Introduction
The goal of translational medicine is to expedite the development of newly identified compounds to enhance the patient’s quality of life. Translational medicine is the synergy between epidemiology, basic research and clinical trials. Translational medicine is the successful application of translational research. Both translational medicine and research are relative newcomers to clinical medicine, not significantly mentioned in peer-reviewed articles prior to year 2000. Since their appearance, interest in both translational research and translational medicine has increased, with translational research driving translational medicine (Fig.1). Translational medicine codifies evidence-based medicine into three basic areas. First, a dynamic exchange is established between the physician and the basic scientist where the clinical question is posed, discussed and focused research is initiated to yield candidate targets for treatment. The dynamic exchange matures to include the commercial sector where potential therapeutic targets are optimized, in some cases employing high throughput peptide, small molecule, phage display libraries, and candidate molecules are brought to clinical trial. At this point, the circle is completed; clinic to bench to clinic. Multicenter clinical trials are expensive in time, labor and money, but necessary to generate safe and useful treatments.
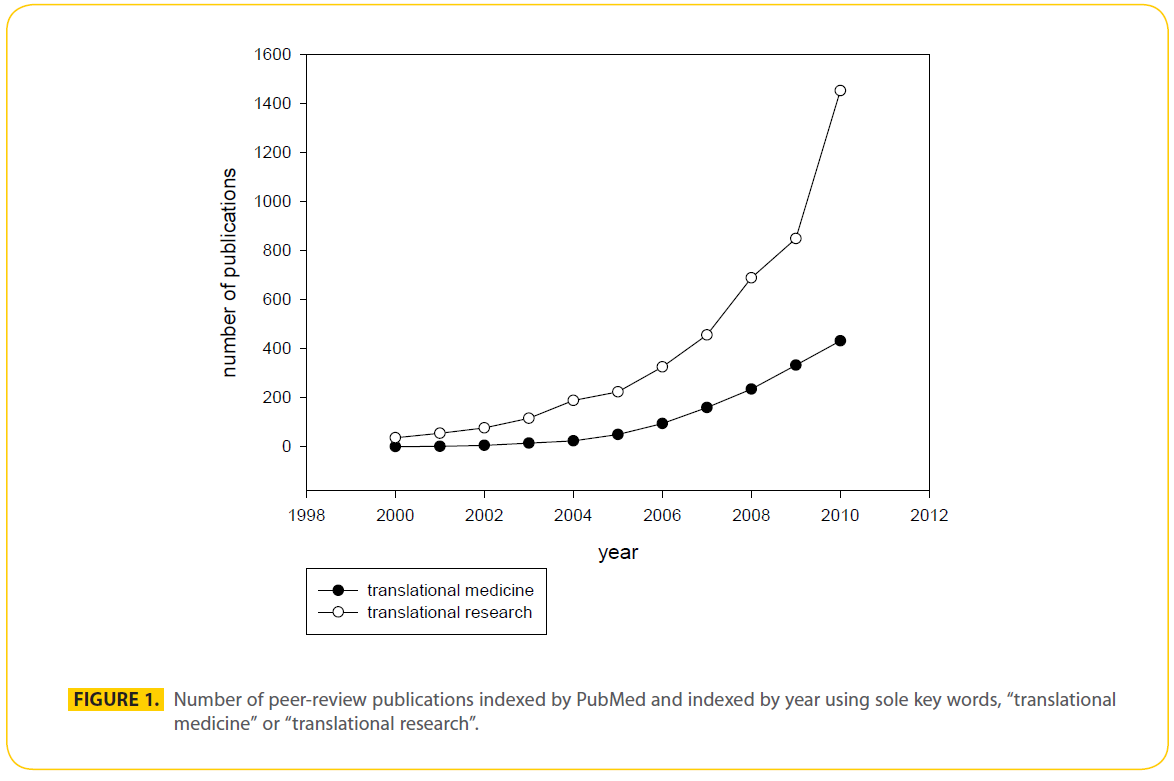
Figure 1: Number of peer-review publications indexed by PubMed and indexed by year using sole key words, “translational medicine” or “translational research”.
