Keywords
Aceclofenac, floating microspheres, Eudragit S 100, Eudragit RL 100, emulsion solvent diffusion developed in an attempt to release the drug slowly in to the GIT and maintain a constant drug concentration in the serum for longer period of time. Such oral drug delivery devices have a restriction due to the gastric retention time (GRT), a physiological limitation[1-2]. Therefore, prolonged gastric retention is important in achieving control over the GRT because this helps to retain the CR system in the stomach for a longer time in a predictable manner[3-4].
Thus the real issue in the development of oral controlled release dosage form is not just to prolong the delivery of the drugs, but to prolong the presence of the dosage forms in the stomach or somewhere in the upper small intestine until all the drug is released for the desired period of time. It may improve bioavailability and reduce drug waste.
Over the last three decades, various approaches have been pursued to increase the retention of an oral dosage form in the stomach, including floatation system[5-6], bioadhesive systems, which adhere to mucosal surface[7-8], density controlled system, which either float or sink in gastric fluid[9], swellable delivery system, which increase in size after swelling and retard the passage through the pylorus[10], modified shape systems[11], magnetic systems[12], superporous hydrogel system[13] and other delayed gastric emptying devices.
In fact, the buoyant dosage unit enhances GRT without affecting the intrinsic rate of emptying[14]. Unfortunately, floating devices administered in a single-unit form such as hydrodyanamically balanced system (HBS)[15] are unreliable in prolonging the GRT owing to their ‘all-or-nothing’ empty process[16] and, thus, they may cause high variability in bioavailability and local irritation due to a large amount of drug delivered at a particular site of the GIT. While multiple unit particulate dosage form (e.g. microspheres) have the advantages that they pass uniformly through the GIT to avoid the vagaries of gastric emptying and provide an adjustable release, thereby reducing the intersubject variability in absorption and risk of local irritation[17]. The concept of floating microspheres can also be utilized to minimize the irritant effect of weakly acidic drugs on the stomach by avoiding direct contact with the mucosa and providing a mean of getting low dosage for prolonged periods.
The object of the present investigation is to develop floating drug delivery system of aceclofenac to minimize the irritant effect of drug on the stomach by avoiding direct contact with the mucosa and providing a mean of low dosage for prolonged period. Aceclofenac is a novel NSAID indicated for the symptomic treatment of pain and inflammation. The mean plasma elimination half life is 4 h. The side effect is dyspepsia, abdominal pain, diarrhea, nausea, dizzieness, and gastric constipation. To reduce the dosing frequency and adverse effect during prolong treatment, it is needed to formulate aceclofenac in long acting dosage form[18].
Material and Methods
Materials
Aceclofenac was received as a gift sample from Aristo Pharmaceutical Pvt Ltd (Mandideep, India). Eudragit S 100 and Eudragit RL 100 were purchased from Rohm Pharma (Darmstadt, Germany. Ethanol was obtained from Sakthi Sugar Pvt Ltd (Erode, India) Dichloromethan and sodium lauryl sulphate was purchased from S.D. Fine Chem. Ltd (Mumbai, India). All other chemicals were of analytical grade.
Preparation of floating microspheres of aceclofenac
Floating microspheres containing aceclofenac were prepared using emulsion- solvent diffusion technique19-20. The drug to polymer ratio used to prepare the different formulations was as shown in table 1. The drug polymer mixture dissolved in a mixture of ethanol (8 mL) and dichloromethane (8 mL) was dropped in to 0.2% sodium lauryl sulfate solution (400 ml). The solution was stirred with a propeller-type agitator at room temperature for 1 h at 500 rpm. The formed floating microspheres were filtered, washed with water and dried at room temperature in a desicator.
