Keywords
|
| Fast dissolving oral film; Montelukast sodium; Musa paradisiaca powder; Solvent casting technique |
Introduction
|
| The oral route is the most acceptable from patient compliance aspects. Formulation of new dosage form for the existing drugs is new area of concern in pharmaceutical field. One of such new dosage form is oral film that rapidly dissolves on the tongue or buccal cavity [1]. Recently, oral film has started gaining popularity and acceptance, for the reason of rapid disintegration or dissolution, self?administration even without water or chewing. Oral films are postage stamp-sized strips made up of film forming polymers. Fast-dissolving films are generally constituted of plasticized hydrocolloids or blends made of thereof that can be laminated by solvent casting or hot-melt extrusion. The manufacture of the dosage forms can present different critical issues. Common problems are caused by foaming during the film formation. The film should be flexible and exhibit a suitable tensile strength and do not stick to the packaging materials and fingers while administration [2]. |
| The first of the kind of oral films (OF) were developed by the major pharmaceutical company Pfizer who named it as Listerine® pocket packs™ and were used for mouth freshening. Chloraseptic® relief strips were the first therapeutic oral thin films (OTF) which contained 7- benzocaine and were used for the treatment of sore throat [3]. |
| Patients who have difficulty in swallowing such as elder persons, pediatric patients and others suffering from mental illness and developmental disorders can be treated with oral films. The surface of oral cavity comprises of stratified squamous epithelium which is essentially separated from the underlying tissue of lamina propria and submucosa by an undulating basement membrane. It is interesting to note that the permeability of buccal mucosa is approximately 4-4,000 times greater than that of the skin, but less than that of the intestine. Hence, the buccal delivery serves as an excellent platform for absorption of molecules that have poor dermal penetration [4]. The buccal mucosa being highly vascularized, drugs can absorbed directly and can enter the systemic circulation without undergoing first-pass hepatic metabolism. This advantage can be exploited in preparing products with improved oral bioavailability of molecules that undergo first pass effect. The sublingual and buccal film of a drug helps to improve the onset of action, lower the dosing and enhance the efficacy and safety profile of medicament [5]. Most of the polymers that are used as film forming agents are predominantly hydrophilic polymers that will swell and allow for chain interactions with the mucin molecules in the buccal mucosa [6]. The critical parameters to formulate a fast dissolving film are choice of polymer and other excipients like superdisintegrant and optimization of concentration of polymer as well as superdisintegrant. The main criteria for fast dissolving film are to disintegrate rapidly on tongue and give rapid onset of action [7]. Several classes of drugs can be formulated as mouth dissolving films including antiulcer, antiasthamatics, antitussives, expectorants, antihistaminics and NSAID’s [8]. |
| Present day researchers are looking for natural excipients as it is believed that anything natural will be more safe and devoid of side effects. Advantage of natural excipients are low cost and natural origin free from side effects, biocompatibility and bioacceptance, renewable source, environment friendly processing, local availability, better patient tolerance as well as public acceptance, they comprise the natural economy by providing inexpensive formulation to people [9]. |
| Musa paradisiaca fruit is available in plenty in India. A literature survey revealed that the Musa paradisiaca powder has not been used to date in oral films and hence it was chosen in the present investigation with an aim to introduce and evaluate it as natural superdisintegrant using Montelukast sodium as model drug in the formulation of oral fast dissolving films [10]. The Montelukast sodium is a leukotriene receptor antagonist (LTRA) used for the maintenance treatment of asthma, chronic asthma attacks and to relive symptoms of seasonal allergies [11]. The main drawback of conventional montelukast sodium formulation is that it undergoes hepatic first pass metabolism. Thus, it shows plasma or biological half-life 2.5 to 5.5 h, thereby decreasing bioavailability upto 64%. Montelukast Sodium is given in a dose of 10 mg once daily [12]. |
Materials and Methods
|
|
Materials
