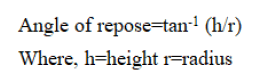Keywords
Matrix tablets; Sustain release; Dry granulation; Diltiazem; Bulk density; Tapped density; Weight variation; Friability; Compatibility study; FTIR studies
Introduction
Present days novel medication conveyance frameworks are critical for transportation of the medicine to the human body. Presently the customary frameworks are recuperated by the novel medication conveyance frameworks [1,2]. In present day pharmaceutical innovation, the continued and controlled medication exchange frameworks are the more celebrated frameworks. Presentation of framework measurements structure as Supported Release (SR) has given another breakthrough for novel dynamic substance move framework within the field of pharmaceutical innovation [3,4]. Diltiazem was generally indicated for the management of chronic stable angina. The Sustained Release (SR) sort of the drug has been found to end in better compliance, reducing the frequency of dosage and within the end, the foremost important thing, maintaining a consistent drug concentration within the body [5,6]. Calcium Channel Blockers (CCBs) are generally used to treat high blood pressure, angina and certain cardiac rhythm abnormalities [7]. CCBs are a category of medicine that ought to not be prescribed as initial or first-line treatment in people with high blood pressure who haven't any other form of heart condition. They're often used as a second or third drug to assist lower blood pressure when other drugs haven't been successful in bringing down the level. However, CCBs could also be considered as initial treatment (usually together with other drugs) for people that have high blood pressure plus angina and/or a high risk of stroke. CCBs shouldn't be given to people with heart failure (often called congestive heart failure). Coronary artery disease has been documented to be one among the most causes of morbidity and mortality in chronic hemodialysis patients with End-Stage Renal Disease (ESRD) [8,9]. It's well established that Heart Rate (HR) lowering, both at rest and during exercise, has beneficial effects in patients with angina. Hypertension has been recognized as a serious public health issue.
Materials and Methods
Materials
Diltiazam, Microcrystalline cellulose 101 (MCC), Ethyl Cellulose, HPMC K4M, Polyvinylpyrrolidone (PVP), Magnesium Stearate, Talc.
Methods
The dry granulation method used for the formulation development of matrix tablets of Diltiazam.
Dispensing: Accurate quantity of drug and chosen excipients are weighed.
Sieving: The dispensed quantity of drug and excipients are sieved through 40 number sieve.
Mixing: The sieved drug and excipients are undergone for manual mixing. The talc and mg stearate added lastly.
Dump mass: After mixing the dump mass is formed by adding IPA to mixed blend. The dump mass is formed.
Size reduction: After preparation of dump mass undergone for size reduction by using sieve number 12. The granules are obtained.
Drying: After size reduction the granules are dried in oven for 30 mins.
Compression: After drying the granules undergo for compression in multipunch compression machine using 10 mm diameter (Table 1).
| Ingredients |
F1 |
F2 |
F3 |
F4 |
F5 |
F6 |
F7 |
F8 |
F9 |
| Diltiazem HCL |
180 |
180 |
180 |
180 |
180 |
180 |
180 |
180 |
180 |
| Microcrystalline cellulose 101 |
237 |
237 |
217 |
217 |
177 |
177 |
207 |
157 |
77 |
| Ethyl cellulose |
- |
40 |
- |
60 |
- |
100 |
40 |
60 |
100 |
| HPMC K4M |
40 |
- |
60 |
- |
100 |
- |
40 |
60 |
100 |
| Polyvinylpyrrolidone (PVP) |
20 |
20 |
20 |
20 |
20 |
20 |
20 |
20 |
20 |
| Mg. stearate |
2 |
2 |
2 |
2 |
2 |
2 |
2 |
2 |
2 |
| Talc |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
1 |
| Tablet weight |
480 |
480 |
480 |
480 |
480 |
480 |
480 |
480 |
480 |
Table 1: Formulation table for diltiazem matrix tablets.
Characterization of tablets
Bulk density: The bulk density is measured as the accurately weighed powder blend is transferred in to the measuring cylinder. First the initial weight of the sample is calculated [10,11].
Tapped density: The tap density is measured as the transferred powder in the cylinder is kept and fix to the tap density apparatus and the tapping is performed about (100).
Percentage compressibility (or) Carr’s index (%): The compressibility index is defined as it comes from the standards of the loose thickness and tap thickness. The compressibility index is calculated.
Hausner’s ratio: It indicates the flow properties of powder and is measured by the ratio of tap density to bulk density.