| Formul ation code |
Mean particle size* (µm) |
True density* (g/cm3) |
Tapped density* (g/cm3) |
Compres sibility index* % |
Porosity* |
Angle of repose* (°) |
| AP1 |
273.70±17.55 |
0.714±0.022 |
0.415 ± 0.005 |
14.43 ± 1.38 |
43.57±1.12 |
28°76' ±0.78' |
| AP2 |
310.33±13.97 |
0.723 ±0.016 |
0.422 ± 0.011 |
15.34 ± 1.89 |
41.43±1.78 |
31°54' ±0.65' |
| AP3 |
338.87 ± 10.29 |
0.743 ±0.026 |
0.432 ± 0.006 |
16.62 ± 1.67 |
39.68±1.73 |
33°45 '±0.92' |
| AP4 |
355.08 ± 13.41 |
0.748 ±0.017 |
0.439 ± 0.01 |
17.32 ± 1.23 |
39.38±1.52 |
32°21' ±1°82' |
| AP5 |
319.47 ± 15.24 |
0.744 ±0.023 |
0.425 ± 0.005 |
16.48 ± 2.12 |
42.4.±1.43 |
34°65' ±0.59' |
| AP6 |
303.89±8.65 |
0.738 ±0.024 |
0.420 ± 0.006 |
18.34 ± 2.32 |
43.45±1.54 |
38°46 '±0.82' |
| AP7 |
293.14±19.15 |
0.729 ±0.034 |
0.418 ± 0.009 |
17.34±1.45 |
45.43±1.54 |
40°07' ±0.53' |
Table 1 : Micromeritics properties of different floating microspheres
Average of three preparation ± S.D.
| Formulation code |
Percentage yield* (%) |
Drug entrapment*
(% w/w) |
| AP1 |
61.24 ±1.71 |
64.78 ±1.76 |
| AP2 |
64.8 ±2.38 |
69.78 ±1.07 |
| AP3 |
68.97±3.81 |
76.94 ±2.14 |
| AP4 |
60.25 ±1.63 |
79.12 ±2.85 |
| AP5 |
71.99 ±1.52 |
76.94 ±2.14 |
| AP6 |
79.04 ±0.6 |
73.18± 2.81 |
| AP7 |
79.55 ± 0.96 |
67.09 ±2.14 |
Table 2: Percentage yield and entrapment drug content of floating microspheres. * Average of three preparation ± S.D.
Micromeritic properties
The microspheres were characterized by their micrometric properties, such as particle size, true density, tapped density, compressibility index (% CI: a value useful in prediction of flowabiltiy), porosity and flow properties. The particle size was measured using an optical microscope and the mean particle size was calculated by measuring around 200 particles with the help of a calibrated ocular micrometer. The tapping method was used to determine the tapped density and percentage compressibility index as follows.
Tapped density = Mass of floating microspheres / Volume of floating microspheres after tapping
% Compressibility index = [1-V/VO] ×100
Where V and V0 are the volumes of the sample after and before the standard tapping respectively. The true density of floating microspheres was determined by liquid displacement method using n-hexane as solvent[21]. Porosity (e) of the floating microspheres was calculated using the following equation[22].

Where Pt and Pp are the true density and tapped density respectively. Angle of repose (q) of the floating microspheres, which measures the resistance to particle flow, was determined by a fixed funnel method and calculated as

where 2 H/D is the surface area of the free standing height of the microshperes heap that is formed on a graph paper after making the microspheres flow from the glass funnel.
Scanning electron microscopy
The floating microsheres were examined for surface morphology and shape using scanning electron microscope (Jeol JSM – 5610, Japan). Samples were fixed on carbon tape and fine gold sputtering was applied in a high vacuum evaporator. The acceleration voltage was set at 20KV during scanning. Microphotographs were taken on different magnification.
Drug entrapment and yield of floating microspheres
The floating microspheres containing drug were dissolved in a mixture of dichloromethane and ethanol (1:1 v/v) by ultrasonication. The dissolved drug amount was measured by UVspectrophotometer (UV-1601 Shimadzu, Japan) at 274 nm after suitable dilution. No interference was found due to the other components at 274 nm. Drug entrapment and yield were calculated as follows.
% Drug entrapment = Calculated drug concentration / Theoretical drug concentration x100
% Yield = [ Total weight of floating microspheres / Total weight of drug and polymer] x 100
Fourier transform infra-red spectroscopy (FT-IR) analysis
The FT–IR analysis was conducted for the analysis of drug polymer interaction and stability of drug during microencapsulation process. Fourier transform infrared spectrum of pure aceclofenac, Eudragit S 100, Eudragit RL 100 and floating microspheres were recorded using FT–IR spectrophotometer (IR-470, Shimadzu, Japan).