Translational research is a recognized as the future of therapeutic drug design, and to succeed, requires collaborations between government, academics, and industry. Ideally collaborations will reduce time and cost involved in bringing products to market. A significant benefit gained through such collaborations would be in the design of therapeutics to treat rare diseases. When advances in translational medicine reduce cost of novel drug development, all will benefit. Herein we describe the current face of translational medicine in countries who lead the world in translational medicine advances.
European initiatives and programs
Seventh Framework Program (FP7) (https://cordis.europa.eu/fp7) is a main source for research funding in EU, with a total budget of over € 50 billion (2007-2013), of which 12% was for health.[1] FP7 gives an emphasis to projects related to translational research in infectious diseases, cancer, cardiovascular disease, diabetes/ obesity, and rare diseases. The Innovative Medicines Initiative (IMI) (https://www.imi.europa.eu), one of European translational medicine (TM) programs, aims at speeding up the development of better and safer medicines. IMI is the largest public-private initiative in Europe, promoting collaborative research projects and actively establishing networks of industrial and academic experts in order to enhance pharmaceutical innovation in Europe.
The European Advanced Translational Research Infrastructure in Medicine (EATRIS) (https://www.eatris.eu) is an initiative taken by the European Strategy Forum on Research Infrastructure (ESFRI) and funded by FP7 program and had a preparatory phase from 2008-10. EATRIS will be responsible for planning of the construction and operation of a European infrastructure for translational research. Currently, EATRIS consortium consists of ten EU countries and will establish translational centres and provide training programs for the next generation of translational researchers.
United Kingdom
The UK has one of the excellent translational medicine (TM) systems in EU. Two types of biomedical research centres are playing critical roles for advancement of Translational research in the country i.e., Comprehensive BRCs and Specialist BRCs. [2] Comprehensive BRCs address a broad spectrum of topics and total five in number. [3] While, Specialist BRCs are seven in total and are more focused on one specific research area. [4,5] These centres have boasted translation research in the country which is evident by increase in total number of clinical trials numbers i.e., 290 in 2005; have risen to more than 850 in 2009- 10. Many UK universities are offering TM related programs at various levels including postgraduate certificate, master and PhD studies. The Wellcome trust along with industrial partners have established translational medicine and therapeutics programmes in various institutes which are offering rigorous basic and clinical science research training. STMTI (Wellcome Trust Scottish Translational Medicine and Therapeutics Initiative) is a collaboration between the four Scottish academic medical centres to prepare a team of academic clinicians with expertise in translational medicine and therapeutics. STMTI is funded by the welcome trust and industry.
Netherlands
Netherlands has made significant progress towards accelerating translational research in the country. The Center for Translational Molecular Medicine (CTMM) (https://www.ctmm.nl) is a joint venture between public-private partnership, dedicated to the development of medical technologies with the ultimate aim to accelerate early diagnoses and personalized treatments for cancer, cardiovascular, neurodegenerative, infectious and autoimmune diseases. CTMM receive funding from academia, industry and the Dutch government. A total of 105 partners are involved in 21 different research projects with a research budget of 275 M€. Research projects are focused on the development of molecular imaging and molecular diagnostics technologies for enhancing patient care. Various foundations are also involved and providing financial support including Netherlands Heart Foundation, the Dutch Kidney Foundation and the Dutch Diabetes Research Foundation.
