of mouth dissolving tablet. It has excellent swelling index that is 433.33%. The loss on drying was also found to be less than 5%. |
| The FTIR spectra of the mucilage (Figure 1) it is observed that, the peaks at 2922.59 cm-1 indicate aldehyde stretching, the peak at 2851.24 cm-1 confirms presence of C-H alkane stretching and the peaks at 3567.66 cm-1 indicate presence of OH and NH groups. |
| Direct compression is highly influenced by powder characteristics such as flow ability, compressibility and dilution potential. A material to be used for direct compression process should possess an adequate level of flow ability when blending with other ingredients in formulation to ensure a uniform die filling of a powder blend during tableting. Coprocessed excipient having good flow ability is an important requirement for a direct compression excipient. Most formulations contain higher amount of excipients compared to the active drug and, as a consequence, excipients play a major role in deciding the formulation’s functionality and processability. However, the numbers of excipients that can actually fulfill such performance requirements are limited. Several bulk powder characteristics have been employed for indirect estimation of the degree of powder flow. |
| To evaluate the flow properties of coprocessed mucilage, the tests like angle of repose, Carr’s index and Hausner ratio was done, which was compared with the parent materials. Table 2 depicts the flow properties before and after coprocessing. It is clear from the evaluation results that coprocessing improved the flow properties. |
| Powder flow depends on 3 general areas: 1) physical properties of the particle (e.g., shape, size, compressibility): 2) bulk powder properties (e.g., size distribution, compaction): and 3) processing environment (e.g., storage, humidity). In coprocessing the substance interacted at sub particle level that comprises the particle shape, size that gives improved flow property. The fundamental powder properties such as morphology, particle size, shape, surface area, porosity and density influences excipient functionalities such as flow ability, compactability, dilution potential, disintegration potential and lubricant potential. Also if a physical mixture of super disintegrant is used in high speed tableting, the problem of segregation of the super disintegrants may be encountered. One of the reasons for preparing the coprocessed super disintegrant was to avoid the problem of segregation. |
| Mucilage showed fair flow properties which after coprocessing when interacted at sub particle level was improved to excellent flow property. The different concentrations of Mannitol: Mucilage and Mannitol: SSG was coprocessed by solvent evaporation method. To investigate the versatility of the coprocessed superdisintegrant, they were compressed into tablet form without API to assess the disintegration time. Different ratios along with its disintegration time have been depicted in Tables 3 and 4 respectively. |
| These coprocessed excipients were compressed into tablet form at different concentrations 2.5%, 5% and 7.5% and evaluated for disintegration time. The optimum disintegration time of the coprocessed mucilage shows the DT upto 8 sec. at the ratio of 1 gm: 65 mg when tablet was formed containing 7.5% coprocessed excipient. The coprocessed SSG shows disintegration time upto 11 sec. The coprocessed mucilage shows the appropriate disintegration time. In water, sodium starch glycolate swells to up to 300 times its volume whereas mucilage swells upto 433.33%. Due to its high swelling index the coprocessed mucilage shows faster disintegration than the coprocessed SSG. The comparative study between coprocessed SSG and coprocessed mucilage was done (Figure 2). |
|
Preparation of mouth dissolving tablet
|
| As given in Table 5, Terbutaline sulphate and coprocessed superdisintegrant were individually weighed and mixed thoroughly for about 5 min. Mannitol and magnesium stearate were blended with the above mixture for about 2 min. The composition was compressed into tablets with 6 mm s/c diameter using a single-punch tablet machine at a fixed compression force. |
|
Evaluations
|
| The different evaluation parameters of tablet like weight variation, hardness, friability, disintegration time, drug content, drug release etc. were measured. Weight variation is important quality attributes for lowdose dosage forms. The weight variation with all of the formulations was very insignificant, that is, all tablets fulfilled the requirements of Indian Pharmacopoeia for weight variation [15]. Hardness of the tablet was measured by using Monsanto hardness tester and expressed as Kg/ cm2. The hardness of the tablets was maintained in the range of 2-3 kg and the friability results were found to be within the acceptable limits (<1%) which suggested that ODTs ability to withstand abrasion in handling, packaging and shipment. |
| To simulate the mechanical stress applied to mouth dissolving tablet in multi-dose containers, the friability method was used. For the friability, twenty undusted tablets were weighted, put in the friabilator (Electrolab, EF-1W) for 100 cycles and re-weighted. The percent loss of a tablet mass was calculated. All results were within the given limits. To simulate the environment of oral cavity, a modified method was developed for the assessment of disintegration time and dissolution profile of drug. A beaker was filled with 10 ml of water. The tablet was put in the beaker and whole assembly was gently stirred, the time for the tablet to completely disintegrate into fine particles was noted [16]. The disintegration time was measured in 10 ml water by considering the small volume of saliva (Figure 3). |
| To determine dissolution profile, same principle was used as in disintegration test that small volume of saliva. Drug release was carried out in 25 ml of phosphate buffer pH 6.8. Water bath containing ample quantity of water was maintained at 37°C. A beaker was filled with dissolution medium and suspended in water bath. The agitation was provided by shaft of mechanical stirrer at 100 rpm. Samples (2.5 mL) were withdrawn at periodic time intervals and replaced with fresh media and analyzed using UV-Vis spectrophotometrically at 276 nm [17]. |
| The coprocessed mucilage having DT upto 7 sec and shows drug release upto 99.1% in 4 min. Coprocessed SSG shows DT upto 11 sec and % CDR upto 98.06% in 6 min. The tablet containing coprocessed mucilage as a superdisintegrant shows fast disintegration and drug release than coprocessed SSG. The results are depicted in Table 6. |
|
Drug excipients compatibility studies
|
| IR study was used to check the compatibility between drug and polymer. Figure 4 shows a sharp peak of drug. This indicates that there is no interaction between drug and polymer used in the formulation i.e., the drug and polymer is compatible with each other. A similar functional group of Terbutaline was observed in Figure 4. This indicates that there is no interaction between drug and polymer used in the formulation i.e., the drug and polymer is compatible with each other. |
|
Stability studies
|
| The stability studies were carried out for the optimized formulation. The optimized formulation did not show any significant change in drug content when kept at different conditions and periods. No significant differences in values of % drug release observed during the stability studies. It indicates that irrespective of concentration of polymer, this formulation was able to retain its stability. |
Conclusion
|
| Mouth dissolving tablet of Terbutaline sulphate containing Ocimum bascilium as a superdisintegrant was prepared successfully. Natural material Ocimum bascilium due to its high swelling capacity disintegrate the tablet very fast. The coprocessed excipient improves its flowability of parent excipients. Hence it may represent as a new alternative, natural and cheaper superdisintegrant for mouth dissolving tablet which may improve the patient compliance. |
Acknowledgements
|
| Authors are thankful to Principal, SSDJ College of Pharmacy, Neminagar, Chandwad for providing all the necessary facilities. Also grateful to Shimoga chemicals, Sangli providing free gift sample of drug (Terbutaline sulphate). |







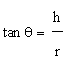
 (2)
(2)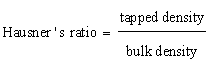 (3)
(3)