Research Article - (2024) Volume 16, Issue 2
Formulation of baclofen microemulsion and validation of analytical method for quantitative estimation by UV spectroscopy
Patidar Mohini*,
Pillai Sujit,
Deshmukh Nitin,
Patel Sumit and
Mahajan Aakansha
Department of Pharmaceutical analytical Science, GRY Institute of Pharmacy, Borawan, India
*Correspondence:
Patidar Mohini, Department of Pharmaceutical analytical Science, GRY Institute of Pharmacy, Borawan,
India,
Email:
Received: 14-Jun-2023, Manuscript No. IJDDR-23-13889;
Editor assigned: 17-Jun-2023, Pre QC No. IJDDR-23-13889 (PQ);
Reviewed: 02-Jul-2023, QC No. IJDDR-23-13889;
Revised: 05-Mar-2024, Manuscript No. IJDDR-23-13889 (R);
Published:
12-Mar-2024
Abstract
Baclofen is a centrally acting skeletal muscle relaxant used to treat muscle spasticity and multiple sclerosis. Baclofen hydrophilic characteristics make it challenging for it to cross the blood brain barrier, resulting in a bioavailability of less than 60%. The current study's objectives are to develop, optimize and validate an analytical method for the quantitative estimation of Baclofen by UV-visible spectrophotometer in accordance with ICH guidelines, as well as to improve the bioavailability and efficacy of drug release by formulating micro emulsion of Baclofen. Four different batches were formulated on the bases of S/Cos ratios 3:1, 3.5:1, 4.5:1 and 1.5:1 respectively by water titration method. Tween 80, span 80 and PEG 400 were blended together to form a homogeneous mixture, Baclofen was dissolved in oil phase. Oil phase and surfactant mixture were mixed slowly with continuous trituration. Drop wise additions of water were made to the existing mixture, which was stirred for 30 minutes at 3000 rpm on a magnetic stirrer. The result of FTIR study were indicated compatibility in between Baclofen and excipients. On the bases of solubility studies 0.1 N NaOH was selected for analytical method validation. The ƛmax and regression equation found to be 221 nm and Y=0.0603x+0.0207, R2=0.999. The result of all analytical validation parameters was not exceeding by 2% RSD value. Out of all batches M1 shows more transparent, no phase separation, pH of 6.7, particle size 100 nm, low viscosity and 96.73% drug content value. It exhibited good stability over the course of the six month stability tests, with no significant changes in its physicochemical characteristics. Hence, it was concluded that the micro emulsion formulation of Baclofen is a potential method for oral delivery that will increase bioavailability.
Keywords
Baclofen; Micro emulsion; Analytical method; 0.1 NaOH; Tween 80;
Castor oil; Polyethylene glycol
Abbreviations
NDDS: Novel Drug Delivery System; FTIR: Fourier Transform
Infrared analysis; UV spectroscopy: Ultra Violet spectroscopy; LOD: Limit of
Detection; LOQ: Limit of Quantitation; SD: Standard Deviation; RSD: Relative
Standard Deviation of the Response; σ: Slope of the calibration curve; PEG:
Polyethylene Glycol
Introduction
The term NDDS refers to entirely novel, innovative approaches for
effectively delivering pharmaceutical compounds inside the body
as needed to achieve their desired beneficial effects. Site targeting
within the body might be a part of it. It frequently worries about
both the quantity and frequency of consumption of drugs. Drug
delivery is a term that directly pertains to dosage and administration
method. The goal of NDDS is to specifically target a medication
to the necessary tissue while also improving medication potency,
controlling medication release to ensure a continued beneficial
impact and providing higher safety [1].