|
| Montelukast sodium was obtained as a gift sample from Unimark remedies (Vapi). Musa paradisiaca powder was purchased from Kalpalaxmi Agro processors and traders (Ahmednagar) and used as received, Aspartame from Hi Media laboratories Pvt. Ltd. (Mumbai), HPMC E 15LV, PEG 400, Tween 80 and Citric acid from Loba chemie Pvt. Ltd. (Mumbai). All chemicals used were of analytical grade. |
| Superdisintegrant characterization: The Musa paradisiaca powder on receipt was sieved to get uniform particle size and was evaluated for the physicochemical properties to determine its applicability as a superdisintegrant. A good superdisintegrant is the one which is insoluble in water, shows poor gel formation, good hydration and good flow property [13]. Effective superdisintegrants provide improved compatibility and have no negative impact on the mechanical properties of the formulation. The natural material was used as a superdisintegrant in formulation of films on the basis of preliminary evaluations. |
| Selection of polymer: Two grades of HPMC, film forming polymer, were tried for the formulation of film. Different concentrations of both the polymers were used in alone as well as in combination. The polymers were dissolved into 10 mL of water and casted in film by casting of the solution in petri plate. The films were dried for 24 h at 60°C and physicochemical evaluation was carried out. The films were observed for absence of whiteness and oiliness and good folding endurance with the fast disintegration. The best polymer was selected for the formulation on basis of the outcome of the evaluation. |
| Selection of plasticizer: Plasticizer play important role for maintaining the flexibility, which is responsible for the good folding capacity of the film. Hence, trials were carried out using various grades of plasticizer like PEG 6000, PEG 400 and PEG 200. Variation in their concentration of plasticizer may affect the flexibility so the trails were carried out at different concentrations. The best suitable plasticizer was selected for formulation of the film on basis of the observations. |
| Preparation of fast dissolving films containing montelukast sodium: The fast dissolving films of Montelukast sodium were prepared by solvent casting technique [14]. Weighed amount of film forming polymer was dissolved completely 5 ml of water. On formation of homogenous solution tween 80 and Musa paradisiaca powder were added with continuous stirring to get uniform dispersion. Drug and remaining excipients were dissolved separately in another beaker containing 5 ml of water. Then the drug solution was added to polymer solution and stirring was carried out on magnetic stirrer for 15-20 minutes and then solution was kept aside till the solution become completely free from air bubble. The solution was casted on to Petri dish, and then kept in hot air oven at 60°C for 24 hrs. The films were then cut in size of 2 cm × 2 cm containing 10 mg of montelukast sodium. |
|
Experimental design
|
| In the present study a natural excipient Musa paradisiaca powder is used and evaluated for its superdisintegrant activity which is directly related to the release of the drug and disintegration time. So to formulate an optimised formulation 32 design was applied using design expert software. Concentration of polymer (X1) and superdisintegrant (X2) were selected as two independent variables based on the results from trial batches. The interaction term (X1 X2) shows how the response changes when two factors are changed simultaneously. Disintegration time (Y1) and % Drug release (Y2) were taken as the response parameters for the design. Changing the concentration of both excipients at three levels i.e., HPMC E15LV (X1) 250, 500 and 750 mg and Musa paradisiaca powder (X2) 50, 100 and 200 mg; 9 batches (F1- F9) were formulated and evaluated for various parameters. Table 1 summarizes these factors with corresponding levels and the responses studied, whereas experimental formulations are listed in Table 2. |
| Characterization of film |
| Visual inspection: Patient acceptance of dosage form is an important factor for the administration of the film. Clarity, transparency and oiliness are the main parameters for inspection. If it was found satisfactory, then further evaluation were carried out. If the formed films were not satisfactory they were discarded [15]. |
| Thickness: The thickness of the films is usually measured using well calibrated electronic digital micrometer screw gauge. Indeed, the measurement of thickness of the film is essential to ascertain the uniformity of the film thickness as it is directly related to the accuracy of dose in the film. In general, an ideal buccal film should exhibit a thickness between 50 and 1000 μm [15]. |