Angle of repose: The point of rest is performed by the pipe technique. Some amount of the powder is taken in a pipe by shutting the hole of the channel. Under the pipe one paper is kept. By opening hole, the powder frames a load like heap. The heap flat width is taken by the scale by the perimeter drawn on the paper. The edge of rest is for the most part for the assurance of the stream property of the powder. Edge of rest is determined by the accompanying recipe,
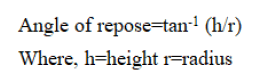
Evaluation of tablets
Physical appearance: The readied tablets are under kept for the perception of the colour, size and shape. For observing of parcel to-part consistency and tablet-to-tablet consistency.
Thickness: The readied tablets are then under kept for watching the thickness of the tablets. The thickness of the tablets are completed by the smaller scale meter or by the vernier calipers. The tablets thickness ought to screen inside the ± 5% variety of standard esteem.
Weight variation: The readied tablets are kept for the weight variety test. The weight variety is performed by the weighing of the individual tablet and all the tablets. Initial weight is taken after that gathering of tablets weight is taken. The weight variety is finished by the accompanying recipe.
% Weight variation=initial weight of the tablet/final weight × 100
Drug content: The drug content was determined by taking ten tablets. It is poured into the motor and pestle and crushed finely. An accurately weighted amount of the powder equal to 10 mg of the diltiazem HCL is taken. From the crushed powder 10 mg of the powder is taken in to the 100 ml of the volumetric flask with 7.4 ph. buffer solution. It is mechanically stirred thorough for 1 hr. The stirred powder solution is filtered through the whatsman filter paper. The absorbance was measured at 237 nm against blank solution.
Friability: Friability is finished through using the Roche friabilator. About 10 tablets are taken for performing the method. First take initial weight of tablets and transfer them into friabilator, the apparatus where they are exposed to the rolling and repeated shocks as they fall from 6 inches in each turn within the apparatus. The tablets revolutions are about 100 per minute. The tablets final weight is compared with the initial weight of the tablets. Aimed at decisive the asset of the tablets the following formula is used; the percentage friability was determined by the formula:

In- vitro drug release study: The prepared tablets are the under kept for the In-vitro drug release educations. For in vitro drug release educations, the USP-II apparatus is used. It is paddle method. The dissolution volume is up to 900 ml [12,13]. It is maintained up to the temperature 37 ± 1°C. The rpm is about 50. The dissolution education is accepted obtainable for 10 hours. The sink conditions are maintained. First start with acid buffer for 2 hrs after that the process is continued in the buffer solution that is 7.4 pH buffer solution. 5 ml of sample is withdrawn in different time intervals and it is replaced with same volume with the buffer solution while the sink conditions are maintained. The withdrawn sample is diluted with the buffer solution and it is under kept for the U.V for knowing of the absorbance of the sample spectrophotometrically at 237 nm.
Results and Discussion
FTIR Studies: IR Spectral investigation diltiazem (medicate) demonstrated the crests at wave quantities of 2317(C-N) 2990(C-H Alkane) 1732(N-H Bending) 1240 (OCH3-extending) 2478(C=C) affirming the virtue of the medication with the standard separately. In physical blend of diltiazem with HPMC K4M and ethyl cellulose real pinnacles of diltiazem were 2318(CN) 2998(C-H Alkane) 1750 (N-H Bending) 1268 (OCH3-extending) 2567 (C=C) wave numbers. Anyway, the extra pinnacles were seen in physical blends which could be because of the nearness of excipients and it tends to be said that there was no concoction cooperation between the medication and excipients from the spectra (Figures 1-3 and Tables 2-4).
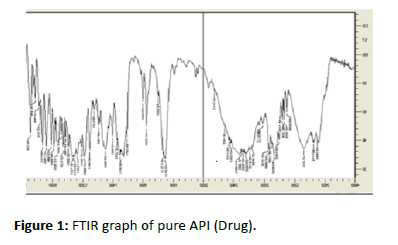
Figure 1:FTIR graph of pure API (Drug).