A micro emulsion was first proposed by Hoar and Schulman in
1943. A type of NDDS technological advances is micro emulsion
[2]. Micro emulsions are liquid combinations of oil, water and a
surfactant that are transparent, stable and isotropic, typically in
combination with another surfactant called a co-surfactant. The
"oil" may actually be a complex blend of various hydrocarbons
and olefins, while the aqueous phase may also contain salt(s) and/
or other substances. Micro emulsions do not require the high
shear conditions that are often employed to create conventional
emulsions, in contrast to ordinary emulsions and they develop
simply by mixing the components. Oil distributed in water is known
as a direct micro emulsion and water dispersed in oil is known as
a reverse micro emulsion. When two immiscible phases (water and
"oil") are present with a surfactant in ternary systems like micro
emulsions, the surfactant molecules may form a monolayer at the
interface between the oil and water. Small droplets or particles in
the dispersed phase typically range in size from 10 to 200 nm [3,4].
Classification of micro emulsion
Winsor identified four different types of micro emulsion phases
that are present in equilibrium and are referred to as Winsor phases.
Winsor I (two phase system): Upper oil layer and lower (o/w)
micro emulsion phase are in equilibrium.
Winsor II (two phase system): The upper (w/o) micro emulsion
and lower surplus water are in equilibrium
Winsor III (three phase system): Middle bi-continuous phase of
o/w and w/o, also known, is in equilibrium with upper phase oil
and lower phase water.
Winsor IV (single phase system): It creates a uniform mixture of
oil, water and surfactant [5].
Micro emulsions are superior to traditional emulsions, suspensions
and micelles solutions as well as the colloidal systems under
examination and they may provide other carriers. They are
promising delivery methods that enable prolonged or controlled
release for the administration of medications via percutaneous,
oral, topical, transdermal, visual and parenteral routes. They
benefit from spontaneous synthesis, simplicity in manufacturing
and scalability, thermodynamic stability, increased hydrophobic
drug solubilisation in medications and bioavailability [6].
Baclofen (γ-amino-β-[p-chlorophenyl]-butyric acid) is a centrally
acting skeletal muscle relaxant derived from the inhibitory
neurotransmitter γ-Amino Butyric Acid (GABA). It is used to treat
muscle spasticity, especially in patients with multiple sclerosis or
with spinal or cerebral disorders [7].
Baclofen has hydrophilic properties and does not readily cross the
blood brain barrier, because of this it’s has less than 60% oral bio
availability. The half-life is 2-6 hours after oral administration.
70%-80% of Baclofen is eliminated in an unchanged form by renal
excretion. Drug concentrations of Baclofen in the cerebrospinal
fluid are approximately 8.5 times lower than in the plasma due to
this it is prescribed to take TDS. In market Baclofen is available in
tablet, solution, packet and suspension formulations [8].
In this study, a Baclofen o/w micro emulsion was created using
polymer, oil and surfactant to increase bioavailability and improve
drug absorption across biological membranes. Low viscosity micro
emulsions have greater absorption into the body, less surfactant
content decreases negative effects and prevent first pass metabolism
which increases the bioavailability of the drug. As a less cost, more
easily scaled up option to the traditional oral, micro emulsion
formulation can be formulated.
A number of analytical methods were available to assess the purity
of the drug Baclofen through UV spectroscopy by using different
solvents. Another goal of this study to develop and optimize a
new analytical method which is quick, simple, precise, sensitive
and cost effective for estimating Baclofen using a UV-visible
spectrophotometer.
Materials and Methods
Instrumentation
UV-1800 Shimadzu with UV probe software system were utilized
for qualitative determination of baclofen, digital pH meter,
laboratory centrifuge, brookfield viscometer, stage microscope,
magnetic stirrer, pycnometer.
Reagents and chemicals
Baclofen was purchased from Yarrow Pharma Mumbai, Tween 80
was purchased from Sd Fine chemicals Ltd, Ahmadabad, Span 80
and PEG 400 was purchased from Loba chemicals Ltd Mumbai,
castor oil was purchased from gliter pharmaceutical Rangwasa, Rao,
Each of the ingredients and chemicals are an analytical grade. They
were procured from the GRY institute of pharmacy’s laboratory in
Borawan, India.
Pre formulation studies
Drug excipients compatibility study: FTIR is an effective method
for examining the interaction between drugs and excipients.
