Key words
|
| |
| FDTS; Losartan Potassium; Direct compression; physicochemical parameters; Disintegration time; Invitro drug release; stability studies. |
| |
INTRODUCTION
|
| |
| Difficulties with and resistance to tablet-taking are most common in all patient groups and can exacerbate compliance problems and undermine treatment efficacy. Physical problems with swallowing (dysphasia) can occur at any age but are particularly prevalent in the elderly and those with dementia, whereas refusal to swallow is often encountered in geriatric, pediatric and psychiatric patients. Nonetheless, oral dosing remains the preferred mode of administration for many types of medication due to its simplicity, versatility, convenience and patient acceptability. In recent years, rapid-dissolving oral drug formulations have been developed to overcome problems related to swallowing difficulties [1]. |
| |
| Fast onset of action is a major concern in the treatment of hypertension. The problem of slow onset of action of drugs can be overcome by development of an appropriate dosage form. Fast disintegrating in mouth tablets are best suited and have gained popularity in the recent years in oral antihypertensive drug therapy. This new formulation of antihypertensive drugs can offer advantages over older formulation in terms of convenience, side-effect profiles, efficacy, and/or a fast onset of action [4]. |
| |
| Losartan Potassium is an antihypertensive drug belongs to the category of Angiotension II receptor antagonist. The molecular weight of Losartan Potassium is 461.01g/mol, half life is 1.5 to 2hr, and its bioavailability is 25 – 35%, metabolized mainly in the liver. FDTS are soluble in saliva are absorbed from the mouth, pharynx and oesophagus as the saliva passes down into stomach, thus enhance the bioavailability by avoiding first pass metabolism. FDTS also leads to an increased patient compliance, and fast onset of action. Keeping all these factors in mind, it was considered appropriate to formulate FDTS of Losartan Potassium. The literature survey reveals that Losartan Potassium is a promising drug candidate for FDTS formulation [10] |
| |
MATERIALS AND METHODS
|
| |
| Losartan potassium was received as gift sample from Karnataka Antibiotics and Pharmaceuticals Ltd.; Croscarmellose sodium, crospovidone, sodium starch glycolate were procured from Micro Labs, Bangalore; Microcrystalline cellulose was kindly gifted by Shrsuti Pharmaceuticals, Bangalore; Mannitol was from Loba Chemicals, Mumbai; Aspartame was obtained from Gland Pharma Ltd., Hyderabad. All others chemicals used were of the best quality commercially available. |
| |
DIRECT COMPRESSION METHOD
|
| |
| The individual ingredients like drug, mannitol, microcrystalline cellulose and aspartame were passed through sieve No. 60 and blended. Lubrication was done using talc and magnesium stearate. |
| |
EVALUATION OF PHYSICOCHEMICAL PARAMETERS
|
| |
|
MICROMERITIC PROPERTIES
|
| |
| ANGLE OF REPOSE61: The angle of repose was determined by funnel method suggested by Newman. Angle of repose is determined by following formula. |
| |
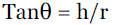 |
| |
| Therefore θ = Tan-1h/r ............(1) |
| |
| Where θ = angle of repose, h= height of the cone, r = radius of the cone base. |
| |
|
BULK DENSITY 61:
|
| |
|
Determination of bulk density:
|
| |
| 10 grams of the granules were transferred to the measuring cylinder of the bulk density apparatus. Noted the final volume as bulk volume. |
| |
| Bulk density is calculated by using formula............(2) |
| |
TAPPED DENSITY:
|
| |
| The measuring cylinder containing known mass of blend was tapped for a fixed time. The minimum volume (Vt ) occupied in the cylinder and weight (M) of the blend as measured. The tapped density (ρt) was calculated using the following formula. |
| |
 |
| |
PERCENTAGE COMPRESSIBILITY:
|
| |
| Percentage compressibility of powder mix was determined by Carr’s compressibility index calculated by following formula. |
| |
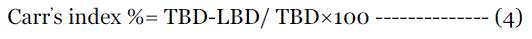 |
| |
|
HAUSNER RATIO:
|
| |
| Hausner ratio is an indirect index of ease of powder flow. |
| |
| It is calculated by the following formula. |
| |
 |
| |
| Where ρt is tapped density and ñb is bulk density |
| |
RESULTS
|
| |
|
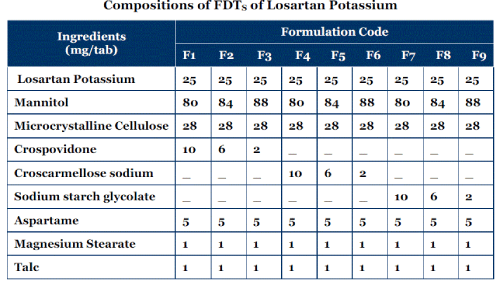
Compositions of FDTS of Losartan Potassium
|
| |
 |
| |
|
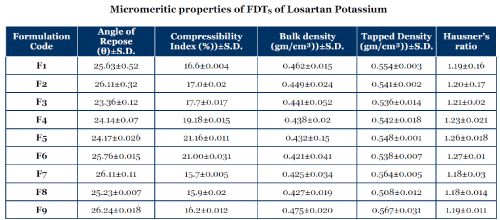
Micromeritic properties of FDTS of Losartan Potassium
|
| |
 |
| |
|
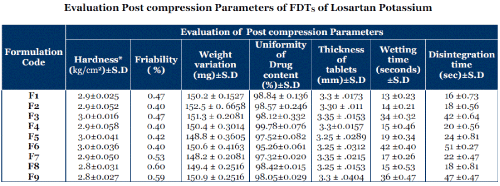
Evaluation Post compression Parameters of FDTS of Losartan Potassium
|
| |
 |
| |
STABILITY STUDIES OF FDTS OF LOSARTAN POTASSIUM
|
| |
|
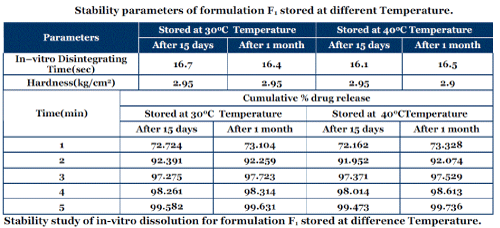
Stability parameters of formulation F1 stored at different Temperature.