| Weight variations: For weight variation, individual films were weighed and the average weights were calculated. Then the average weight of the patches is subtracted from the individual weight of the patches. A large variation in weight indicates the inefficiency of the method employed and is likely to have non-uniform drug content [16]. |
| Surface pH: The surface pH of the oral dissolving film is evaluated in order to investigate the risk of any side effects. The surface pH of the film should be close to that of pH of the buccal cavity i.e., 6.8. The oral film was slightly moistened with the help of water. The pH was measured by bringing the electrode in contact with the surface of the oral film [17]. |
| Drug content determination: Drug content uniformity was determined by dissolving the film (4 cm2) in 100 ml of phosphate buffer pH 6.8 with occasional shaking. Then 5 ml solution was taken and diluted with phosphate buffer pH 6.8 and the resulting solution was filtered through a 0.45 μm Whatman filter paper. The drug content was then determined after proper dilution at 283 nm using UV Vis spectrophotometer [18]. |
|
Mechanical characterization of the film
|
| The flexibility of buccal patches is an important physical character needed for easy application on the site of administration. To know the flexibility, the mechanical characterization of the films needs to be determined. The casted films after drying were carefully cut into film strips (length 42.4 mm × width 19.8 mm) and investigated for the mechanical properties like tensile strength, percent elongation and young’s modulus using Instron Instrument (model 4467, Instron Corp., Canton, MA) by ASTM standard test principle [19]. Measurements were made at a crosshead speed of 5 mm/min and gauge length of 50 mm at 50% relative humidity (RH) and 23°C temperature. For each film specimen all the parameters were determined in triplicate. The folding endurance was determined by repeatedly folding the film at 180°. The folding endurance was determined by repeatedly folding one film at the same place till it breaks. The number of times the film could be folded at the same place without breaking gives the value of the folding endurance. |
| Disintegration time: The disintegration time limit of 30 second or less for orally disintegrating tablets described in CDER guidance can be applied to fast dissolving oral strips. Although, no official guidance is available for oral fast disintegrating films/strips, this may be used as a qualitative guideline for quality control test or at development stage. 10 ml of pH 6.8 phosphate buffer was taken in a petri plate and the film was placed on the surface of it. The time taken to disintegrate the film was recorded [20]. |
| In vitro drug release: Release of drug from the prepared films is a prerequisite for permeation through the buccal epithelium. Release studies determine the cumulative drug release from the formulation in a given period of time. Each film was placed in a 100 mL glass beaker containing 20 mL of phosphate buffer (pH 6.8) with the help of forceps. Mechanical stirrer was used to provide stirring speed of 100 rpm. 0.5 mL of aliquots were withdrawn every 30 second and this was done for 5 minutes. On each withdrawal, 0.5 mL of phosphate buffer solution was added to the beaker to maintain sink condition. Spectrophotometric determination of each sample was done at 283 nm and % drug released was calculated. |
| Dissolution kinetics: In order to predict and correlate the In vitro release behavior of Montelukast sodium from formulated oral fast dissolving films, it is necessary to fit into a suitable mathematical model. The In vitro drug release data were evaluated kinetically using important mathematical models: |
| Zero-order model: Q=kt+Q0; where Q represents the drug released amount in time t, and Q0 is the start value of Q; k is the rate constant. |
| First-order model: Q=Q0ek·t, where Q represents the drug released amount in time t and Q0 is the start value of Q; k is the rate constant. |
| Higuchi model: Q=kt0.5, where Q represents the drug released amount in time t and k is the rate constant. |
| Korsmeyer-Peppas model: Q=ktn, where Q represents the drug released amount in time t, k is the rate constant and n is the release exponent, indicative of drug release mechanism [21]. |