Surfactant, co-surfactant and oil were added to the pure medication
individually then KBr pellets are prepared and the resulting mixture
was scanned in the 400 cm-1–4000 cm-1 range. To reduce the
possibility of significant functional groups in the drug interacting
with the excipients, the comparison was conducted using the FTIR
spectrum of the pure drug [9].
Drug solubility analysis: A solubility analysis was carried out in
order to create a new analytical method. Baclofen powder was added
in excess to the solvents water, ethanol, methanol, 0.1 N HCl and
0.1 N NaOH and the mixture was then vortexes. The samples were
left at room temperature for 30 minutes to achieve equilibrium. The
un-dissolved drug was subsequently removed from the equilibrated
samples using sonication for 30 minutes. Then filter the solution
by Whatman filter paper. Before formulating micro emulsion
choose appropriate oil, surfactant, and co surfactant in which drug
shows maximum solubility. The same approach was conducted to
check the solubility of Baclofen in different oil, surfactants and co
surfactants.
Description of analytical method
Selection of wavelength: 100 mg pure Baclofen was dissolved in
100 ml of 0.1 N NaOH solution to create a primary stock solution
with a concentration of 1000 μg/ml. This solution was further used
to determine the wavelength of maximum absorption (ƛmax) of
baclofen. Then, 10 ml of primary stock solution was diluted with
100 ml of 0.1 N NaOH solution to make a concentration 100 μg/
ml secondary stock solution. Different concentrations of the drug
dilution (2 μg/ml, 4 μg/ml, 6 μg/ml, 8 μg/ml and 10 μg/ml) were
prepared with the same solvent and then scanned with a UV-visible
spectrophotometer between the wavelengths of 200 nm-400 nm
with a 0.1 N NaOH solution serving as a blank.
Validation of analytical method
Analytical method for Baclofen is validate as per ICH guideline.
The main validation parameters such as linearity and range,
accuracy and precision, recovery, ruggedness, LOD and LOQ were
evaluated in developed method [10-13].
Accuracy: From the stock solutions 4, 6 and 8 μg/ml concentration
dilution were made in 0.1 N NaOH and samples were scan at 221
nm to measure the accuracy. From the absorbance value % purity
was determined and calculates SD and RSD value.
Precision: The precision was determined in the terms of
repeatability, intermediate and reproducibility. 4, 6 and 8 μg/ml
concentration solutions was analysed to determine all parameter
and the relative standard deviation was calculated.
Linearity: A series of dilutions of Baclofen at 1, 2, 3, 4, 5, 6, 7,
8 and 10 g/ml were made and then scanned under a UV light.
A calibration curve was created using the resulting absorbance
data. Further calculations were made by Y-intercept equation's
correlation coefficient.
Determination of LOQ and LOD: LOD and LOQ were
determined to determine the proposed method's sensitivity. The
lowest amount of analyte quantify is LOQ, whereas LOD is the
lowest amount of analyte detectable by the technique. The ICH
recommendations were used to compute the baclofen LOD and
LOQ using the formulas LOD=3.3 /S=0.825 g/ml and LOQ=10
/S=2.5 g /ml.
Range: By comparing the results interval of lower and upper levels
of LOD, it has been possible to define the range of analytical
methods with a reasonable degree of precision and accuracy.
Robustness: The ability of an analytical method to stay unaffected
by slight but intentional changes in the method parameters is
measured as robustness, which gives a clue as to how reliable the
method will be in typical conditions. The robustness of this method
was determined by analyzing of 3 μg/ml and 6 μg/ml concentration
solutions within the day and calculating SD and RSD.
Ruggedness: The robustness of this procedure was assessed by two
analysts analyzing solutions with concentrations of 5 μg/ml and
10 μg/ml on the same equipment. After that, SD and RSD were
calculated.
