Key words
|
| |
| Clerodendron inerme, Paracetamol, Hepatoprotective activity |
| |
Introduction
|
| |
| Liver diseases remain as one of the serious health problems. However we do not have satisfactory liver protective drugs in allopathic medical practice for serious liver disorders. Herbal drugs play a role in the management of various liver disorders most of which speed up the natural healing processes of the liver. Numerous medicinal plants and their formulations are used for liver disorders in ethnomedical practice as well as traditional system of medicine in India. More than fifteen of these plants are evaluated for their hepatoprotective activities in light of modern medicine [1]. |
| |
| Clerodendron inerme, green stem shrub grows to the light. Height is about 2-3meters, width varies from 15 to 25 cm, light reqirement is Low to High, Temperature is 20oC to 30oC, Hardness tolerance is very soft to very hard, pH tolerance is 4 to 7,growth is moderate. Common name is Bonghhama in Bengali, synonym is Clerodendrum inerme, English name is Glory Bower genus, Family – verbenaceae, Genus – clerodendron, Species - inerme. Clerodendron inerme is a branched shrub, 2 to 3 meters high, the stems woody and smooth. Leaves are simple and opposite. Flowers are rotationally symmetric, salverform, white--symmetry, sex, arrangement of male and female flowers if unisexual. |
| |
| Geographical distribution: Clerodendron inerme is mainly found in India, Nepal and Bangladesh. It is mainly found in the terrestrial and tropical regions. Cultural usage: Leaves used for fever,cough and diarrhoea, also used in conjunction with other plants leaves for ghost diseases. |
| |
| Clerodendron inerme, a tropical plant, widely distributed in villages of West Bengal and reported as medicinally important plant. We have chosen this plant for the detail pharmacological studies to prove whether this plant can be used as an enormous source future medicine development. |
| |
| Herbal Liv 52 is a unique, all-natural, complex multiingredient formula. It is safe and effective in protecting the liver against harmful toxins from drugs, alcohol, food and water. Herbal Liv 52 helps regulate levels of enzymes and optimizes assimilation. Recent work shows that Herbal Liv 52 has cholesterol-regulating action. Clinically, it helps maintain healthy levels of serum cholesterol, lipoproteins, phospholipids and triglycerides. |
| |
