Introduction
It is common for most review papers on radiation therapy to begin by acknowledging a novel discovery that was awarded the first Nobel Prize in Physics: the X-ray by Röntgen in 1895. As vital as this discovery may be, perhaps since to the X-ray we owe many of the modern achievements in radiotherapy, we will focus on another startling fact. The birth of radiotherapy took place less than a year after Röntgen took the first ever Xray, belonging to his wife’s hand (Figure 1). It was in fact a Viennese physician, Leopold Freund, who first demonstrated the therapeutic use of X-rays as a form of treatment on a 5- year old girl inflicted with nevus pigmentosus pilosus [1]. The following first half of the twentieth century witnessed an unprecedented growth and development surrounding this potentially curative technique not only in terms of invested resources but also interdisciplinary collaborations among physicists, engineers, technologists, and biologists.
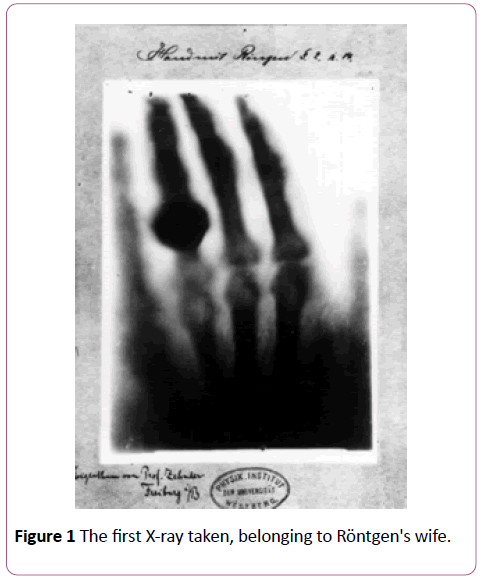
Figure 1: The first X-ray taken, belonging to Röntgen's wife.
The progress from the primitive X-raying capabilities in the early twentieth century, which reached maximum energies in the keV range, was dramatic as by the mid twentieth century peak energy maximums could be produced in the MeV range along with more efficient and cheaper methods of delivery.
One concern that has always been present in the developing course of radiation therapy is the actual method of delivery. Surprising as it may seem, even in the early stages of this technique, radiotherapists have always been aware of the vital need to tailor the delivery of radiation in order to spare normal tissue and increase accuracy to target, or cancerous tissue. The limitations to achieving this accuracy have not been due to lack of imagination but rather a lack in technology. Nevertheless, innovation has strived once more to produce the current method of custom radiation delivery known as conformal radiotherapy (CFRT). Advances in mathematics used to create algorithms and computer programs coupled with modern computer processing capabilities and 3-D imaging has marked radiation therapy as a most unique curative technique versatile enough to approach most cancers.
The purpose of this paper is to be introduced to and familiarize with the language of radiation therapy, along with some important concepts pertaining to it. We will then lead into a more focused discussion on a particular form of radiation therapy-Intensity Modulated Radiation Therapy. IMRT is a landmark therapy format which uses modulated beam profiles for appropriate levels of radiation where specifically, or even slightly generally, required. Finally, we will explore a second form of radiation therapy-Image Guided Radiation Therapy-one which greatly enhances the technique of curative therapy by localizing cancerous tissue and irradiate appropriate doses required with extreme accuracy. IGRT is described by using near real-time imaging “during treatment delivery to reduce uncertainties in target position’’ [2]. The future of IGRT is one which is soon to become a reality, building on all preceding knowledge and techniques in efforts to produce a highly effective method to detect, control, or eradicate cancer [1].
Radiation therapy
Radiation therapy may be prescribed to patients afflicted by some sort of cancer as a therapeutic, curative, adjuvant or palliative treatment whereby radiation is used to damage or destroy cell DNA. The radiation functions to directly or indirectly ionize the composite atoms of DNA, and is targeted to cancerous cells which inherit this damage and as a result, over generations, lose their ability to reproduce.