Formulation of micro emulsion
Castor oil and sesame oil were chosen as the oil phases for the
creation of the o/w micro emulsion based on the solubility
investigation of the drug Baclofen. Tween 80 and span 80 mixture
together used as surfactants and co surfactants; propylene glycol is
used as the polymer and distilled water as the aqueous phase. Four
surfactant-co surfactant ratios (S/Cos ratios) batches, including
3:1, 3.5:1, 4.5:1 and 1.5:1, were tested in the current study. To
make a micro emulsion, Baclofen were dissolved in oil phase and
Surfactant, co surfactant and PEG was blended together to form
a homogeneous mixture (Smix). Oil phase and Smix phase were
mixed slowly with continuous trituration. Water was added drop in
drop to the present mixture which was mixed on a magnetic stirrer
at 3000 rpm at for 30 minutes. The slow and continuous stirring
allows equilibration between oily and aqueous phases. The resulted
mixtures ranged from a milky white, highly turbid, translucent,
and transparent liquid phase. The formation of transparent, freeflowing
mixtures indicated the endpoint of the titration. The
percentage of four different phases oil, water and mixture of
surfactant and co surfactant were calculated [7].
Evaluation parameters of baclofen microemulsion
Optical transparency: After each addition of water, the mixture
of oil, surfactant, or surfactant and co-surfactant was visually
inspected. By visual inspection formulation's colour, phase
separation, creaming and clarity were examined [14].
Viscosity measurement: Using a brook field viscometer, the
optimized formulation's viscosity was assessed without dilution
(DV-E Brookfield Viscometer Model-LVDVE). 500 ml sample
formulation was keeping in beaker, at 350°C ± 2 room temperature
and spindle no. 6, 7 at 60 rpm was used to measure the viscosity at.
For reproducible results, these processes were repeated three times.
Particle size determination: The stage microscopic method is
used to determine particle size. In this technique, a created micro
emulsion droplet was placed on a piece of glass using a stage
microscope. A 100X eyepiece lens was used to observe the droplet's
size and then particle size calculated [15].
Specific gravity: The density of the micro emulsion and the water
was obtained by using a density bottle and the specific gravity of
the micro emulsion was then calculated using the formula [16].
pH determination: The pH of the micro emulsion obtained was
measured using a digital pH meter at 25°C and calibrated with
phosphate buffer. For greater accuracy, every reading was obtained
in triplicate and the estimate of the triplicates was obtained [17].
Drug content analysis: 5 ml drug micro emulsion was dissolved in
100 ml of 0.1 N NaOH, shake the solution till the micro emulsion
is completely mixed, then kept in centrifugation tube at 3000
rpm for 30 mins. The excess was divided and filtered. From this
solution 0.1 ml solution was diluted to 10 ml with same solvent
then again filtered it. The absorbance was measured at 221 nm
by UV Spectrophotometer using the placebo micro emulsion as a
blank solution and the drug content was calculated.
Stability testing: According to ICH guidelines, the accelerated
ambient stability investigation was carried out in stability chambers
(Thermo laboratories) at temperature at 40 ± 2°C, 25°C and 10°C,
respectively and humidity (75 ± 5% RH). The formulation was kept
in a hermetically screw capped bottle with a volume of around 100
ml. In order to evaluate the physical appearance, phase separation
at accelerated gravity and drug content during the course of the
study's 6 month duration, sample analysis were taken in three
month intervals [18].
Results and Discussion
Interpretation of IR spectra of baclofen and other
excipients
Characteristic absorption bands in the IR spectra of baclofen
can be identified. They are connected to stretching vibrations
of the N-H group of primary amines (3447.89 cm-1), stretching
vibrations of the C-Cl group (664.48 cm-1) and different COOH
group (1729.65 cm-1) vibrations. The IR spectra of combinations
of baclofen with tween 80, span 80, PEG 400, castor oil and honey
contain those particular bands. There has been no change in the
chemical structure of baclofen or its compatibility with other
substances, as evidenced by the presence of all typical bands of the
drug and excipients in the IR spectra of combinations with varied
concentrations of both components (Fig. 1. and Tab. 1.).