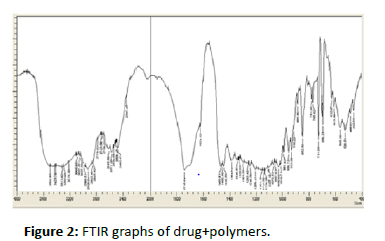
Figure 2:FTIR graphs of drug+polymers
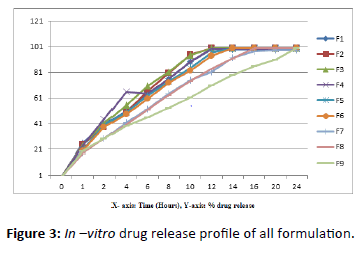
Figure 3:In –vitro drug release profile of all formulation.
| Formulation |
Angle of repose (θ) |
Bulk density (gm/ml) |
Tapped density (gm/ml) |
Carr’s index (%) |
Hausner’s ratio |
| F1 |
28.53 |
3.40 ± 0.01 |
4.4 ± 0.06 |
0.612 |
95.24 ± 0.22 |
| F2 |
28.26 |
3.55 ± 0.00 |
4.5 ± 0.06 |
0.646 |
94.57 ± 0.42 |
| F3 |
26.59 |
3.6 ± 0.01 |
4.4 ± 0.00 |
0.686 |
96.43 ± 0.13 |
| F4 |
28.54 |
3.48 ± 0.01 |
4.70 ± 0.06 |
0.526 |
96.83 ± 0.42 |
| F5 |
26.05 |
3.32 ± 0.01 |
4.60 ± 0.10 |
0.546 |
97.86 ± 0.32 |
| F6 |
29.52 |
3.40 ± 0.01 |
4.40 ± 0.06 |
0.612 |
95.24 ± 0.22 |
| F7 |
32.52 |
3.55 ± 0.00 |
4.50 ± 0.06 |
0.646 |
96.57 ± 0.42 |
| F8 |
31.45 |
3.60 ± 0.01 |
4.40 ± 0.00 |
0.686 |
94.43 ± 0.13 |
| F9 |
35.25 |
3.48 ± 0.01 |
4.70 ± 0.06 |
0.53 |
96.83 ± 0.42 |
Table 2: Pre-compression results of the diltiazam matrix tablets.
| Formulation |
Weight variation |
Thickness (mm) |
Hardness (Kg/cm2) |
Friability (%) |
Drug content (%) |
Disintigration (min) |
| F1 |
399.23 ± 0.001 |
3.51 ± 0.01 |
4.7 ± 0.06 |
0.258 |
98.25 ± 0.2 |
25 |
| F2 |
399.8 ± 0.01 |
3.52 ± 0.00 |
4.5 ± 0.06 |
0.41 |
99.23 ± 0.1 |
26 |
| F3 |
399.44 ± 0.01 |
3.49 ± 0.01 |
4.5 ± 0.00 |
0.235 |
99.52 ± 0.1 |
26 |
| F4 |
400 ± 0.00 |
3.54 ± 0.01 |
4.7 ± 0.06 |
0.42 |
98.52 ± 0.2 |
25 |
| F5 |
399.58 ± 0.01 |
3.50 ± 0.01 |
4.5 ± 0.10 |
0.52 |
99.55 ± 0.1 |
24 |
| F6 |
398.85 ± 0.02 |
3.48 ± 0.01 |
4.6 ± 0.06 |
0.4 |
100.12 ± 0.01 |
27 |
| F7 |
399.2 ± 0.01 |
3.63 ± 0.00 |
4.5 ± 0.06 |
0.243 |
99.80 ± 0.1 |
28 |
| F8 |
399.5 ± 0.01 |
3.62 ± 0.01 |
4.6 ± 0.00 |
0.402 |
98.90 ± 0.1 |
28 |
| F9 |
399.2 ± 0.01 |
3.59 ± 0.01 |
4.7 ± 0.06 |
0.421 |
99.32 ± 0.1 |
29 |
Table 3: Post-compression results of the diltiazam matrix tablets.
| Time (Hrs.) |
F1 |
F2 |
F3 |
F4 |
F5 |
F6 |
F7 |
F8 |
F9 |
| 0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
| 1 |
25.63 |
24.65 |
24.25 |
23.87 |
20.14 |
19.86 |
18.52 |
18.46 |
19.52 |
| 2 |
40.23 |
38.25 |
41.23 |
44.23 |
39.55 |
38.43 |
29.3 |
29.3 |
29.3 |
| 4 |
50.54 |
49.55 |
55.43 |
65.54 |
49.82 |
48.23 |
41.52 |
40.2 |
39.2 |
| 6 |
64.25 |
66.35 |
70.52 |
64.25 |
62.54 |
60.48 |
52.2 |
51.52 |
45.52 |
| 8 |
76 |
80.5 |
81.52 |
7
6 |
73.56 |
72.65 |
64.24 |
63.1 |
53.1 |
| 10 |
89.5 |
94.56 |
94.2 |
89.5 |
84.2 |
82.45 |
74.54 |
74.2 |
61.2 |
| 12 |
98.58 |
100.02 |
99.65 |
99.52 |
96.52 |
93.59 |
81.36 |
83.85 |
70.85 |
| 14 |
98.86 |
100.02 |
99.72 |
99.54 |
99.68 |
100 |
92.52 |
91.56 |
78.56 |
| 16 |
98.86 |
100.02 |
99.72 |
99.54 |
99.69 |
100 |
97.52 |
99.7 |
85.7 |
| 20 |
98.86 |
100.02 |
99.72 |
99.54 |
99.69 |
100 |
98.14 |
99.81 |
90.8 |
| 24 |
98.86 |
100.02 |
99.72 |
99.54 |
99.69 |
100 |
98.32 |
99.85 |
99.62 |
Table 4: In–vitro drug release profile of all formulation.