The European Advanced Translational Research InfraStructure in Medicine (EATRIS) (https://www.eatris.eu) is going to establish their headquarters in the Netherlands. The Netherlands will receive a boost by this decision. The BioMedical Materials (BMM) (https://www.bmm-program.nl) program is an initiative taken by universities, university medical centers, companies and patient organizations with ultimate goal to develop novel BioMedical Materials and for their potential applications to achieve the functional repair and regeneration of tissue. The program is extended over a period of five years with funding of M€ 90.Like CTMM and BMM, Top Institute Pharma (TI Pharma) is another public-private partnership with a focus to the development of socially valuable medicines. All these three programs are playing a significant role for progress of translational research in the country.
Austria
Translational medicine is an emerging field in Austria. In the past few years, academics have undertaken initiatives for establishment of translational research infrastructure in Austria. The Austrian Science Fund (FWF) which is one of the main research funding bodies in Austria has started a translational Research Programme which is part of the BRIDGE Initiative, commissioned by the Federal Ministry for Transport, Innovation and Technology (BMVIT). (https://www.fwf.ac.at/ en/projects/translational_research.html) The programme’s goal is to support extended or “oriented” basic research at the interface to applied research. In addition, a visiting scientist porgramme “Translational Brainpower” has been stated with aim to involve international researchers in translational Research projects in Austria. (https://www.fwf.ac.at/de/applications/trptranslational- research.html).
The Austrian Research Promotion Agency (FFG) is the central organization for promoting and financing research, development and innovation in Austria. The aim of the FFG is to strengthen research and innovation in Austria. The Austrian Research Promotion Agency (FFG) offers various programs to meet the demand of enterprises, research and academic institutions. FFG also acts as a national contact point for research programmes of the European Union. By its various programs and services, FFG is actively supporting the cooperation between science and industry in Austria.
There is increasing awareness in Austrian academia about the promising role and significance of translational research. The Medical university of Vienna which is the country’s biggest medical university has established a “Center of Translational Research” (https://www.meduniwien.ac.at/orgs/ index.php?id=1041). In addition, research institutes are now focused on translational research in some specific diseases e.g., Austrian Institute of Technology (AIT). To meet the demand of industry, the University of Vienna is planning to start the country’s first quality educational programs in the field of translational medicine with the specific aim to produce high quality future’s leaders in translational medicine field.
Drivers for Translational Medicine in the Asia Pacific Region
Singapore
Singapore since the late 1990’s has transformed from being an information technology development powerhouse to one that is now focused on government sponsored partnerships with biotechnology and pharmaceutical companies, including significant educational partnerships [6]. Singapore has become a life science knowledge economy and in doing so, Singapore has invested in academic PI’s and clinical research [7]. Strategic university investments in the early part of the last decade 2000-2004 had a focus on supporting new innovations arising from experimental medicine units that could be commercialized by investigator led spin-off companies. During the previous decade many Singapore graduates wished to train in both undergraduate science and postgraduate MBA courses, however there has been a recent explosion of Singapore scientists training to PhD level. This is opposite to what was happening 10 years ago, where it was difficult to recruit Singapore PhD students, and the significant shortfall being made up of students from China, India and Malaysia. This transition in Singapore to local PhD training was likely, in part, a result of start-up companies spun out of the University system finding it difficult to attract funding without patient translational data - this led to an adjustment of the model, where the Singapore government formed partnerships with large pharmaceutical companies and expanded the number of A*STAR Institutes [7]. Partnerships have also been formed with the National University of Singapore (NUS) and Duke University, with a focus on graduate medical training and translational research with the Duke-NUS being located on the same site as Singapore General Hospital [8]. Because Singapore has close ties with sponsored biotechnology and pharmaceutical industry, educational internships are possible.
Additionally, Singapore has developed key talent development initiatives include the NUS Academy of GxP Excellence (NUSAGE) that offers post-graduate training in pharmaceutical manufacturing, and the GSKEDB partnership to build up capabilities in sustainable manufacturing. Early exposure to research training is encouraged during undergraduate science courses at NUS, with the undergraduate Research Opportunities Programme in Science (UROPS). A*STAR drives a significant component of Singapore’s translational medical science PhD training. The recent development of Singapore’s Institute for Clinical Science (SICS) in 2007 has resulted in a flagship program in neuroscience, metabolic medicine, diabetes and developmental epigenetic studies. SICS also has a strong pharmacological translational medicine program in partnership with Center for Clinical Pharmacology at the National University Hospital and Pharmaceutical companies.