In vitro evaluation of floating ability
Fifty milligrams of the floating microspheres were placed in 0.1 N HCl (100 mL) containing 0.02% Tween 20. The mixture was stirred at 100 rpm in a magnetic stirrer. The layer of buoyant microspheres was pipetted and separated by filtration at 1, 2, 4 and 8 hours. The collected microspheres were dried in a desiccator over night. The percentage of floating microspheres was calculated by the following equation : %Floating microspheres = (Weight of floating microspheres / Initial weight of floating microspheres) x100
In vitro release studies
The drug release rate from floating microspheres was carried out using the USP dissolution paddle assembly (Elico Lab, Mumbai). A weighed amount of floating microspheres equivalent to 100 mg drug were dispersed in 900 mL of 0.1 N HCl (pH 1.2) maintained at 37 ± 0.5°C and stirred at 100 rpm. At preselected time intervals sample was withdrawn. The collected samples were suitably diluted with 0.1 N HCl and analysed spectrophotometrically at 274 nm. The initial volume of the dissolution fluid was maintained by adding same volume of fresh dissolution fluid after each withdrawal. The dissolution studies were repeated using phosphate buffer pH 6.8 as dissolution medium.
All the previous experiment were done in triplicate
Kinetic modeling of drug release
The dissolution profile of all the batches was fitted to zero-order, first order, Higuchi[23] models to ascertain the kinetic modeling of drug release.
Results and Discussion
Micromeritic properties
The particle size was determined by optical microscopy. The particle size of floating microspheres plays important role in floating ability and release character of drug from microspheres. Smaller the microspheres floating ability will be less and faster will be the release rate of the drug form the microspheres, while larger the size, floating ability will be more and sustained will be the release of drug. In Eudragit microspheres the mean particle size of microspheres was increased with the increasing Eudragit S 100 concentration. The viscosity of the medium increase at a higher polymer concentration resulting in enhanced interfacial tension. Shearing efficiency is also diminished at higher viscosities, result in the formation of larger particles. The mean size was influenced by the content and type of Eudragit used and its ratio in the formulation. The mean particle size ranged from 273.7 ± 17.55 to 355.08 ± 13.41 for Eudragit S 100 (table 2). The tapped density values ranged from 0.415 ± 0.005 to 0.439 ± 0.01 g/cm3, while their true density ranged between 0.714 ± 0.022 to 0.748 ± 0.017 g/cm3 of all the formulations obviously, the density values of the floating microspheres were less than that of the gastric fluid (~1.004 g/cm3) thereby, employing that these floating microspheres will have the propensity to exhibit an good buoyancy effect in vivo. The porosity of all the formulations was found to be in the range of 39.38 ± 1.52 – 45.43 ± 1.54%. All formulation showed excellent flowability as represented in terms of angle of repose. Percentage compressibility value ranged between 14.43 ± 1.38 to 18.34 ± 2.32% suggested excellent flowability of floating microspheres (table 1).
Morphology
Scanning electron microscopic photographs of floating microspheres are shown in fig. 1 (A to D). Floating microspheres were spherical with no visible major surface irregularity. Few wrinkles and inward dents were appeared at the surface. It may be due to the collapse of the floating microspheres during the in situ drying process. Formulation formed entirely of ES 100 were observed to be fairly spherical, sphericity was decreasing when the content of Eudragit RL 100 increased. The surface morphology of floating microspheres was examined at higher magnifications (fig 1 C & D), which illustrate the smooth surface of floating microspheres. Some small pores and cavities were present on the surface of floating microspheres, probably arising as a trace of solvent evaporation during the process.