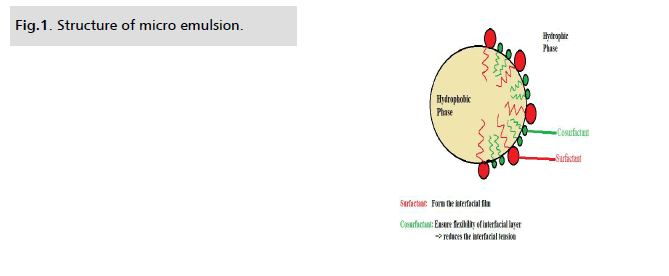
Figure 1: Structure of micro emulsion.
| Wave number (cm-1) |
Corresponding functional group and type of molecular vibration |
| 1527.15 |
Aromatic C=C (S) |
| 2817.44 |
Alkane (S) |
| 3447.89 |
Amine N-H (S) |
| 664.48 |
Halogen C-Cl (S) |
| 1729.65 |
Carboxylic acid C=O (S) |
Tab. 1. Peaks observed in infrared spectrum of baclofen.
Solubility
Baclofen shows better solubility with 0.1 N NaOH so it is used as
solvent for analytical method development (Tab. 2.).
| Solvent |
Solubility |
| Water |
Slightly soluble |
| Ethanol |
Partial soluble |
| Methanol |
Partial soluble |
| 0.1 N NaOH |
Soluble |
| 0.1 N HCl |
Soluble |
Tab. 2. Solubility study.
Analytical method results
Different dilutions ranging from 2 g/ml to 10 g/ml were prepared
for the purpose of determining the analytical wavelength and scanned at wavelengths between 200 and 400 nm in a UV visible
spectrophotometer. Maximum absorbance was detected in the
dilutions at 221 nm. Consequently, 221 nm was chosen as the
analytical wavelength (Fig. 2.)
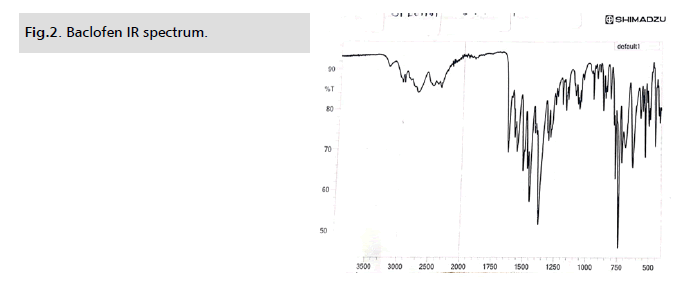
Figure 2: Baclofen IR spectrum.
Validation of analytical method
Analytical method was validated as per ICH guideline. According
to the results of linearity value concentration range from 1 μg/ml
to 10 μg/ml, correlation coefficient equation found to be y=0.0603 X+0.0207 and regression value found to be R2=0.999 (Fig. 3. and Fig. 4.). Accuracy, precision, robustness and ruggedness were
calculated and result value of the relative standard deviation was
not exceeding by 2%. Results of LOD LOQ, range were 0.825 μg/
ml, 2.5 μg/ml, 1 μg/ml, 10 μg/ml respectively (Tab. 3.).
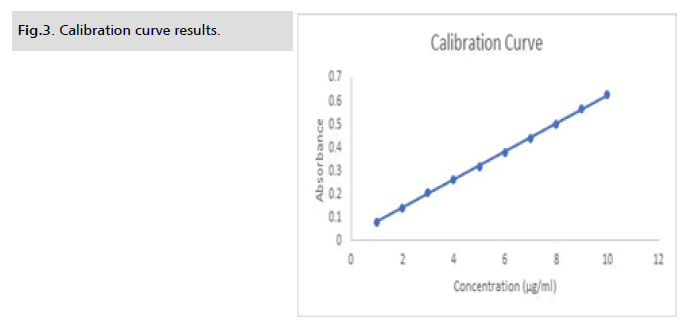
Figure 3: Calibration curve results.