One key limitation of radiation therapy is the distinct characteristic of solid tumours becoming oxygen deficient, as they eventually outgrow their blood supply. The resultant state of hypoxia surrounding the tumour makes them inadvertently more resistant to the radiation since it is the oxygen which makes the damage to DNA permanent.
The idea behind radiation therapy may be simple; however the procedure is not that straightforward. There are many conditions which may govern the intensity, duration or type of radiation therapy administered. Treatment plans are created as specific as can be for each patient and depend on the stage, grade and type of cancer. Although the side effects induced by radiation therapy are usually minimal in most cases, they might be present in a slightly higher degree with higher doses. Acute side effects, including surface damage to epithelial cells, fatigue, edema, and infertility-should the treatment be focused on gonads-may result after months of treatment. Long-term side effects, acquired from years of exposure to radiation, include hair loss, fatigue, dryness in the salivary and lacrimal glands, fibrosis, and in rare cases cancer since radiation is a carcinogen [3]. Since most common forms of radiation therapy ultimately revolve around the concept of sending high energy photons, in the form of X-rays or γ-rays, that damage cancerous and normal, healthy cells, a proper treatment plan requires a trade-off between the chance of relapse and the chance of complications resulting from treatment. The concepts of physical phenomenon and dosing behind radiation therapy will not be discussed, as they are outside the scope of this article. What will be discussed, however, are a few important terms which denote the language used in radiology:
• Gross tumour volume (GVT) is an outlined volume that has definitive parameters which delineate tumour growth. Should any volume of GVT not receive the prescribed dosage of radiation so as to eradicate cancer, the remaining cancerous cells will have a chance to proliferate, increase chances of cancer and even metastasize.
• Clinical target volume (CTV) is defined as the volume which usually extends a few millimetres beyond the GVT to ensure proper irradiation of the tumour.
The actual dose of radiation administered, however, is defined as the planning target volume (PVT) and delineates a region which holds both the previous volumes discussed and extends a bit outwards from CTV. Although this method exposes normal tissue to radiation, it ensures complete eradication of cancerous tissue.
The organs, tissues or glands that are particularly at risk from radiation and are essential in the improvement or maintenance of the patient’s lifestyle are termed organs at risk (OAR). Damage that is too extensive to OARs may lead to loss of, or abnormalities in function or paralysis. Examples of OARs include the brain stem, spinal cord, optic nerve, mandible, parotid gland and gonads.
Oncologists have a method of determining the probability of a particular method of radiation therapy’s effectiveness and appropriate dosage. Tumor control probability (TCP) is a probability index which collects information on the proportion of patients which exhibit some level of response, or control or cure to therapy and treatment.
One form of radiation therapy is conventional radiation therapy. This form of delivery is appropriated using 2-D beams from a linear accelerator (LINAC). The radiation beam is administered to the patient from several angles and directions, front, back, or from both sides. This method is termed conventional as it recreates the actions of the LINAC, and as rudimentary as it may seem, it is being replaced by other therapeutic methods as it minimizes exposure to normal tissue.
Another form, 3-D conformal radiotherapy (3DCRT), uses computed tomography or magnetic resonance imaging, or the two coupled together to map out tumours or cancerous tissue and surrounding normal tissue. The advantage of 3DCRT lies in the profile of the beam, which is conformed to fit the parameters of the planning target volume using wedges and collimators. Collimators adjust the width, allowing for narrowing of the beam and wedges block out regions of tissue not meant to be irradiated. 3DCRT, in summation, provides more accurate exposure to cancerous tissue and spares a higher percentage of normal tissue when compared with conventional radiotherapy. The use of rotating beams, integrated with collimators and wedges greatly enhances the conformity of the beam to the actual PVT and highly increases the efficiency of the treatment.