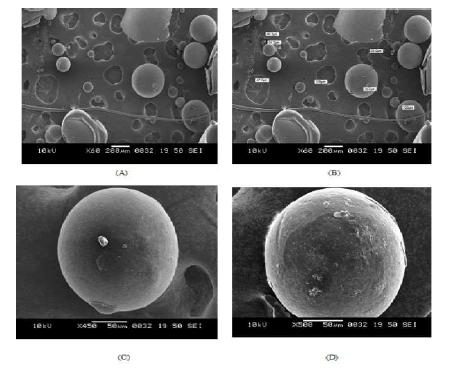
Figure 1. Scanning electro microphotographs of Eudragit floating microspheres. (A and B) lower magnification (C and D) higher magnification
Drug entrapment and yield of floating microspheres
The entrapment efficiency of the drug depended on the solubility of the drug in the solvent and continuous phase. An increase in the concentration of polymer in a fixed volume of organic solvent resulted in an increase in entrapment efficiency. Notably, In case of Eudragit S 100 microspheres, the entrapment efficiencies of the microspheres prepared with Eudragit S 100 were higher than those of microspheres prepared with the Eudragit S/RL combination. The drug retained decreased with the increase in Eudragit RL 100 content of floating microparticle. This could be due to the permeation characteristics of this polymer that could facilitate the diffusion of a part of entrapped drug to the surrounding medium during preparation of floating microspheres. The entrapment efficiencies ranged from 64.78 ±1.76% to 79.12 ±2.85% for Eudragit S microspheres.. The percentage yield of different formulations was determined by weighing the floating microspheres after drying. In preliminary trial, the percentage yield of different formulations for Eudragit S 100 was in the range of from 60.25 ±1.63 to 79.55 ± 0.96. In general, percentage yield was increasing with increased concentration of Eudragit S 100 up to a certain concentration than deceased but on adding Eudragit RL 100, percentage yield was increased.
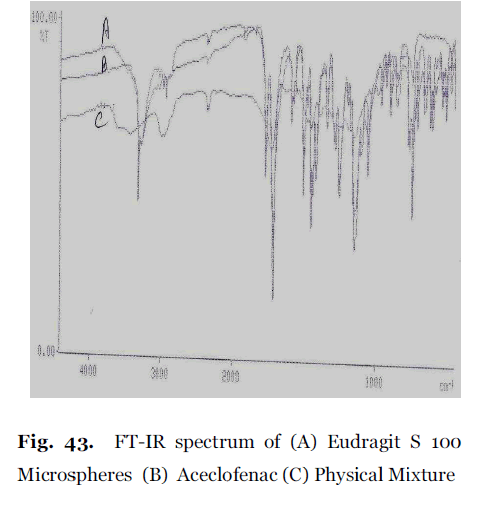
Figure 2. FT-IR spectrum of (A) Eudragit S 100 Microspheres (B) Aceclofenac (C) Physical Mixture
Fourier transform infrared spectroscopy(FT-IR) analysis
The FT-IR spectra of the aceclofenac, polymer, physical mixture of drug- polymer and formulated floating microspheres were recorded. Pure aceclofenac showed 3319.7, 2971.0, 2937.5, 1835.6, 1772.0, 1749.1, 1717.5, 1583.6, 1508.3, 1362.0, 1271.6, and 669.3 wave number as major peaks due to C=O stretching, OH stretching CH stretching superimposed on OH stretching, NH stretching and C-CL respectively. In Eudragit S 100 microspheres, The characteristic peaks due to pure aceclofenac appeared at 3319.9, 2972.1, 2937.5, 1835.6, 1772.1, 1748.5, 1717.6, 1583.9, 1508.4, 1366.2, 1271.6, and 670, without any markable change in their position after successful encapsulation, indicating no chemical interaction between aceclofenac, Eudragit S 100 and Eudragit RL 100. It also confirmed the stability of drug during microencapsulation process.
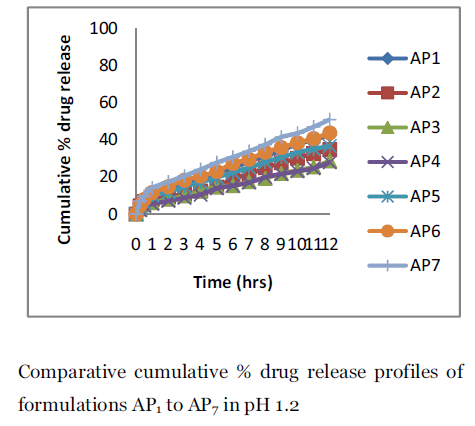
Figure 3. Comparative cumulative % drug release profiles of formulations AP1 to AP7 in pH 1.2.