China
China has no formal translational educational program, although in the last year a number of expanded PI positions have been created for translational scientist appointments. This has in part been regulated by the government to promote biotechnology development through strategic support in its ‘Medium and Long Term S&T [science and technology] Development Plan’, overseas talent-attraction programmes, commercialization initiatives, and the development of high-technology and science parks [9]. All of the leading global pharmaceutical companies have established a presence in China and many establishing R&D units. Global pharmaceutical companies in China are well placed to offer practical placements for PhD graduates and clinicians wishing to gain industry experience. Additionally the presence of pharmaceutical companies supports a central mandate for translational research outcomes [10]. However it is recognized that significant man power is now required for new pharmaceutical compound discovery, indeed it has been well cited that development of new pharmaceuticals and biologics for the world stage will only be successful if a new generation of translational scientists are trained.
Australia
Australia promotes a more traditional UK system of PhD translational research that incorporates an apprenticeship model of research training, predominately without graduate research laboratory rotations or course work programs. Australia’s main funding body National Medical and Research Council (NHMRC) has a mandate to fund translational research and support educational research training and development [11]. Reviewers of NHMRC policy have suggested that NHMRC needs to integrate its translational research within the frame work of three pillars which include research, education and clinical care. In doing so this would create Advanced Health Research Centers where significant educational translational research could be performed [12, 13]. For a hospital campus/ university/ institute collaborative to be nominated an AHRC it should be able to demonstrate it is a place that has been able to coalesce a single research and education-supported patient care mission out of all three functions. Furthermore, evidence of integrated leadership with shared purpose and investment priorities by the stakeholder organizations will be important [12, 13]. Emerging educational models with a high level of integration for clinical care, research and education include the first University owned hospital (Macquarie University Hospital), where hospital and university are located on the same grounds [14]. This model is providing graduate educational research opportunities to develop treatments relevant to clinical conditions such as neuro-vascular aneurysms. Australian National University has recently developed a master’s course work program in Translational Medicine and offers a PhD research program.
Japan
Japan also has no established translational research specific course work programs, however has established a pharmaceutical translational agenda since early 2001 in partnership with leading universities in Japan. Many Japanese medical schools are now offering integrated training in translational research [15]. Japan has also been a pioneer in regenerative medicine with Olympus Inc. exploring research opportunities in bone tissue engineering for translational applications [16]. In 1998, the Institute for Frontier Medical Sciences (IFMS) was established through the reorganization of the Chest Disease Institute and Research Center for Biomedical Engineering with the main aim of performing regenerative medicine research. Since its establishment, IFMS has been contributing to the development of human embryonic stem (ES) cells and induced pluripotent stem (iPS) cells [17] and the institute has been responsible for training many PhD graduates in translational stem cell research. The nation’s most prestige universities have each been assigned their own biological pharmaceutical targets, as directed via government.
India
India does not have a formal translational educational agenda nor has it significantly invested in translational medicine education, and current debate surrounds the integration of its curriculum and what this means for specific education domains such as Pharmacology. The Ministry of Human Resource Development (MHRD) has recently set up five new Indian Institutes of Science Education and Research (IISERs) and is in the process of establishing 30 new federal universities. The primary goal of IISERs is to integrate highquality interdisciplinary research with undergraduate teaching to improve science education and quality of future researchers in the country. India is currently developing a Translational Health Science and Technology Institute and is benefiting from a partnership with the Harvard-MIT HST[18]. Founded in 1970, HM-HST is a multi-disciplinary, multi-professional and multiinstitutional academic enterprise for research and educational, built on the premise that significant challenges to advancing working together. HM-HST has partnered with the Government of India to oversee the development of Translational Health Science and Technology Institute, and to mentor and train its faculty wherever necessary.