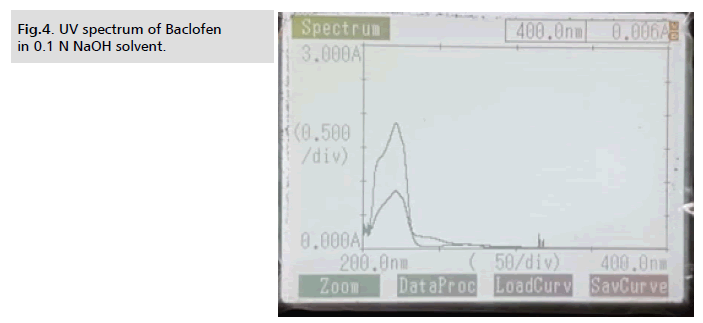
Figure 4: UV spectrum of Baclofen
in 0.1 N NaOH solvent.
| S. no. |
Parameter |
Results |
| 1 |
ƛmax |
221 nm |
| 2 |
Regression equation (y=mx+c) |
Y=0.0603x+0.0207 |
| 3 |
Slope |
0.0603 |
| 4 |
Intercept |
0.0207 |
| 5 |
Correlation coefficient (R2) |
0.9995 |
| 6 |
Accuracy (RSD) |
Less than 2% |
| 7 |
Interday precision (RSD) |
Less than 2% |
| 8 |
Intraday precision (RSD) |
Less than 2% |
| 9 |
Linearity range |
1 µg/ml-10 µg/ml |
| 10 |
LOD |
0.825 µg/ml |
| 11 |
LOQ |
2.5 µg/ml |
| 12 |
Ruggedness1 |
Less than 2% |
| 13 |
Ruggedness2 |
Less than 2% |
| 14 |
Robustness |
Less than 2% |
| 15 |
% Purity of formulation |
99.30% |
Tab. 3. Summary of validation parameters.
Composition formula optimization
Span 80 has a low HLB value with lipophilic properties, which
can be used to form oil-in-water micro emulsions when used in
combination with tween 80 having hydrophilic properties and high
HLB value. The emulsifier’s tween 80 and span 60 are frequently
used in combination. In comparison to using only one surfactant,
the combination of these two surfactants can increase the solubility of drug in water and physical stability of the micro emulsion.
PEG-400 was used as co solvents and polymer for improving the
aqueous solubility of weakly water soluble Baclofen which will
help in improving drug bioavailability. Four different composition
formulas were optimized for fixing the S/Cos ratios (Fig. 5.). Out
of four formulas M1 appear as clear, thermodynamically stable
isotropic liquid mixtures of oil, water and surfactant and their
result of evaluation are mentioned below (Tab. 4.).

Figure 5: Baclofen micro emulsion
formulations.
| Ingredients |
Formulation code (100 ml) |
Purpose |
| F1 |
F2 |
F3 |
F4 |
| Baclofen |
100 mg |
100 mg |
100 mg |
100 mg |
Drug |
| Tween 80 |
30 ml |
33 ml |
28 ml |
32 ml |
Surfactant |
| Span 80 |
10 ml |
6 ml |
13 ml |
10 ml |
Co-surfactant |
| PEG 400 |
8 ml |
8 ml |
8 ml |
10 ml |
Polymer |
| Sesame oil |
- |
32 ml |
16 ml |
14 ml |
Oil phase |
| Castor oil |
32 ml |
- |
16 ml |
16 ml |
Oil phase |
| Rose water |
4 ml |
5 ml |
3 ml |
4 ml |
Fragrance |
| Water |
16 ml |
16 ml |
16 ml |
16 ml |
Aqueous phase |
Tab. 4. Composition formula of baclofen micro emlusion.
Evaluation of formulation
Optical transparency: Micro emulsions are regularly inspected
to check the formulation's color change, phase separation in oil
and water phase, upward and down ward creaming. Through the
examination of physical parameter it was concluded that there
is no colour change and creaming of formulation’s found. Both
the phase’s oil and water were immiscible with each other. Micro
emulsion of Baclofen M1 was transparent and cleared solution.