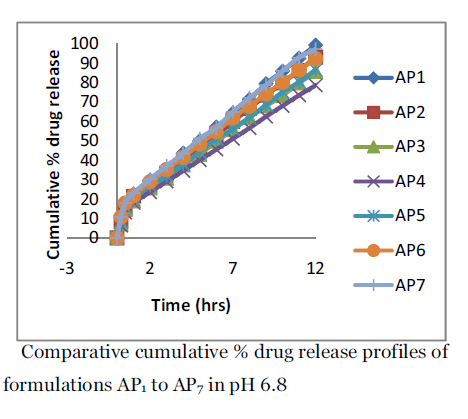
Figure 4. Comparative cumulative % drug release profiles of formulations AP1 to AP7 in pH 6.8.
In vitro evaluation of floating ability
The purpose of preparing floating microspheres was to extend the GRT of the drug. The floating ability test was carried out to investigate the floatability of the prepared microspheres. The floating test was carried out to investigate the floatability of the prepared microspheres in 0.1 N HCl (pH 1.2) containing 0.02 % Tween 20. Tween 20 was added to counteract the downward pulling at the liquid surface by lowering surface tension.
In Eudragit floating microspheres, the floating ability pattern differed according to the formulation tested and the medium used suggesting interplay between various factors. In preliminary trial, formulation AP6 gave the best floating ability as evidenced by the percentage of particles floated at different time intervals (Table 4). Although the formulation contains a percentage of the permeable ERL, yet the surface of floating microspheres is regular. This would decrease points of contact and therefore the opportunity of aggregate formation upon hydration. The floating properties of microspheres may be attributed to the low bulk density and the porosity of the floating microspheres, implying that the floating microspheres will have the propensity to exhibit an excellent buoyancy effect in vivo. Stirring speed had negative effect on the floating ability. This may be due to decreasing the particle size of floating microspheres on increasing the stirring speed.
| Form ulatio n code |
1 hrs* |
2 hrs* |
4 hrs* |
8 hrs* |
| AP1 |
88.31 ±1.13 |
85.67±1.45 |
80.87±1.93 |
64.53 ± 3.78 |
| AP2 |
94.62 ±1.81 |
91.89±2.12 |
83.78±1.23 |
69.32 ± 1.58 |
| AP3 |
97.54±1.32 |
92.43±1.45 |
88.34±1.56 |
75.30 ±2.27 |
| AP4 |
98.32±1.42 |
96.54±1.78 |
92.55±2.13 |
80.97 ±3.78 |
| AP5 |
96.45±0.98 |
93.67±1.98 |
89.13±1.86 |
72.22 ± 2.83 |
| AP6 |
98.78±0.43 |
95.45±1.76 |
92.87±2.87 |
80.64 ± 1.89 |
| AP7 |
92.72±2.24 |
87.53±1.65 |
82.65±2.87 |
68.15 ± 3.10 |
Table 3 : Percentage floating of different formulations of floating microspheres in 0.1 N HCl (ph 1.2) containing 0.02 % Tween 20.
In vitro drug release study
In vitro dissolution studies of aceclofenac from floating microspheres were performed in 0.1 N HCl (pH 1.2) and pH 6.8 for 12 hours using USP dissolution test apparatus I. It was found that formulations AP1 showed 42.83±0.77 release while AP4 showed of 27.89±1.032 release in 0.1N HCl in 12 hours.. Eudragit S100 polymer, which is present in all formulations, is of low permeability and insoluble in acid medium. It is an anionic copolymer of methacrylic acid, methyl methacrylate containing free carboxylic and ester groups. It’s very low permeability results from high intermolecular attraction between its molecules. Hydrogen bonding between the hydroxyl groups of the carboxylic moiety and the carbonyl oxygen of ester groups increase the degree of compactness of the polymer and decrease its porosity and permeability. Because of these characters, drug release rate from floating microspheres prepared only by Eudragit S 100 was very slow and incomplete in 0.1 N HCl. The rate of drug release from the microspheres depended on the polymer concentration of the prepared devices, which indicates that the release rate decrease with increasing the amount of the polymer.