Malaysia
Malaysia has targeted biotechnology as a national priority to enhance productivity and sustainability, as well as build wealth and economic growth. The Malaysia Biotech Corporation was established by the government to coordinate all biotechnology industry activity. The Malaysian government has launched economic initiatives like the Ninth Malaysia Plan that allocated over US$1 billion to biotechnology industry development (2006–2010), as well as the National Biotechnology Policy and Third Industrial Master Plan (2006– 2020), to spur sector growth [13,19]. The training of PhD researchers in Malaysia to meet the demand for biotechnology research is significantly lower when compared to other Asian countries [20]. On the other hand medical training has continually expanded in Malaysia, where a growing number of offshore medical schools have been established or are under development. For example UK based University Newcastle medical school will establish a campus near the Singapore border in Malaysia which has a mandate to focus on translational research. The University Malaya Centre of Addiction Sciences (UMCAS) is a newly formed translational center of research under the Health and Translational Medicine (HTM) Cluster of University Malaya with collaborations with other research centres within UM like CeRiA and outside the university e.g., INFORRM (USM), National Anti-Drug Agency (NADA).
South Korea
South Korea has a translational education focus on meeting its demand for clinical trial educational demands. The government formed Korea National Enterprise for Clinical Trials (KoNECT) which has been operating nationwide education programs for clinical trial professionals since 2008 mainly through government funds [21]. Education centers have been selected on a competition basis for their experience, capabilities, nationwide network capacity, quality of lecturers/ tutors and excellence of program operational plans. There are currently eight institutions for different educational programs such as investigator, clinical pharmacologist, CRC, CRA, pharmaceutical medicine, pharmacoepidemiologist/ biostatistician/ data manager, and trial pharmacists. In 2012 and 2013, Clinical Trials Training Academy of Korea will focus on nurturing professionals in the special area of clinical trials, and establishing accreditation systems [21]. KoNECT hopes that these efforts will help in improving and assuring high quality of its professionals [21].
In summary, at present no translational educational metrics have been developed to assess the success of these translational programs in Asia and Australia, though many distinct and differentiated translational educational programs have emerged across Australasia. Indeed the changing landscape of translational science away from the individual PI in the lab to large funded collaborations will also impact on educational outcomes in this region of the world.
United States initiatives and programs
This fall marks the beginning of a new center, the National Center for Advancing Translational Sciences (NCATS) at the National Institutes of Health (NIH). NCATS is the vision of NIH director Francis S. Collins and involves incorporation of 5 existing programs that have a $720 million annual budget [22]. NCATS was formed in order to focus on the development of innovative strategies to overcome identified bottlenecks in new drug development that currently take 13 years, have a >95% failure rate, and cost over $1 billion dollars [22-24]. The current approach relies on NIH-funded scientists to make basic science discoveries and compliment industries efforts to develop and capitalize on new therapies [25]. Ultimately, the diminished success in new drug approval relative to the investment made has resulted in decreased industry and venture capital investment into industry research. NCATS was organized to identify and work through limitations that make the current approach unsustainable to enable translation of successful advances that resulted in increased numbers of candidate drug targets including genetically implicated disease pathways into effective therapies.
Some areas of research to focus on were identified by NCATS [22] and include the development of drugs that target pathways that are implicated in many diseases and those targeting functional genetic variants implicated by genome wide association studies (GWAS) [26]. There is a need to assess different types of molecules used as drugs and develop new ways to identify and deliver promising drugs. Ways to overcome computational limitations to predicting therapeutic structures is needed. It will be necessary to enhance the predictability of drug safety and efficacy using cell-based systems and develop treatment-response biomarkers. Phase (0) zero clinical trials using low doses of drugs in humans were proposed as a means to assess target distribution and determine formulation, dosing, pharmacokinetics, and pharmacodynamics. Large scale efforts to repurpose approved drugs for treatment of other diseases were requested along with developing more adaptive clinical trials. Finally, NCATS suggested focusing on continuing to assess efficacy and safety on FDA approved therapeutics. The outlined approach is supported with examples of success and that by focusing efforts here obstacles to therapeutic advances may be overcome [22].