| List of research studies and clinical trials on Liv-52: Liv-52 by Himalaya is one of the most researched herbal products one can find. More than 300 clinical studies, many double-blind, placebo-controlled, have been performed on Liv-52 (Liver Care) since its introduction more than 47 years ago. Since then, it has become one of the most widely sold medications in the world with more than two billion tablets produced annually. |
| |
MATERIALS AND METHODS:
|
| |
|
Plant Materials
|
| |
| The plant Clerodendron inerme (Family: verbenaceae) was collected from south 24 paraganas district, West Bengal, India. The plant material was taxonomically identified by the taxonomists of Botanical Survey of India, Kolkata. A voucher specimen (NO.CNH/I-I (291)/2009/Tech.II/333) has been preserved in our laboratory for future reference. |
| |
|
Extraction
|
| |
| Leaves were dried under shade and then powered with a mechanical grinder to obtain a coarse powder, which was then subjected to soak in petroleum ether overnight and subsequently packed in a Soxhlet apparatus using ethanol (600-650C).The resulting ethanolic extract was then used for studies. |
| |
|
Experimental Animals
|
| |
| Studies were carried out using male Swiss albino rats (150-200g). The animals were grouped and housed in polyacrylic cages (38 x 23 x10 cm) with not more than six animals per cage and maintained under standard laboratory conditions (temperature 250 ± 20C) with dark and light cycle (12/12 h). The animals were fed with standard pellet diet supplied by Hindustan Lever Ltd., Kolkata, India and fresh water ad libitum. All the animals were acclimatized to laboratory condition for a week before commencement of experiment. |
| |
|
Drugs and Chemicals
|
| |
| Paracetamol 500mg I.P. tab from The Martina Biogenic Pvt. Ltd., Liv.52 syrup from The Himalayan Drug Company, Bangalore, India. SGOT, SGPT, ALP, Bilirubin and Total Protein kits were procured from Span Diagnostics, Surat, India. And the rest of the chemicals utilized were of analytical grade. |
| |
|
Paracetamol-induced hepatotoxicity in rats (Acute model)
|
| |
| Male Swiss rats were randomized and divided into five groups (I-V) of six animals in each group. Group I served as untreated control and fed orally with normal saline 5ml/kg body weight daily for seven days. Group II (paracetamol treated) rats were similarly treated as group I. Group III and IV were treated with 200mg/kg i.p. and 400mg/kg i.p. body weight of the ethanolic extract daily for