On the other hand, ERL is a copolymer of acrylic and methacrylic acid esters with a low content of quaternary ammonium groups. The ammonium groups present as salts give rise to permeability and act, after their dissolution, as channeling agents for the entrance of the dissolution medium through the floating microparticle wall causing its swelling. This gives an opportunity for the dissolved drug to diffuse out to the bulk medium. To increase drug release considerably, the more quantity of Eudragit RL 100 was added to achieve further drug release. For formulations AP5, AP6 and AP7, the drug release was 36.68±1.37% to 50.96±1.81% in 12 hours. The results obtained at pH 6.8 showed a different trend. While the formulation formed of ES100 only, showed the least amount released in 0.1 N HCl, it gave an appreciable release in buffer pH 6.8. ES100 is an enteric coating material. So, it will start to dissolve at pH 6.8 due to ionization of carboxylic acid groups, thus allowing penetration of solvent and liberation of the drug. Formulation AP1 gave 99.25 ± 1.53% release and AP4 gave 78.55 ± 1.69% release in pH 6.8, while from formulation AP5 to AP7 showed a mixed effect due to the presence of two different types of Eudragit. Both the high degree of hydration of ERL leading to formation of hydrated channels, associated with the dissolution of ES100, determined the release of drug at pH 6.8. Formulation AP5 to AP7 gave 86.58 ± 2.11% to 97.14 ± 2.76 % drug releases.
Kinetic modeling
The release data of formulations were fitted to models representing zero order, first-order and Higuchi’s square-root of time model to predict the drug release kinetics and mechanism. The release constant was calculated from the slop of appropriate plots, and the regression coefficient (r2) was determined. It was found that the in-vitro drug release of floating microspheres was best explained by zero order kinetic as the plots showed the highest linearity and it indicated a time-independent release process.
Conclusion
Gastro-retentive floating microspheres have emerged as an efficient means of enhancing the bioavailability and controlled delivery of many drugs. The present formulation study of aceclofenac was performed in an attempt to prepare floating drug delivery system of floating multiple unit system using emulsion-solvent diffusion technique and the performance of these formulations was evaluated. The ideal properties of floating microspheres are a high buoyancy and sufficient release of drugs in pH 6.8 solution. In developing a desired intragastric floating system employing these microspheres, it is necessary to select an appropriate balance between buoyancy and drug releasing rate and formulation AP6 showed the best appropriate balance between buoyancy and drug release rate.
The designed system AP6 combining high buoyant ability and suitable drug release rate, could possibilily be advantageous in terms of increased bioavailability of aceclofenac. The designed system AP6might be able to float in the stomach. This phenomenon could prolong the gastric residence time (GRT) and delay drug arrival at the absorption site; consequently, the sustained action provided, in addition, floating microspheres enabled increased drug absorption rate of drug as the floating microspheres in the stomach gradually sank and arrived at the absorption site. Same time the prepared formulation will minimize the irritant effect of aceclofenac on the stomach by avoiding direct contact with the mucosa and providing a mean of low dosage for prolonged period. Therefore, multiple unit floating system, i.e, floating microspheres should be possibility beneficial with subject to sustain action. The developed formulation overcomes and alleviates the drawbacks and limitations of sustained release preparations. Major advantages of the prepared formulations include.
? Easy of Preparation
? Good Buoyancy
? Sustained Release Over Several Hours.
Conflict of Interest: NIL
Source of Support: NONE
5651
References
- Arora S, Ali J, Ahuja A, Khar RK, Baboota S.Floating drug delivery systems : a review.nAAPS PharmSciTech 2005; 06: E 372-E390.
- Singh BN, Kim KH. Floating drug deliverynsystem : an approach to oral controlled drug delivery via gastric retention. J ControlnRelease 200; 63: 235-259.
- El-Gibaly I. Development and in vitronevaluation of novel floating chitosannmicrocapsule for oral use: comparison withnnon-floating chitosan microspheres. Int JnPharm 2002; 249 : 7-21.
- Sato Y, Kawashima Y, Takeuchi H,nYamamoto H, Fujibayashi Y (2004).nPharmacoscintigraphic evaluation ofnriboflavin-containing microballoons for anfloating controlled drug delivery system innhealthy humans. .J Control Release 2004;n98 : 75-85.
- Rouge N, Cole ET, Doelker E, Buri P.nBuoyancy and drug release pattern ofnfloating minitablets containing piretanidenand aenolol as model drug. Pharm DevnTechnol. 1994; 3: 73-84.