Some concerns regarding the NCATS, proposed approach to facilitating therapeutic development were also addressed [22] and include assurance there will little change in relative levels of the essential basic research funding that has fueled top drug advances [27] and that NIH investigators have a record of successfully contributing to the discovery of up to 21% of FDA-approved new drugs [25]. In a time of technological revolution and an abundance of promising scientific leads it is appropriate to address business challenges as a major contributor impeding the translational potential of the times [28]. NCATS includes the interagency partnership, NIH-FDA Regulatory Science Initiative, which supports the development of advanced regulatory approaches, standards and tools to assess safety, efficacy and quality.
Also included in NCATS is the Clinical and Translational Science Awards (CTSA) program started in 2006 with similar goals of promoting collaborations between academia, government and industry to transform medical research [29-32]. There is a network of 60 CTSA centers in 30 U.S. states and the District of Columbia. CTSA centers promote advances in community engagement [33-35], comparative effectiveness research [36- 38], biostatistics, epidemiology and research design (BERD) [38], and investigator initiated clinical research involving drugs or devices [39]. The centers include predoctoral and postdoctoral clinical and translational scientist training programs [37,40,41]. The training programs attract and promote diversity and interdisciplinary collaboration primarily based in academia with opportunities to become familiar with industry and government [41,42]. The CTSA program has been successful [43,44] and was noted to double the number of collaborative papers and grants at the University of Pennsylvania [45]. CTSA researchers continue to identify and overcome challenges to translational medicine [44,46-50].
In addition, NCATS includes the Components of the Molecular Libraries Program that has the complimentary goal of developing high-throughput methodologies, the Therapeutics for Rare and Neglected Diseases program that focuses on drug development, the Rapid Access to Interventional Development program that provides access to resources for therapeutic development and the Office of Rare Diseases Research program that supports research on rare disease. NCATS will include a new program, Cures Acceleration Network which is focused on translational research on high-need medical problems. The overall goal is to facilitate drug development by advancing technologies, and commercialization would continue to be the focus of industry. Ultimately successfully overcoming the challenges inherent to translational medicine will depend on collaboration [28,51,52].
The International Society for Translational Medicine (ISTM)
The International Society for Translational Medicine (ISTM) (https://www.istmed.org) is a non-profit organization which was established in early 2011. ISTM provides a forum for interdisciplinary exchange of current knowledge and concepts on translational medicine through the society’s membership, professional development programs and conferences. ISTM is working actively to produce future TM leaders through education, training, and internship programs.[53]
2531
References
- The E.U. total health budget for framework 7 Available from: https://ec.europa.eu/research/fp7/index_en.cfm?pg=health.
- Lord, G.M. and K. Soderquest, Strategies for Translational Research in the United Kingdom. Science Translational Medicine, 2010. 2(53).
- Lord, G.M., K. Snape, and R.C. Trembath, Translational medicine and the NIHR Biomedical Research Centre concept. Qjm-an International Journal of Medicine, 2008. 101(11): p. 901-906.
- Lord, G.M. and R.C. Trembath, A strategy for translation. Lancet, 2007. 369(9575): p. 1771-1773.
- Lee, Y. and Y. Tee, Reprising the role of the developmental state in cluster development: The biomedical industry in Singapore. Singapore Journal of Tropical Geography, 2009. 30: p. 86-97.
- Edelson, E. Edelson, S (2010) SciBX 3(42); doi:10.1038/ scibx.2010.1256. SciBX, 2010. 3, 1-2 DOI: 10.1038/scibx.2010.1256.