Viscosity measurement: Using a Brookfield Viscometer, all
the formulation's viscosity was assessed at 35°C ± 2°C room
temperature and 60 rpm. Viscosity of formulation M1 and M4
were found to be 82 and 110 dynes/cm2.
Particle size determination: The rate of phase separation is directly
propositional to the radius of the globules. The particle size of the
formulation found to be in between 100 nm-125 nm, acceptable
range (Fig. 6.).
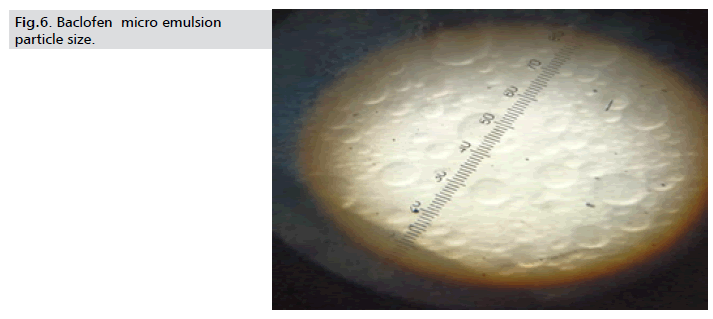
Figure 6: Baclofen micro emulsion
particle size.
Specific gravity: The density of the micro emulsion was calculated
as compared to water. The specific gravity of the micro emulsion
was found to be 0.89-1.00, equivalent to water.
pH determination: The pH of the micro emulsion was measured
using a digital pH meter. The pH of the formulation M1 found to
be 6.7, which in slightly acids in nature.
Drug content analysis
For each batch of formulation, drug content studies were completed
in triplicate. The calculated drug content was found to be in range
of 89.19% to 96.73%. According to the data, formulation M3
has the least drug, whereas formulation M1 contains the highest
amount shown in (Tab. 5.).
| Parameters |
Formulations |
| M1 |
M2 |
M3 |
M4 |
| Phase separation |
No |
No |
Yes |
Yes |
| Clarity |
Transparent |
Milky |
Milky |
Milky |
| Colour |
Yellowish |
Brown |
White |
Whitish brown |
| Viscosity (dynes/cm2) |
82 |
87 |
95 |
110 |
| Particle size(nm) |
100 |
110 |
118 |
125 |
| Specific gravity |
1 |
0.89 |
0.8 |
0.6 |
| pH |
6.7 |
6.2 |
6.4 |
6.5 |
| Drug content |
96.73%. |
90.32% |
89.19% |
92.45% |
Tab. 5. Result of evaluation parameters of baclofen micro emulsion.
Stability testing
The stability study was carried out to refine the ideal micro emulsion
formulation for harsh environments. After 3 month periods sample
was collected and evaluated. Drug content, pH and viscosity of
best formulation M1 was 95.98%, 6.6 and 81.4 respectively. There
is no phase separation, cracking and coalescence was examined in
the samples.
Conclusion
A micro emulsion of baclofen was successfully formulated. Micro
emulsions have improved the bioavailability, effectiveness, and rate
of absorption of Baclofen in accordance with the objectives of the
current investigation. The optimized formula consists of Tween 80, span 80 and PEG 400 in (3:1) ration. A FTIR investigation found
that the excipients and the medication were compatible. Utilising
0.1 N NaOH solution the analytical method was validated and It
was accurate, precise and economical. M1 batch had all the desired
characteristics and a drug content of 96.73% across all batches.
On the bases of results, it was concluded that the micro emulsion
formulation of Baclofen is a potential and effective formulation for
oral delivery that will increase bioavailability.
Acknowledgements
Without the assistance of Dr. Sujit Pillai, Mr. Sumit Patel, Ms.
Aakansha Mahajan and my family, this research would not have
been feasible. I'd like to take this initiative to thank GRY Institute
of Pharmacy for providing the required equipment and materials to
enable me to conduct the research project.