Drug content: The drug content was determined by taking ten tablets. It is poured into the motor and pestle and crushed finely. An accurately weighted amount of the powder equal to 10 mg of the diltiazem HCL is taken. From the crushed powder 10 mg of the powder is taken in to the 100 ml of the volumetric flask with 7.4 ph. buffer solution. It is mechanically stirred thorough for 1 hr. The stirred powder solution is filtered through the whatsman filter paper. The absorbance was measured at 237 nm against blank solution.
Thickness: The readied tablets are then under kept for watching the thickness of the tablets. The thickness of the tablets are completed by the smaller scale meter or by the vernier calipers. The tablets thickness ought to screen inside the ± 5% variety of standard esteem.
All the formulations (F1-F9) Angle of repose, bulk density, tapped density, hansures ratio, compressability all are within the limits.
Conclusion
Diltiazem HCL tablet was prepared by using different types of excipients and controlled release polymer (HPMC K4M, Ethyl Cellulose). The tablet was formulated by using dry granulation method. The use of the combination of controlled release polymer is extends the release of the drug up to the 24 hours. The in vitro drug release study was done by using USP-II apparatus, the Phosphate Buffer pH 6.8 was used. The optimized formulation F9 shows sustained-release of the drug diltiazem by controlled manner up to the 24 hours.
38402
References
- Mandal S, Ratan GN, Mulla JS, Thimmasetty J, Kaneriya A (2010) Plan and calculation of gastro retentive sustained discharge tablets of tizanidine Hydrochloride, Indian J Nov Drug Deliv 2: 144-152.
- Lee BJ, Ryu SG, Cui JH (1999) Preparation and discharge characters of hydroxypropyl methylcellulose matrix tablet having melatonin. Drug Dev Ind Pharm 25: 493-501.
- Prajapati ST, Patel LD, Patel DM (2008) Floating matrix tablets: Preparae and optimization using combination of polymers, Acta 58: 221-229.
- Jantzen GM, Robinson JR (1995) The review on the controlled-release active substance delivery systems, In Banker and Expanded. Drugs and The Pharma Sci 72: 1995; 575-609.
- Patel PN, Patel MM, Rathod DM, Patel JN, Modasiya MMK (2012) Sustain release drug delivery: A theoretical prospective.Journal of Pharmacy Research 5: 4165-4168.
- Gambhire MN, Ambade KW, Kurmi SD, Kadam VJ, Jadhav KR (2007) Development and in vitro evaluation of an oral floating matrix tablet formulation of diltiazem hydrochloride.Aaps Pharm Sci Tech 8: E166-E174.
- Baviskar D, Sharma R, Jain D (2013) Formulation and in vitro/in vivo evaluation of sustained release diltiazem matrix tablets.Trop J Pharm Res 12: 311-316.
- De Lemos JA, Hillis LD (1996) Diagnosis and management of coronary artery disease in patients with end-stage renal disease on hemodialysis.J Am Soc Nephrol 7: 2044-2054.
- Goldsmith DJ, Covic A (2001) Coronary artery disease in uremia: Etiology, diagnosis, and therapy.Kidney int 60: 2059-2078.
- Government of India Ministry of Health and Family Welfare (1996) The pharmacopoeia of India, Controller of Publication 2: 734 – 736 15.
- Odeku OA, Fell JT (2006) Effects of the method of preparation on the compression, mechanical, and release properties of khaya gum matrices. Pharm Dev Technol 11: 435-441.
- Gupta NC, Aggarwal N (2007) A gastro-retentive floating delivery system for 5-fl uorouracil. Asian J Pharm Sci 2: 143-9.
- Bhise SB, Aloorkar NH (2008) Formulation and in vitro evaluation of floating capsules of theophylene. Indian J Pharm Sci 70: 224-7.