| The accuracy and prediction ability of these models were compared by calculation of squared correlation coefficient (R2). The model giving correlation coefficient close to unity was taken as the best fit model. The value of n indicates the drug release mechanism. The ‘n’ value is used to characterise different release mechanism concluding that value n=0.5 indicates Fickian diffusion and values of n between 0.5 and 1.0 or n=1.0 indicate non- Fickian mechanism. |
| Stability studies: The stability studies were carried out as per ICH Q1A (R2) guidelines for the optimized formulation. The formulations were packed in aluminum foil and placed in self-sealing bag at 40 ± 2°C and 75 ± 5% RH for duration of three months and evaluated for any change in the appearance, weight variation, drug content, drug release, disintegration time and surface pH [22]. |
Results and Discussion
|
| The aim of the present research was to formulate oral fast dissolving film of Montelukast sodium to evaluate a natural superdisintegrant. Preliminary trails were carried out for characterization of various excipients, so as to choose proper excipient. |
|
Superdisintegrant characterization
|
| The characterization of Musa paradisiaca powder was carried out to understand its suitability as a Pharmaceutical excipient in the mouth dissolving film. The results obtained for characterization are depicted in Table 3. The powder was found to be practically insoluble in water. Water soluble materials tend to dissolve rather than disintegrate, while insoluble materials generally produce rapid disintegration. Liquid is drawn up or “wicked” through capillary action and rupture the interparticulate bonds causing the formulation to break apart [23]. The swelling index was found to be 0.571, which indicates good hydration capacity. The bulk density, tapped density, Carr’s index and Hausner’s ratio were found to be in the range of Pharmacopoeial limit. The angle of repose 33.25°, indicates better flow property. On the basis of the observation Musa paradisiaca powder was used in the formulation as superdisintegrant. |
|
Selection of polymer
|
| Polymer is the major component in the film formation. The selection of the polymer is based on its property to produce a clear, transparent, non-sticky and flexible film. HPMC is the cellulose derivative which is widely used in film formulation due to its ability to form a thin uniform film. HPMC E15 LV and HPMC 6 cps were two grades of the film forming polymer tried in the preliminary trails. Whiteness and oiliness was the main problem observed with films formulated using HPMC 6 cps. These films also had the problem with peeling from the petri plate. On the other hand the films formulated with HPMC E15 LV had no whiteness and oiliness and also the peeling of the film from petri plate was easier. Hence, HPMC E15 LV was selected as the suitable polymer for the formulation of the oral films. |
|
Selection of plasticizer
|
| The flexibility is the important factor to be considered while formulating the oral film. The flexibility of the film depends on proper selection of the plasticizer. PEG 6000, PEG 400 and PEG 200 are the different grades of the plasticizer which were used in the trails. Whiteness was the major problem observed with films formulated with PEG 6000. These films also had a very small folding endurance value. Film formulated with PEG 200, no whiteness was observed but the folding endurance value was found to be small. The film containing PEG 400 had good folding endurance and no whiteness was observed. Hence, on the basis of these results PEG 400 was selected for the formulation. |
|
Development of mouth dissolving film and its characterisation
|
| To formulate an optimised formulation 32 factorial design was applied using design expert software. The concentration of two independent variables were varied at three levels i.e., HPMC E15LV (X1) 250, 500 and 750 mg and Musa paradisiaca powder (X2) 50, 100 and 200 mg. The optimisation batches so developed (Table 2) were evaluated for the physicochemical parameter such as thickness, weight variation, folding endurance, surface pH, disintegration time, % drug content and In vitro drug release. The results obtained for the evaluation are depicted in Table 4. |
|
Visual inspection
|
| Clarity, transparency and oiliness are the main parameters for visual inspection. The films were found to be clear and transparent in appearance that indicates the uniformity of the film. The film were non-oily in nature which helps to avoid the sticking of the film while administration. The films were having smooth surface and they were elegant enough to see. The clear, transparent and non-oily films were thus obtained the further evaluation was carried out. |
