INTRODUCTION
|
| |
| Medicinal plants are used in traditional treatments to cure variety of diseases. In the last few decades there has been an exponential growth in the field of herbal medicine. Natural products have been a source of drugs for centuries. Rumex vesicarius L. is an annual plant belong to family Polygonaceae[1] , commonly known as “Bladder dock”. The importance of Rumex genus was based on its biological activities, like antimicrobial, anti-inflammatory, antidiarrhoeal and antiviral properties[2] Rumex vesicarius L. is an annual, glabrous herb, 15-30cm height, branched, leaves elliptic, ovate (or) oblong with monoecious flowers. The plant is widely cultivated as green leafy vegetable in many parts of India. According to Unnani systems of medicine, the herb is used as an analgestic, astringent, antiulcer, hepato-protective agent and also useful for scabies, leucoderma, toothache, asthma, heart troubles, tumors, and scurvy. The leaves are used as asperient, diuretic, cooling and used as an antidote for snake venom, seeds are also used as cooling agent curing dysentery and considered as an antidote for scorpion venom [5,6,7,8]. In the present study four extracts of the plant (EAE, EE, CE, and HE) were analyzed by GC-MS technique to study the major and minor phytoconstituents of the aerial edible vegetative parts of the plant. |
| |
MATERIALS AND METHODS
|
| |
|
Plant material
|
| |
| Rumex vesicarius L. was collected from fields at Tiruvannamalai, Tiruvannamalai District of Tamilnadu. The plant was well preserved and identified by BSI, South regional centre, Coimbatore, India. Type specimen was deposited in the Department of Botany, Government Arts College (Autonomous), Kumbakonam, Tamilnadu. |
| |
|
Preparation of plant extracts
|
| |
| The collected plant materials were shade dried, powered and passed through 40mm meshes and stored in closed vessel for further use. The dried powder material was extracted separately with different solvents viz., ethyl alcohol, ethyl acetate, chloroform and n-Hexane. The extract contains both polar and non-polar phytocomponents. |
| |
|
Gas chromatography-Mass spectrometry analysis
|
| |
| The Gas chromatography-Mass spectrometry (GC-MS) analysis of the extracts was performed using a GC-MS (Model; QP 2010 series, Shimadzu, Tokyo, Japan) equipped with a VF-5ms fused silica capillary column of 30m length, 0.25mm dia., and 0.25μm film thickness. For GC-MS detection, an electron ionization system with ionization energy of 70eV was used. Helieum gas (99.99%) was used as a carrier gas at a constant flow rate of 1.51ml/min. injector and mass transfer line temperature were set at 200 and 240°C respectively. The oven temperature was programmed from 70to 220°C at 10°C/min, held isothermal for 1min and finally raised to 300°C AT 10°C/min. 2μl of respective diluted samples was manually injected in the split less mode, with split ratio of 1:40 and with mass scan of 50-600 amu. Total running time of GC-MS is 35min. |
| |
| The relative percentage of the each extract constituents was expressed as percentage with peak area normalisation. |
| |
|
Identification of components
|
| |
| The identity of the components in the extracts was assigned by the comparison of their retention indices and mass spectra fragmentation patterns with those stored on the computer library and also with published literatures. NIST08.LIB[9], WILEY8.LIB[10], PESTEI_3.LIB, and FA_ME.LIB library sources were used for matching the identified components from the plant material. |
| |
RESULTS AND DISCUSSION
|
| |
| The GC-MS analysis of the extracts showed the presence of phytocomponents, the phytocomponents of each extract are presented separately in Table 1-4 and the GC-MS chromatogram with peak area of each extract is also given figure1-4. Totally 211 constituents was identified in the present study from all the four extracts of the plant. Hexane extract recorded highest number of (61) phytoconstituents, while in ethanol extract lower number of (45) phytoconstituents was observed which including both major and minor constituents. More than 10 constituents were commonly present in all the four extracts. |
| |
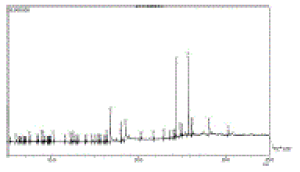
| The Hexane extract (HE) of the plant showed 61 constituents, the major constituents were n- Hexatriacontane (18.52%), Palmitic acid (16.20%), Tetra tetra conatane (14.56%), α-Linoienic acid (12.31%), gamma-Sitosterol (9.87%), Cyclohexyl amine (3.79%), α-Tocopherol (2.79%) and Methyl linolenate (2.44%) along with minor constituents were also reported. (Table1). The GC-MS chromatogram with peak area has shown in Figure1. |
| |
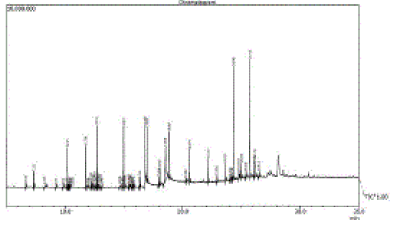
| The Chloroform extract (CE) of the plant showed 56 constituents (Table2), the major constituents were n- Tetracontane (14.19%), Docosane (11.38%), Hexadecanoic acid (9.61%), Octadecatrien (9.05%), α-Octadecene (6.77%) and l-docosene (4.80%) along with major constituents, minor constituents were also recorded. The GC-MS chromatogram with peak area was given in Figure 2. |
| |
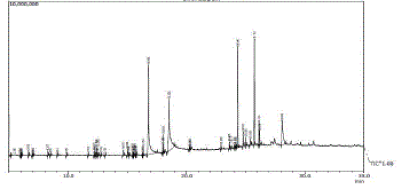
| The Ethyl acetate extract (EE) of the plant reveled 49 constituents (Table 3), the major constituents were Palmitic acid (24.38%), α-Linoienic acid (22.12%), n- Hexatriacontane (11.82%), Stigmastenol (9.96%), Tetratetracontane (9.50%) and alpha Tocopherol (3.12%) along with major constituents, minor constituents were also reported. The GC-MS chromatogram with peak area was presented in Figure 3 |
| |
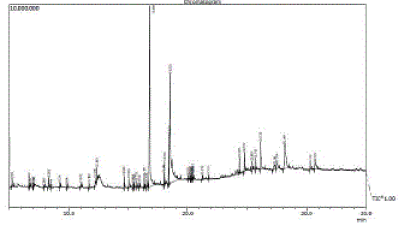
| The Ethylalcohol extract (EAE) of the plant reveled that the presence of 45 constituents (Table 4), the major constituents were Hexadecanoic acid (27.40%), α-Linoienic acid (26.53%), gamma- Sitosterol (9.61%), Methylcycloheptene (6.52%), and along with major constituents, minor constituents were also reported. The GC-MS chromatogram with peak area has shown in Figure 4. |
| |
| The aim of the present study is to provide more information about the essential phytoconstituents of Rumex vesicarius L. the results from the present investigation are very encouraging and indicates that all the plants should be studied more extensively to explore its potential to use as plant medicinal nutritive. |
| |
Tables at a glance
|
 |
 |
 |
 |
| Table 1 |
Table 2 |
Table 3 |
Table 4 |
|
| |
Figures at a glance
|
 |
 |
 |
 |
| Figure 1 |
Figure 2 |
Figure 3 |
Figure 4 |
|
| |