seven days respectively [3]. Animals of Group V were fed with standard drug Liv.52 syrup at a dose level of 0.5 mL/100g body weight (b. wt.) [4] p.o. daily for seven days. On the seventh day, paracetamol suspension was given by oral route; in a dose of 750mg/kg body weight to all rats except the rats in group I .The biochemical parameters were estimated after an 18h fast following the last dose [5]. |
| |
| Biochemical studies The blood was obtained from all animals by puncturing retro-orbital plexus. The blood samples were allowed to clot for 45 min at room temperature. Serum was separated by centrifugation at 2500 rpm at 300C for 15 min and utilized for the estimation of various biochemical parameters namely SGOT, SGPT [6], SALP [7], serum bilirubin [8] and total protein [9]. |
| |
|
Serum hepatospecific markers
|
| |
| Activities of serum glutamate oxaloacetate transaminase (SGOT) and serum glutamate pyruvate transaminase (SGPT) were estimated by the method of Reitman and Frankel [6]. 0.05 ml of serum with 0.25 ml of substrate (aspartate and á-ketoglutarate for SGOT; alanine and á - keto glutarate for SGPT, in phosphate buffer pH 7.4) was incubated for an hour in case of SGOT and 30 min. for SGPT. 0.25 ml of DNPH solution was added to arrest the reaction and kept for 20 min in room temperature. After incubation 1 ml of 0.4N NaOH was added and absorbance was read at 505 nm in uv-vis spectrophotometer. Activities were expressed as IU/L. |
| |
| Based on the method of King and Armstrong [7] alkaline phosphatase activity was assayed using disodium phenyl phosphate as substrate. The colour developed was read at 510 nm in uv-vis spectrophotometer after 10 min. Activities of ALP was expressed as IU/L. Serum total bilirubin level was estimated based on the method of Malloy and Evelyn [8]. Diazotised sulphonilic acid (0.25 ml) reacts with bilirubin in diluted serum (0.1 ml serum + 0.9 ml distilled water) and forms purple colored azobilirubin, which was measured at 540 nm in uvvis spectrophotometer. Activities of total bilirubin were expressed as mg/dl. Serum total protein level was estimated based on the method of Gornall et al [9]. Biuret reagent (1.0 ml) reacts with serum (10 ìL) and the colour developed was read at 578 nm in uvvis spectrophotometer. Activities of total protein were expressed as mg/dl |
| |
|
Statistical Analysis:
|
| |