|
Thickness
|
| The measurement of thickness of the film is essential to ascertain the uniformity of the film thickness as it is directly related to the accuracy of dose in the film. The thickness of the film of optimization batches were found in the range 78 to 87 μm. this shows that the film were thin enough and the low SD value indicate the uniformity of the thickness. |
|
Weight variation
|
| The individual patches were weighed and average of the weight was calculated. A large variation in weight indicates the inefficiency of the method employed and is likely to have non-uniform drug content. The films showed weight variation ranging from 40.3 to 66.7 mg for the optimisation batches. The SD value is low which suggests the prepared films are uniform in weight. |
|
Surface pH
|
| The pH of the film to be administered in oral cavity must be close to that salivary pH. The surface pH was found to be in the range of 6.31 to 6.72 which is close to salivary pH. The closeness of the pH value indicates that films may be comfortable for administration and not irritate the oral mucosa. |
|
Drug content
|
| The drug content is determined to know the actual amount of drug incorporated the film. Drug content determination is important so as to get accuracy in dosing. The drug content was found to be in the range of 95 to 103.57%. The results obtained indicated that the drug is uniformly distributed throughout the film. |
|
Mechanical characterization
|
| Optimized formulation (F7) showed moderate tensile strength and high elongation with sufficient flexibility to be bent in the dried state. The results of mechanical property testing (Table 5) revealed that plasticizer addition was effective for positively modifying the nonplasticized film. All the films exhibited excellent folding endurance. |
|
Disintegration time
|
| The disintegration time of all the films was in the range of 19 to 52 sec. Films devoid of superdisintegrant took around 3-4 mins for dissolve in the salivary solution (Data not shown). Batch F7 was found to be promising and showed a disintegration time of 19 sec. From the results it can be said that at higher concentration of superdisintegrants and lower concentration of polymer, the film take a lesser time to disintegrate. Thus addition of superdisintegrant helps the faster breakdown of the film and hence fast release is obtained. |
|
In vitro drug release
|
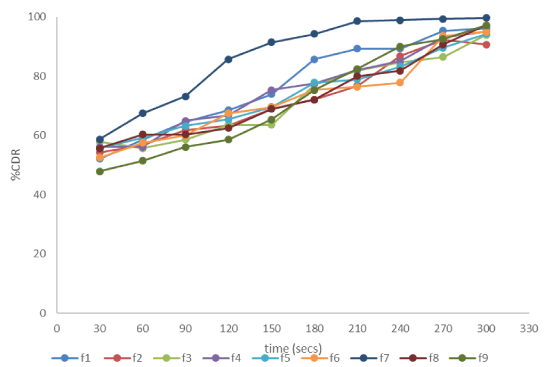
| The drug release study was carried out for 5 mins at time interval of 30 secs. The drug release profile for optimisation batches is shown Figure 1. Rapid release of drug from the films was observed on addition of the superdisintegrant to the formulation. As the concentration of the superdisintegrant was increased there was considerable increase in the drug release. But with the increase in the polymer concentration the inverse results were observed. The F7 batch containing 250 mg of HPMC and 200 mg of superdisintegrant showed the drug release of 58.71% in initial 30 secs and upto 99.64% in 5 mins. |
|
Drug release kinetics
|
| In vitro drug release data of all formulation was subjected to goodness of fit test by linear regression analysis according to zero order, first order kinetic equation, Higuchi and Korsmeyer Peppas model to ascertain the mechanism of drug release. The squared correlation coefficient (R2) and the diffusion exponent ‘n’ were calculated. The ‘n’ value of can be calculated using the Korsmeyer Peppas equation. The results obtained are depicted in Table 6. The dissolution kinetics for the films were analysed and zero order kinetic equation was found to be a good fit for the release profiles, with R2 values close to unity. The n values determined lies in between 0.5 to 1, that means it follows non- Fickian diffusion. In other words, the mouth dissolving films follow zero order release profile and the same amount of drug by unit of time. The release of the drug from the formulation occurs due to swelling and erosion of the polymer. |
|
Fitting of the model
|
| 32 factorial experimental design was selected and as required 9 batches were prepared. The ranges of Y1 and Y2 are 19-52 sec and 95- 99.64% respectively. For all the responses observed for 9 formulations prepared were simultaneously fitted to Linear, 2FI, Quadratic and Cubic models using Design Expert. It was observed that the best-fitted model were 2FI and linear for disintegration time and % drug release respectively. It is evident that all the two independent variables, namely the concentration of polymer (X1), concentration of superdisintegrant (X2), respectively have interactive effects on the two responses, Y1 and Y2. A positive value represents an effect that favors the optimization, while a negative value indicates an inverse relationship between the factor and the response. |