- Halliwell, B., The National University of Singapore and what it does. Biointerphases, 2010. 5(3): p. FA15-FA18.
- Zhang, F., P. Cooke, and F. Wu, State-sponsored Research and Development: A Case Study of China’s Biotechnology. Regional Studies, 2011. 45(5): p. 575-595.
- Wang, X., E. Wang, and F. Marincola, Translational Medicine is developing in China: a new venue for collaboration. J Transl Med, 2011. 4(9): p. 3.
- Grose, S., Australian committees set to advise on translational medicine. Nat Med, 2009. 15(11): p. 1238.
- Wartman, S.A., Commentary: Academic health centers: the compelling need for recalibration. Acad Med, 2010. 85(12): p. 1821-2.
- Fisk, N., et al., Academic health science centres in Australia: let’s get competitive MJA, 2011. 194: p. 59-60.
- Clarke, R., Education programs at the new Australian School of Advanced Medicine at Macquarie University. Medical journal of Australia, 2007(187): p. 685.
- Ito, Y., New Japanese Graduate School Programs for Human Resources Development in Clinical Research. Quarterly Review 2008. 26: p. 11-25.
- Tsubouchi, M., et al., Overview of the clinical application of regenerative medicine products in Japan. Health Policy, 2008. 88(1): p. 62-72.
- Shineha, R., et al., Familiarity and Prudence of the Japanese Public with Research into Induced Pluripotent Stem Cells, and Their Desire for its Proper Regulation. Stem Cell Reviews and Reports, 2010. 6(1): p. 1-7.
- Bayry, J., S.V. Kaveri, and P. Follette, Indian science: steps to excellence. Science, 2011. 331(6013): p. 29-30.
- Ahn, M. and A. York, Resource-based and institution-based approaches to biotechnology industry development in Malaysia. Asia Pacific Journal of Management, 2011. 28(2): p. 257.
- Ng, S., et al., Influential factors to pursue doctorate degree in Malaysia. Procedia - Social and Behavioural Sciences, 2011. 15: p. 2028-2032.
- Tejasvi, A., Striving to be a global trial hub, in BioSpectrum Asia2011.
- Collins, F.S., Reengineering translational science: the time is right. Sci Transl Med, 2011. 3(90): p. 90cm17.
- Paul, S.M., et al., How to improve R&D productivity: the pharmaceutical industry’s grand challenge. Nat Rev Drug Discov, 2010. 9(3): p. 203-14.
- Munos, B., Lessons from 60 years of pharmaceutical innovation. Nat Rev Drug Discov, 2009. 8(12): p. 959-68.
- Stevens, A.J., et al., The role of public-sector research in the discovery of drugs and vaccines. N Engl J Med, 2011. 364(6): p. 535-41.
- Manolio, T.A., Genomewide association studies and assessment of the risk of disease. N Engl J Med, 2010. 363(2): p. 166-76.
- Zycher, B., J.A. DiMasi, and C.P. Milne, Private sector contributions to pharmaceutical science: thirty-five summary case histories. Am J Ther, 2010. 17(1): p. 101-20.
- Williams, R.S. and S. Desmond-Hellmann, Making translation work. Science, 2011. 332(6036): p. 1359.
- Linehan, J.H. and A. Chaney, Academic/Industry challenges for medical device development. Sci Transl Med, 2010. 2(63): p. 63mr6.
- Portilla, L.M., et al., Advancing translational research collaborations. Sci Transl Med, 2010. 2(63): p. 63cm30.
- Steele, S.J., Working with the CTSA Consortium: what we bring to the table. Sci Transl Med, 2010. 2(63): p. 63mr5.
- Evans, G. and F. Austin, Collaborations among academia, government, and industry in the diagnostics space: barriers and some ideas for solutions. Sci Transl Med, 2010. 2(63): p. 63mr3.