No matter how much conformity 3DCRT may achieve around cancerous tissue however, true dose conformity, unfortunately, cannot be achieved through the use of wedges and collimators. What is required, is an even more accurate and precise method to deliver high doses of radiation at the center of the target, and lower doses around the normal periphery. Such a method is employed through another form of radiotherapy: Intensity Modulated Radiation Therapy.
Intensity modulated radiation therapy
Intensity Modulated Radiation Therapy (IMRT) is growing to become very popular as a mode of radiation therapy. In fact, there has been a recent explosion in research conducted to explore and refine this method as it, to boldly put it, supersedes the previous methods discussed. There is much united appeal and support between oncologists, medical physicists and technologists alike to push for the use of IMRT in clinical settings strictly due to its efficient nature.
In fact, the idea behind IMRT is very simple once we take into account the striving innovation that was mentioned earlier. Current technologies allow us to, in a sense, sculpt dose delivery, ever so increasing that lacking accuracy and precision with the use of multi-leaf collimator.
The remarkable phenomenon about IMRT is in the name itself, intensity modulation. Indeed, with the method provided by IMRT it is possible to modulate the beam profile and target the desired tissue with accurate intensity in the appropriate areas three dimensionally. There are two methods of irradiation which allow for the operation of this phenomenon: temporal and spatial variation in radiation delivery.
Spatial variation exploits the exponential decay of X-rays when attenuated. By using compensators, or block of metals, placed between the radiation source and the patient, the amount of radiation administered can be adjusted appropriately. In addition, different metals and their different dimensions will allow for varying amounts of radiation to pass through per unit time.
Secondly, radiation modulation can also be achieved by the use of multi-leaf collimators. When a patient is irradiated-with a constant amount-over an area, a beam profile can be created with the use of the leaves of the multi-leaf collimator. These leaves pass across the path of the radiation beam at a predetermined rate, thus effectively blocking out radiation over a given area and creating a distinct amount of dose delivered as a function of area [4]. This modulation in dose delivery is vital since the geometry of target tissue and OARs is “highly patient-dependent,” and provides for the dynamic blocking of different parts of the beam.
The use of multi-leaf collimators to contour the desired volume is governed by the treatment plan assigned by the collaborative efforts of oncologists, clinicians and radiotherapists. The previously discussed target volumes are outlined and mapped on an image generated through computed tomography. An alternate method called inverse treatment planning involves-guided by oncologists and the like-the prescribing of dose across a given volume which is thought to be most effective, through inference. Current models and algorithms are responsible for the creation of appropriate dose planning and administration, for example, the mapping of the multi-leaf collimator shown in Figure 1.
As novel as IMRT may seem however, there are a few obstacles yet to overcome [5]. Treatment planning, for instance, is not always simple and straightforward. In which, complications lie mostly within the patient as it might be difficult to arrive at a decision to irradiate if there is a history of extensive previous radiotherapy and a risk of compromising lifestyle. In addition, even with multi-leaf collimators it might yet be difficult to devise a treatment which spares OAR.
One major obstacle is the unexpected occurrences of hot spots in uncontoured regions surrounding the PTV, or tumour movement during the course of therapy.
Image guided radiation therapy
Widely acclaimed as the future of radiotherapy, image guided radiation therapy (IGRT) provides a most unique and advantageous spin on the previously discussed conventional methods. IGRT uses near real-time imaging techniques to achieve a highly accurate form of dose delivery. The procedure dictating IGRT can be summarized into six steps:
Like in any other method of radiotherapy, the initial step is the detection and diagnosis of cancerous tissue which can be carried out non-invasively owing to the novel discovery of Xrays. An example of detection and diagnosis is the use of highresolution CT scans or other methods, such as virtual endoscopy.
The next step is the delineation of target tissue and OARs, which is possible through previously discussed methods. With the advent of high speed helical scanners, such as CT and PET/CT, delineation comes with somewhat relative ease since it is now possible to co-register metabolic and anatomical data. There is potential for using MRI techniques coupled with LINACs for an even more advanced approach where target localization and treatment are more accurately and precisely coupled together.