|
Contour plots and response surface analysis
|
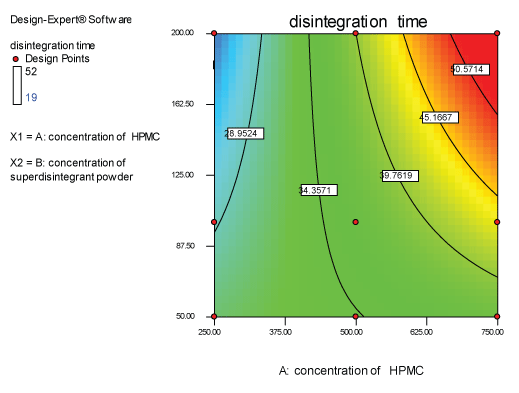
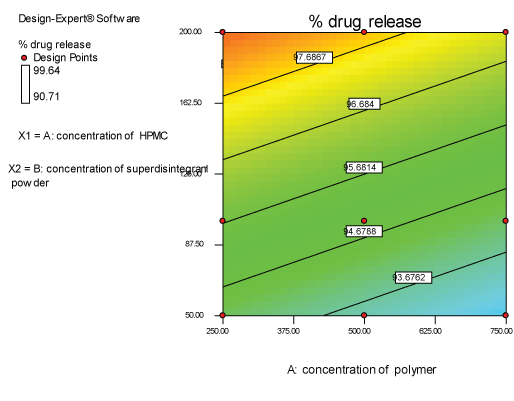
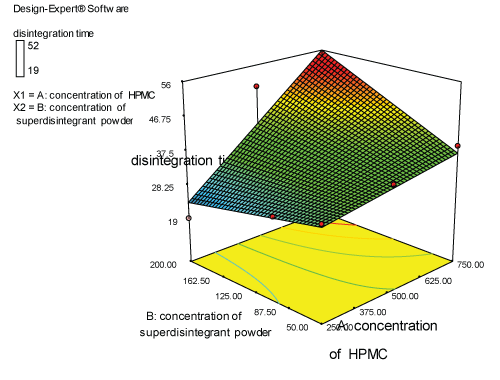
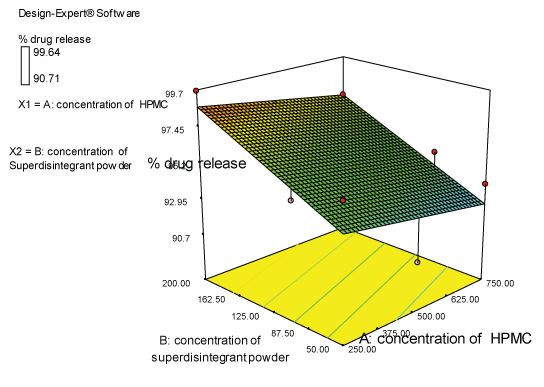
| Two dimensional contour plots were prepared for both the responses and are as shown in Figures 2 and 3 for responses Y1 and Y2 respectively. The 3D surface plots for both responses are depicted in Figures 4 and 5 for responses Y1 and Y2 respectively. These plots are known to study the interaction effects of the factors on the responses |
|
Response 1 (Y1): effect on disintegration time
|
| The model proposes the following polynomial equation for disintegration time |
| Y1=+35.33333-6.00000E-003X1+0.14000X2+3.54286E-004X1X2 |
| Where, Y1 is disintegration time, X1 is the polymer concentration, and X2 is the concentration of superdisintegration. The Model F-value of 7.09 implies the model is significant. There is only a 2.99% chance that a “Model F-Value” this large could occur due to noise. Values of “Prob>F” less than 0.0500 indicate model terms are significant. Values greater than 0.1000 indicate the model terms are not significant. If there are many insignificant model terms, model reduction may improve your model. The ANOVA data is depicted in Table 7. |
|
Response 2 (Y2): effect on % drug release
|
| The model proposes the following polynomial equation for % drug release- |
| (Y2)=+93.5100-3.10000E-003X1+0.029771X2 |
| Where, Y1 is disintegration time, X1 is the concentration of polymer, and X2 is the concentration of superdisintegration. The Model F-value of 6.66 implies the model is significant. There is only a 3.00% chance that a “Model F-Value” this large could occur due to noise. Values of “Prob>F” less than 0.0500 indicate model terms are significant. Values greater than 0.1000 indicate the model terms are not significant. If there are many insignificant model terms, model reduction may improve your model. The ANOVA data is depicted in Table 8. |
|
Optimisation
|
| The formulation of 9 batches of oral films according to 32 factorial design was carried out. The formulated batches were evaluated for various physicochemical parameters. After feeding the results in design expert software and analysing the data provided, the batch (F7) containing 250 mg of HPMC E15 LV and 200 mg of Musa paradisiaca was suggested as optimized batch. The optimized films were found to disintegrate in 19 seconds and released around 99.64% drug in 5 mins. Therefore it can be said that fast release of drug occurs from the film at higher concentration of superdisintegrant and lower concentration of the polymer. It was found that enhancing the polymer concentration shows negative effect on disintegration time and the drug release. But when the concentration of the Musa paradisiaca powder was increased, it had a positive effect on the disintegration time and drug release. |
|
Stability studies
|
| The stability studies for the optimized batch were carried out and the results of the evaluation are depicted in Table 9. The results of stability studies show no considerable variations in the appearance, weight variation and thickness. Also the drug content, surface pH, disintegration time and drug release do not show any variations. Hence, it indicates that the formulation is stable physically as well as chemically. |
Conclusion
|
| The main objective of the present study was to formulate and evaluate fast dissolving films of Montelukast sodium. The main drawback of conventional Montelukast sodium formulation is that it undergoes hepatic first pass metabolism. Thus show bioavailability upto 64%. The fast dissolving film was formulated using superdisintegrant to increase the bioavailability of drug by enhancing the drug release rate. On evaluation of the different batches of films it was found that F7 batch shows the desired results. The film starts to disintegrate rapidly and it showed almost 99.64% of drug release in just 5 minutes. The addition of superdisintegrant helped in the rapid breakdown of the film. The aim to use Musa paradisiaca powder as superdisintegrant in the oral film was satisfactorily achieved. |
Tables at a glance
|
 |
 |
 |
 |
 |
| Table 1 |
Table 2 |
Table 3 |
Table 4 |
Table 5 |
 |
 |
 |
 |
| Table 6 |
Table 7 |
Table 8 |
Table 9 |
|
Figures at a glance
|
 |
 |
 |
 |
 |
| Figure 1 |
Figure 2 |
Figure 3 |
Figure 4 |
Figure 5 |
|