|
| |
 |
| |
DISCUSSION
|
| |
| FDTS were developed for widely used drug Losartan Potassium to improved bioavailability, patient compliance and for quick onset of action as it is meant for hypertensive patients. |
| |
| An attempt has been made to formulate and investigate the physicochemical parameters, in vitro disintegration time and in vitro drug release of FDTS of Losartan Potassium. |
| |
PREFORMULATION STUDIES
|
| |
METHOD DEVELOPMENT FOR DRUG ESTIMATION
|
| |
| At the outset, method for the estimation for the drug was developed. Standard Stock Solution was prepared and scanned by UV Spectrophotometer and the l max was found to be 206.5 nm against phosphate buffer pH 6.8 as blank. |
| |
| The Calibration Curve and the data were obtained and the linear plot between concentrations versus absorbance showed that, Beer-Lambert’s law was obeyed in concentration range of 2-50 µg/ml which one subjected to regression analysis. The r2 value (regression co-efficient) was found to be 0.998 which showed linear relationship between concentration and absorbance. |
| |
DRUG EXCIPIENT COMPATIBILITY STUDIES
|
| |
| In order to investigate the possible interaction between drug and excipients, IR studies were carried out. After spectral comparison it was confirmed that no incompatibility reaction took place between drug and excipients. |
| |
| The infrared spectrum of standard drug Losartan Potassium and physical mixture with excipients corresponds the similar wave numbers. Therefore, the study revealed that there is no interaction between the drug and the excipients indicating that the excipients and the active pharmaceutical ingredient (API) are compatible with each other. |
| |
FORMULATION STUDIES
|
| |
|
FORMULATION DEVELOPMENT
|
| |
| Various formulations of FDTS were developed for Losartan Potassium using various superdisintegrants like SSG, CCS, CP; directly compressible vehicle like MCC and filler Mannitol. Magnesium stearate and talc were used as lubricant, aspartame used as sweetening agent. The various developed formulations of FDTS were prepared by direct compression technique and were compressed using 8 mm flat punches to an average weight of 150 mg. |
| |
EVALUATION OF PHYSICOCHEMICAL PARAMETERS
|
| |
MICROMERITIC PROPERTIES
|
| |
| The angle of repose values ranged from 23 -27(θ) which indicate good flow properties of powders; the compressibility index values ranged from 15%-21%. Bulk densities of various formulations were found to be 0.40-0.49g/ml. From the result it was concluded that the powder blends had good flow property. |
| |
THICKNESS, HARDNESS, FRIABILITY OF TABLETS
|
| |
| Thickness of all the formulations was in the acceptable range of 3.25 mm to 3.35 mm. The average hardness of all the tablet formulations lies in the range of 2.8-3 kg/cm2. The average friability of all the formulations lies in the range of 0.40%-0.60% which was less than less than 1% as per official requirement of IP. |
| |
WETTING TIME
|
| |
| The average wetting time of all the formulations was obtained in the range of 10-45 seconds. The maximum wetting time of 42±0.4097 seconds which showed by formulation F6 and the minimum wetting time was shown by formulation F1 13±0.2378 seconds. |
| |
MOISTURE UPTAKE
|
| |
| The moisture uptake by the developed FDTS formulations was due to the water uptake and the swelling behavior of superdisintegrants. Since all the formulations contained almost same excipients, any difference in moisture uptake may be due to different concentrations of superdisintegrants. Due to maximum moisture uptake the increased percentage of weight showed by formulation F1 and the minimum in formulation F9. |
| |
WEIGHT VARIATION
|
| |
| The average weight of 20 tablets was calculated for each formulation which varied from148.2±0.2081mg to 152.5±0.6658mg which comply the official requirement as per I. |
| |
UNIFORMITY OF DRUG CONTENT
|
| |
| The uniformity of content of all the formulations varied from 95.26± 0.0611 % w/w to 99.78 ± 0.076 %w/w which was as per I.P. requirements. |
| |
IN VITRO DISINTEGRATION TIME
|
| |
| Formulation F6 showed the maximum disintegration time of 51 ± 0.27 seconds and formulation F1 showed the least disintegration time of 16 ±0.7 seconds. To shorten the disintegration time in the oral cavity for the tablet, the addition of the disintegrate having a property of quick water uptake in the formulation would be preferable. It was considered that rapid disintegration would be due to its wettability.5 |
| |
| |
EFFECTIVENESS OF TASTE MASKING
|
| |
| The numerical of all formulations were getting in the range 0 to1 that means tasteless to slight taste. |