The determination of biological attributes is a key factor in treatment. The best treatment plan is created using the current existing conditions of the tumour. Hypoxia alluded to earlier, is one important factor which governs the effectiveness of radiotherapy. Currently, there is much research effort awarded to the management of tumour hypoxia and there is promise in uncovering approaches which will non-invasively image and evaluate this condition (perhaps using PET) by means of hypoxia-specific radiotracers [2].
The fourth step involves the use of IMRT techniques, along with the real-time image evaluation discussed. This stage, where dose-distribution is designed, follows the format of IMRT treatment planning and radiation dose is sculpted or painted. In essence, the tumour volumes mentioned earlier are mapped out and algorithms used to create precise margins within which irradiation is intended.
The concept of dose delivery assurance, as two points out, holds three key elements:
• 3D volumetric of soft tissues including tumours
• efficient acquisition and comparison of the 3D volumetric
• an efficacious process for clinically meaningful intervention
Although these ideas may seem ideal on this date, they are not far from becoming a reality in the near future. The main concept needed to realize is the ability to mark and track tumour kinetics. This is presently possible in a number of ways, such as assigning specific bones or static objects within the body as landmarks. In addition, radio-opaque markers, such as seeds used in brachytherapy could be inserted as reference points. When RT is monitored using image-guidance, normal tissue is greatly spared, and dose escalation and control at target site is enforced and improved.
Lastly, it is important to monitor treatment response. Through the powerful tool of molecular imaging, treatment response can be interpreted and logged for future reference. The effectiveness of one treatment can be used to devise treatments for future cases. PET scans can be used to assess and monitor tumour progression, and similarly the eradication. The use of FDG, an analog of glucose, is a popular radioisotope used to detect the presence of cancer cells-as cancer cells metabolize glucose in high levels. Among many others, MRI is another ever-increasing popular tool to evaluate the effectiveness of radiation therapy [2].
Conclusion
Although the technique of IGRT is not yet fully employed as the leading method of radiotherapy, there is ample support for it among oncologists and radiotherapists alike. Imaging is playing an ever-increasing role in diagnostics and staging. The procedure listed above, which can perhaps be taken as a guideline, outlines critical steps that lead to addressing a diagnosis with proper care. IGRT is not so dramatically different from IMRT, yet has a rather remarkable aspect to it.
The ultimate goal of IGRT is to deliver carefully calibrated amounts of dose to an exceptionally accurate and precise target volume. Though IMRT resolves the issue of modulating radiation intensity to target volumes; IGRT uses real-time images to not only track the movement of tumours and deliver dose appropriately in order to spare normal tissue, but also uses existing radiology techniques, such as CT, PET and MRI scanning to evaluate the condition of tumours or cancerous tissues in order to determine the effectiveness of radiation therapy.
23595
References
- Kogelnik HD (1998) 100 years of radiotherapy. On the birth of a new specialty. Wien Klin Wochenschr 110: 313-320.
- Greco C, Ling C (2008) Broadening the scope of image-guided radiotherapy (IGRT). Acta Oncol 47(7): 1193-1200.
- Mossman K, Shatzman A, Chencharick J (1982) Long-term effects of radiotherapy on taste and salivary function in man. Int J Radiat Oncol Biol Phys 8(6): 991-997.
- Orlandini LC, Betti M, Fulcheri C, Coppola M, Cionini L (2015) Dosimetric impact of different multileaf collimators on prostate intensity modulated treatment planning. Rep Pract Oncol Radiother 20(5): 358-364.
- Deasy JO, Alaly JR, Zakaryan K (2007) Obstacles and advances in intensity-modulated radiation therapy treatment planning. Front Radiat Ther Oncol 40: 42-58.