- Lee JH, Park TG and Choi HK. Developmentnof oral drug delivery sytem using floatingnmicrospheres. J. Microencapsul. 1999; 16:n715-729 .
- Akiyama Y, Nagahara N, Kashihara T, HirainS, and Toguchi H. In vitro and in vivonevaluation of mucoadhesive microspheresnprepared for the gastro-intestinal tract usingnpolyglycerol esters of fatty acids and anpoly(acrylic acid) derivative. Pharm Resn1995; 12: 397-405.
- Yeole, P.G., Khan, S., Patel, V.F., 2005.nFloating drug delivery systems: need andndevelopment, Indian J. Pharm. Sci. 67(3),n265-272.
- Whitehead L, Fell JT, Collett JH, SharmanHL, and Smith AM . An in vivo studyndemonstrating prolonged gastric retention.nJ Control. Release 1998; 55: 3-12.
- Fix JA, Cargill R and Engle K. Controlledngastric emptying, Part 3. Gastric residence time of a non disintegrating geometric shinnhuman volunteers. Pharm Res 1993; 10:n1087-1089.
- Kedzierwicz F, Houvenot P, Lemut J,nEtienne A and Hoffman M. Evaluation ofnperoral silicon dosage forms in human byngamma-scintigraphy. J Control Releasen1999; 58: 195-205.
- Groning R, Bertgen M and Georgarakis.nAcyclovir serum concentration followingnperoral administration of magnetic depotntablets and the influence of extracoporalnmagnets to control gastrointestinal transit.nEur J Pharm 1998; 46: 285-291.
- Park K (1988) Enzyme-digestible swellingnhydrogels as platforms for long term oralndrug delivery : Synthesis andncharacterization, Biomaterials 1988; 9: 435-n441.
- Stithit S, Chin W and Price JC. Developmentnand characterization of buoyantntheophylline microspheres with near zeronorder release kinetics. J Microencapsulationn1998; 15: 725-737.
- Sheth PR and Tossounian J. Thenhydrodynamically balanced system HBSTM :na novel drug delivery system for oral use.nDrug Dev Ind Pharm 1984; 10: 313-339.
- Kawashima Y, Niwa T, Takeuchi H, Hino Tnand Ito Y. Preparation of multiple unitnhollow microspheres (microballoons) withnacrylic resin containing tranilast and theirndrug release characteristics (in vitro) andnfloating behavior (in vivo). J ControlnRelease. 1991; 16: 279-290.
- Rouge N, Leroux JC, Cole ET, Doelker E andnBuri P. Prevention of the sticking tendencynof floating minitabletes filled into hardngelatin capsules. Eur J Pharm Biopharmn1997; 43: 165-171.
- upta, V., Barupal, A.K., Ramteke, S., 2008.nFormulation development and in vitroncharacterization of proliposomes for topicalndelivery of aceclofenac. Indian J Pharm Scin70, 768-775
- Kawashima Y, Niwa T, Takeuchi H, Hino Tnand Itoh Y. Hollow microspheres for use asna floating controlled drug delivery system innthe stomach. J Pharm Sci 1992; 81: 35-140.
- El-Kamal AH, Sokar MS, Al Gamal SS andnNaggar VF. Preparation and evaluation ofnketoprofen floating oral delivery system. IntnJ Pharm 2001; 220: 13-21.
- Soppimath KS, Kulkarni AR andnAminabhavi TM. Development of hollownmicrospheres as floating controlled systemsnfor cardiovascular drugs ; preparation andnrelease characteristics. Drug Dev Ind Pharmn2001; 27: 507-515.
- Sato Y, Kawashima Y, Takeuchi H andnYamamoto H. Physicochemical properties tondetermine the buoyancy of hollownmicrospheres (microballons) prepared bynthe emulsion solvent diffusion method. EurnJ Pharm Biopharm. 2003; 55: 297-304.
- Higuchi T. Mechanism of sustained-actionnmedication: theoretical analysis of rate ofnrelease of solid drugs dispersed in solidnmatrices. J Pharm Sci 1963; 52: 1145-1149.