| The data were expressed as mean ±SEM (Standard error mean).The statistical significance between groups was analyzed using Student’s t-test and P value of 0.05 or less was considered significant. |
| |
RESULTS
|
| |
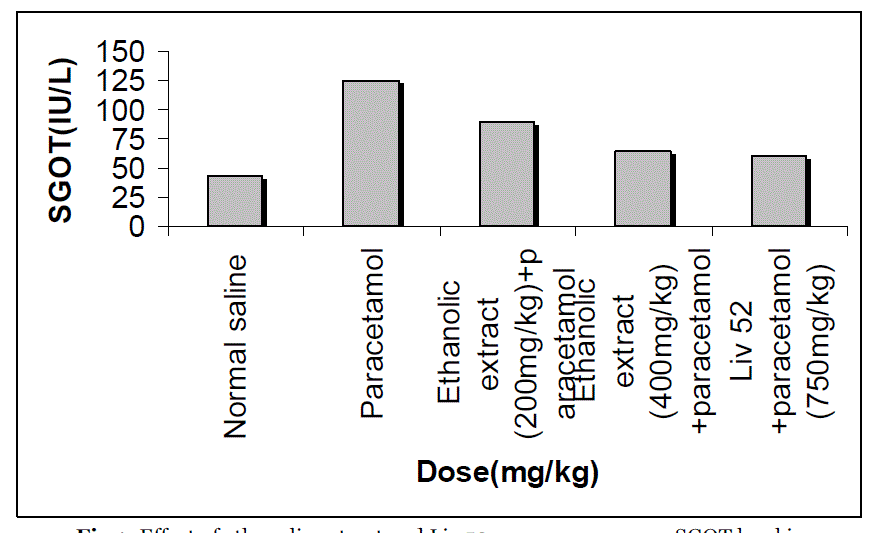
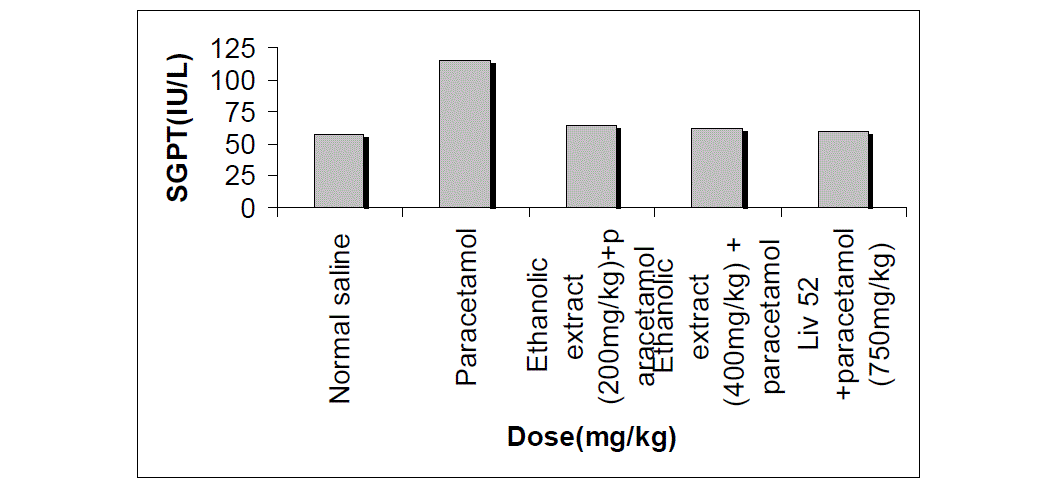
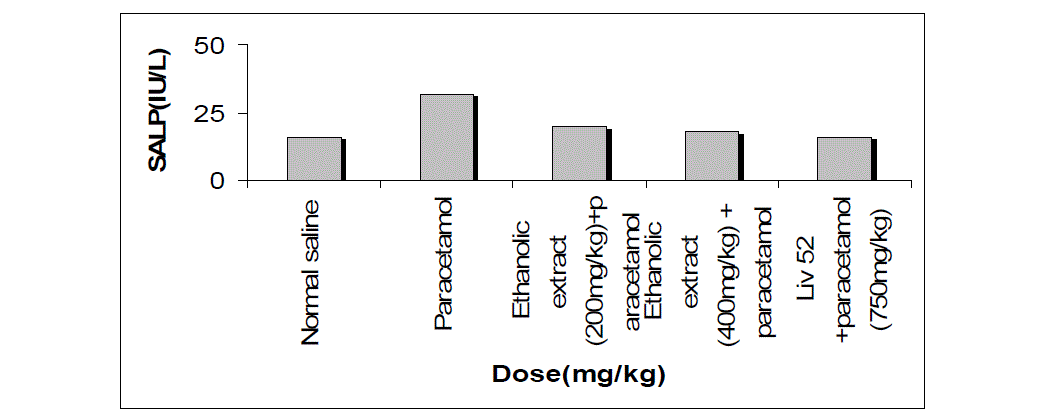
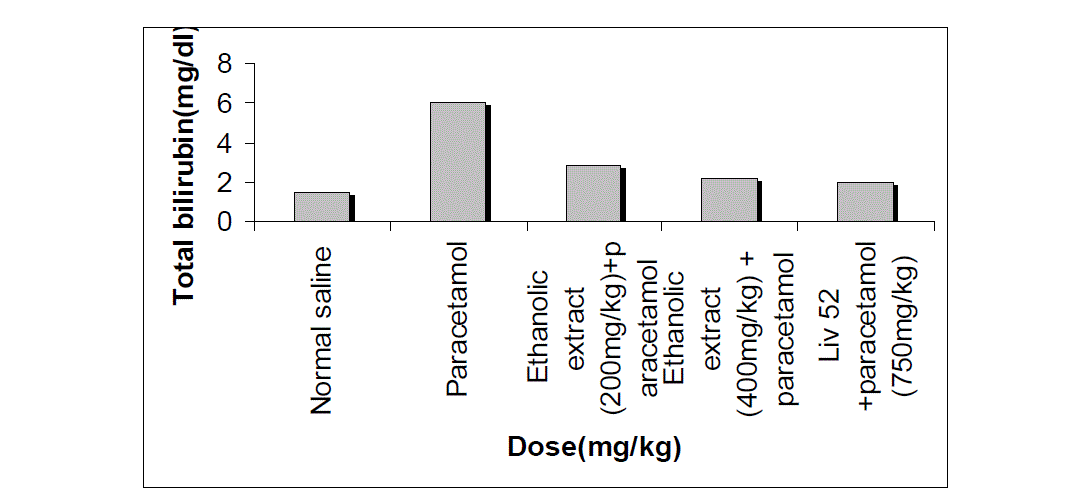
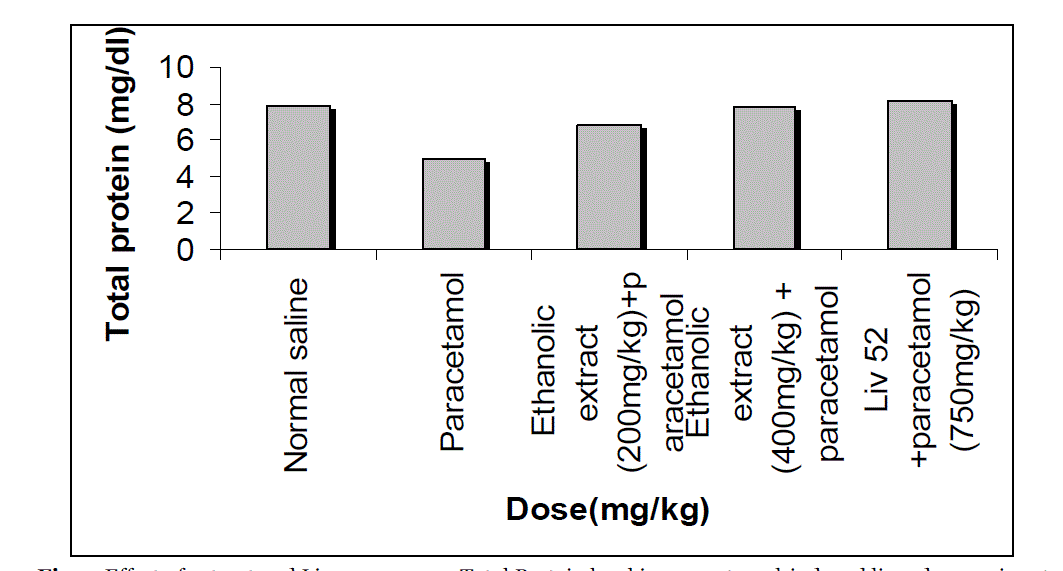
| The effects extract on serum transaminase, alkaline phosphatase, bilirubin and total protein levels in paracetamol-induced liver damage in rats are summarized in Table no. -1, 2, 3, 4 & 5. Administration of paracetamol (750mg/kg; body weight), after 18 hours of intoxication resulted a significant (P<0.05) elevation of hepatospecific serum markers SGOT, SGPT, SALP, bilirubin and total protein in paracetamol-treated group, in comparison with the normal control group. On administration of extract (Group III & IV) and Liv 52 (Group V) the level of these enzymes were found retrieving towards normalcy. |
| |
| All the results of extract on serum transaminase, alkaline phosphatase, bilirubin and total protein levels in paracetamol-induced liver damage in rats are shown by bar chart in figure no.-1, 2, 3, 4 & 5. |
| |
DISCUSSION
|
| |
| Paracetamol (acetaminophen) is a commonly and widely used analgesic and antipyretic agent. Hepatotoxic doses of acetaminophen deplete the normal levels of hepatic glutathione, when (N-acetylp- benzo-quinone imine) NAPQI covalently binds to cysteine groups on proteins to form 3-(cystein-S-yl) acetaminophen adducts [10]. The glutathione protects hepatocytes by combining with the reactive metabolite of paracetamol thus preventing their covalent binding to liver proteins [11]. |
| |
| In living systems, liver is considered to be highly sensitive to toxic agents. The study of different enzyme activities such as SGOT, SGPT, SALP, total bilirubin and total protein have been found to be of great value in the assessment of clinical and experimental liver damage [12]. In the present investigation it was observed that the animals treated with acetaminophen resulted in significant hepatic damage as shown by the elevated levels of serum markers. These changes in the marker levels will reflect in hepatic structural integrity. The rise in the SGOT is usually accompanied by an elevation in the levels of SGPT, which play a vital role in the conversion of amino acids to keto acids [13]. The pretreatment with ethanolic extract, both at the dose of 200mg/kg and 400mg/kg, significantly attenuated