| |
IN VITRO DISSOLUTION STUDIES
|
| |
| As there is no specific dissolution test available for mouth dissolving tablets, dissolution rate was studied as per USP specifications for conventional tablets using paddle type of dissolution apparatus. Phosphate buffer pH 6.8 was used as dissolution medium. The higher concentration of crospovidone results in the rapid disintegration of tablet in dissolution medium resulting in optimized drug release from tablets. |
| |
| Overall the FDTS formulations of Losartan Potassium showed an average of 95 % to 99 % drug release range at the end of 5 minutes and it was also observed that formulations took shortest time to release the maximum amount of drug whereas the other formulations took more than 6 min to release the drug. |
| |
STABILITY STUDIES OF FDTS OF LOSARTAN POTASSIUM
|
| |
| The best formulation i.e. F1 was packed in bottle, which was tightly plugged with cotton and capped. Then the formulation was exposed to various temperature and humidity conditions (30°C/ 65% RH and 40°C/ 75% RH) for 1 month to assess their stability as per ICH guidelines Q1C62. At the various time intervals samples were evaluated for physicochemical parameters, in vitro disintegration time and in vitro dissolution profiles. There was no significant variation in the physicochemical parameters, in vitro disintegration time, and in vitro dissolution profiles after one month stability study. |
| |
CONCLUSION
|
| |
| Losartan Potassium is an antihypertensive drug belongs to the category of Angiotensin II receptor antagonist. Even though Losartan Potassium is well absorbed after oral dosing, it has first pass metabolism which leads to a reduced oral bioavailability of the drug (25 – 35%). |
| |
| The present investigation is concerned with the development of the FDTS which are soluble in saliva are absorbed from the mouth, pharynx and oesophagus as the saliva passes down into stomach, thus enhance the bioavailability by avoiding first pass metabolism. |
| |
| From the IR spectra alone, it is possible to conclude that the selected excipients are likely to be suitable for the preparation of tablets formulation, since no significant incompatibility were detected. |
| |
| Based on preliminary studies various formulations were developed by using a different concentration of three superdisintegrants, MCC, mannitol, aspartame followed by direct compression adding lubricants using 8mm flat punch. The total tablet weight was 150mg. |
| |
| Developed FDTS gave satisfactory results for various physicochemical evaluations like hardness, friability, weight variation, drug content, in-vitro disintegration time and in- vitro dissolution profiles. |
| |
| Disintegration time of FDTS depends on concentration of superdisintegrants. The disintegration time of all the formulation varied from 16 ± 0.73 to 51 ± 0.27 seconds. |
| |
| In vitro drug release for various formulations ranges from 95.23 to 99.90 % end of 5 minutes. And it was also observed that some formulations took shortest time to release the maximum amount of drug whereas the other formulations took more than 6min to release the drug. |
| |
| The analysis of the release profile of the most satisfactory formulation led to conclusion that highest water uptake due to capillary action of CP led to shortest disintegration time and maximum drug release at the end of 5th min. The concentrations used are 2, 6 and 10mg, in that 10mg acts as the best and can be employed in the formulation of the FDTS. Though tablets prepared by croscarmellose sodium and sodium starch glycolate disintegrates in the mean time. |
| |
| The most satisfactory formulation showed no significant change in physicochemical properties, drug content, in vitro disintegration time, in vitro dissolution pattern after storage at 30°C/ 65% RH, 400C(75%RH) in stability chamber for 1month and in concerned with dissolution profile was found superior than conventional formulation. |
| |
| Therefore it was concluded that the most satisfactory formulation satisfied the micromeritic parameters, post compression properties, disintegration time requirements, in vitro drug release profile requirements and stability requirements. |
| |
| Thus, the objective of my work of FDTS of Losartan Potassium by using different proportions of superdisintegrants can be successfully prepared and undoubtedly the availability of various technologies and the manifold advantages of FDTS will surely enhance the patient compliance and its popularity in the near future. |
| |
Conflict of Interest
|
| |
| NIL |
| |
Source of Support
|
| |
| NONE |
| |