Author's Contribution
(A) Study Design · (B) Data Collection . (C) Statistical
Analysis · (D) Data Interpretation · (E) Manuscript Preparation · (F) Literature Search
· (G) No Fund Collection
References
- Chauhan L, Thakur P, Sharma S. Microemulsions: New vista in novel drug delivery system. Innov Pharm Pharmacother. 2019; 7:37-44.
[Google Scholar]
- Gibaud S, Attivi D. Micro emulsions for oral administration and their therapeutic applications. Expert Opin Drug Deliv. 2012; 9: 937-951.
[Crossref] [Google Scholar] [PubMed]
- Madhav S, Gupta D. A review on micro emulsion based system. Int J Pharm Sci Res. 2011; 2(8): 1888-1899.
[Google Scholar]
- Saini JK, Nautiyal U, Kumar M, et al. Micro emulsions: A potential novel drug delivery system. Int J Pharm Sci Res. 2014;2: 15-20.
[Google Scholar]
- Jadhav A, Daundkar A, Morale D, et al. Review on: Micro emulsion a novel approach for drug delivery. Int J Pharm Sci Rev Res. 2018;11: 60-65.
[Google Scholar]
- Katiyar BS, Katiyar SS, Mishra PS, et al. Micro emulsions: A novel drug carrier system. Int J Pharm Sci Rev Res. 2013;20: 138-148.
[Google Scholar]
- Adhao VS, Thenge RR. Development and validation of stability indicating high performance liquid chromatography method for determination of baclofen. Res Artic American J Pharm Tech Res. 2017;7:544-556.
[Google Scholar]
- Rojek B, Wesolowski M, Suchacz B. Detection of compatibility between baclofen and excipients with aid of infrared spectroscopy and chemo metry. Spectrochim Acta A Mol Biomol Spectrosc. 2013;116: 532-538.
[Crossref] [Google Scholar] [PubMed]
- Potdar M. Pharmaceutical quality assurance. Nirali prakashan. 2017;6: 8.29-8.31.
- ICH Harmonised Tripartite Guideline. Validation of analytical procedures: Text and methodology, Geneva: IFPMA. 1996;1-5.
- Gabhane KB, Jaiswal A, Tapae KK. Simple and validated ultraviolet spectrophotometric method for the estimation of baclofen in bulk form. Res J Pharm Biol Chem Sci. 2014;5: 104-109.
[Google Scholar]
- Lotfi B, Ghorbel A, HassineT. Baclofen pharma profile. Article in compendium yardley, PA. 2007: 629-632.
- Katiyar B, Katiyar S. Micro emulsions: A novel drug carrier system. Int J Pharm Sci. 2013;20: 138.
- Gui Sh Y, Wu L, Peng DY, et al. Preparation and evaluation of a micro emulsion for oral delivery of berberine. Pharmazie. 2088;63: 516–519.
[Crossref] [Google Scholar] [PubMed]
- Yadav V, Jadhav P, Kanase K, et al. Preparation and evaluation of microemulsion containing antihypertensive drug. Int J Appl Pharm. 2018;10: 138-146. [Crossref] [Google Scholar] [PubMed]
- Panghal A, Sachdeva M, Agarwal V. Formulation and development of baclofen micro emulsion incorporated into transdermal patch. J Drug deliv Ther. 2022; 12: 55-63.
[Google Scholar]
- Nath R, Bhowmik R, Chakraborty R, et al. Preparation and evaluation of a novel oral micro emulsion drug delivery system for enhancing the bioavailability of Diltiazem. Int J Pharm Sci Rev Res. 2021;70: 164-70.
[Google Scholar]
- Badawi A, Nour S, Sakran W, et al. Preparation and evaluation of micro emulsion systems containing salicylic acid. AAPS Pharm Sci Tech. 2009;10: 1081-1084.
[Crossref] [Google Scholar] [PubMed]