the elevated levels of the serum markers. The normalization of serum markers by ethanolic extract suggests that they are able to condition the hepatocytes so as to protect the membrane integrity against acetaminophen induced leakage of marker enzymes into the circulation. The above changes can be considered as an expression of the functional improvement of hepatocytes, which may be caused by an accelerated regeneration of parenchyma cells. Serum ALP and bilirubin levels, on the other hand are related to hepatic cell damage Increase in serum level of ALP is due to increased synthesis in presence of increasing billiary pressure [14]. Effective control of bilirubin level and alkaline phosphatase activity points towards an early improvement in the secretory mechanism of the hepatic cell. |
| |
| Our studies showed that ethanolic extract of Clerodendron inerme leaves in the dose of 200mg/kg and 400mg/kg has significant effects. Therefore, ethanolic extract appears to be useful in the attenuation of paracetamol induced liver damage and showed more prominent action. |
| |
| The ethanolic extract of Clerodendron inerme leaves showed significant activity against paracetamol induced liver damage in rats when compared with that of standard drug Liv 52. |
| |
CONCLUSION
|
| |
| The present investigation indicates that ethanolic extract of Clerodendron inerme leaves exhibits significant barrier against paracetamol-induced toxicity. Our studies also showed that ethanolic extract at the dose of 400mg/kg has more effect than the extract of 200mg/kg dose level. Therefore, ethanolic extract of Clerodendron inerme leaves in the dose of 400mg/kg appears to be more useful and showed more prominent effect than in the dose of 200mg/kg . Both the extracts showed significant activities against paracetamol induced liver damage in Swiss albino rats when compared with that of standard drug Liv.52. Further investigation is necessary to determine the pharmacologically active molecules in the ethanolic extracts that are responsible for its hepatoprotective effect. |
| |
Acknowledgment
|
| |
| The work has been supported by University Grant Commission, New Delhi, India. |
| |
Conflict of Interest
|
| |
| NIL |
| |
Source of Support
|
| |
| NONE |
| |
Tables at a glance
|
 |
 |
 |
 |
 |
| Table 1 |
Table 2 |
Table 3 |
Table 4 |
Table 5 |
|
| |
Figures at a glance
|
 |
 |
 |
 |
 |
| Figure 1 |
Figure 2 |
Figure 3 |
Figure 4 |
Figure 5 |
|
| |