- Milne, C.P. and K.I. Kaitin, Translational medicine: an engine of change for bringing new technology to community health. Sci Transl Med, 2009. 1(5): p. 5cm5.
- Ross, L.F., 360 Degrees of human subjects protections in community-engaged research. Sci Transl Med, 2010. 2(45): p. 45cm23.
- Consortium, C.a.T.S.A., C.E.K.F.C. Task, and F.o.t.P.o.C. Engagement. Principals of Community Engagement. 2011 August 1, 2011; 2nd:[Available from: https://www.atsdr.cdc.gov/ communityengagement/.
- Selker, H.P., et al., White paper on CTSA consortium role in facilitating comparative effectiveness research: September 23, 2009 CTSA consortium strategic goal committee on comparative effectiveness research. Clin Transl Sci, 2010. 3(1): p. 29-37.
- Kroenke, K., et al., Training and career development for comparative effectiveness research workforce development: CTSA Consortium Strategic Goal Committee on comparative effectiveness research workgroup on workforce development. Clin Transl Sci, 2010. 3(5): p. 258-62.
- Helfand, M., et al., A CTSA agenda to advance methods for comparative effectiveness research. Clin Transl Sci, 2011. 4(3): p. 188-98.
- Berro, M., et al., Support for investigator-initiated clinical research involving investigational drugs or devices: the Clinical and Translational Science Award experience. Acad Med, 2011. 86(2): p. 217-23.
- Clinical and Translational Science Awards, 2010, https://www.ncrr. nih.gov/clinical_research_resources/clinical_and_translational_ science_awards/.
- Jackson, R.D., et al., Training the translational scientist. Sci Transl Med, 2010. 2(63): p. 63mr2.
- DuBois, J.M., et al., Instruction in the responsible conduct of research: an inventory of programs and materials within CTSAs. Clin Transl Sci, 2010. 3(3): p. 109-11.
- Fiske, W.H., et al., Efficacy of cetuximab in the treatment of Menetrier’s disease. Sci Transl Med, 2009. 1(8): p. 8ra18. 44.
- Rose, L.M. and R. Neale, Development of the first inhaled antibiotic for the treatment of cystic fibrosis. Sci Transl Med, 2010. 2(63): p. 63mr4.
- Hughes, M.E., J. Peeler, and J.B. Hogenesch, Network dynamics to evaluate performance of an academic institution. Sci Transl Med, 2010. 2(53): p. 53ps49.
- Borner, K., et al., A multi-level systems perspective for the science of team science. Sci Transl Med, 2010. 2(49): p. 49cm24. 47.
- Yock, P.G., T.J. Brinton, and S.A. Zenios, Teaching biomedical technology innovation as a discipline. Sci Transl Med, 2011. 3(92): p. 92cm18.
- Connor, E., D. Lombardi, and J. van den Anker, More than baby steps: perspectives on pediatric translational research. Sci Transl Med, 2009. 1(2): p. 2cm2.
- Portilla, L.M. and B. Alving, Reaping the benefits of biomedical research: partnerships required. Sci Transl Med, 2010. 2(35): p. 35cm17.
- Rudick, R.A. and D.M. Cosgrove, A radical proposal: integrate clinical investigation into the U.S. health care system. Sci Transl Med, 2009. 1(4): p. 4cm4.
- Munos, B.H. and W.W. Chin, A call for sharing: adapting pharmaceutical research to new realities. Sci Transl Med, 2009. 1(9): p. 9cm8.
- Deftos, L.J., Courts complicate industry-academia ties. Science, 2011. 333(6044): p. 823.
- Aamir Shahzad, R.J.C., Roland Andersson, Xiangdong Wang & Gottfried Köhler, Recommendations for comprehensive translational medicine education and training. Translational Biomedicine, (2011). Vol. 2 (No. 2:1).






