Keywords
Left/right isomerism, Heterotaxy, Situs ambiguous, Congenital malformation, Human foetus, Foetal autopsy, Embryology
Introduction
Heterotaxy is a rare malformation syndrome with an incidence of 1-1.5/10,000 live births and a high mortality rate [1-3]. It is defined as an abnormal arrangement of viscera across the left/right axis primarily induced by disorders of laterality determination during early embryonic development. The presence of bilateral right- or left-sided “situs ambiguous” should be differentiated from the usual “situs solitus” and the mirror image “situs inversus”. The classic term “bilateral sidedness” implies the presence of bilateral equivalent lungs and bronchi as well as bilateral symmetric atria. In this context the arrangement of the bronchopulmonar tract and the atrial appendages is of particular importance for correct classification. Heterotaxy can be classified into bilateral right-sided -“right isomerism”- and left-sided -“left isomerism”- [4]. Typical prenatal findings in both varieties are viscerocardiac heterotaxy, complex cardiac malformations and anomalies of the caval veins [5]. In most cases, however, a wide variety of cardiac and extracardiac congenital malformations, with a considerable overlap of the anatomical features, can occur. As a consequence, the prenatal differentiation between the two main settings is sometimes unclear. A detailed knowledge of the morphogenesis in cases of heterotaxy is essential for understanding the common varieties of different organ systems in view of an exact prenatal diagnosis. This is mandatory for adequate parental counselling regarding further diagnostic steps and the planning of perinatal management [6].
Material and Methods
Specific signs of heterotaxy were prenatally detected in 5 human foetuses. The 4 foetuses with suspected left isomerism were female, and the foetus with right isomerism was male. Following the termination of the pregnancy in the second trimester (14 weeks/4 days, 16 weeks/0 days, 17 weeks/3 days; 20 weeks/2 days; 22 weeks/6 days) a detailed anatomical survey in accordance with the guidelines of the sequential segmental approach was performed [7]. We report the complete spectrum of autoptic findings with respect to the underlying disorders in the early embryonic development.
Results
Of the 5 foetuses with prenatally suspected left isomerism, the diagnosis was confirmed by post mortem in 4 cases, in 1 case, however, right isomerism was detected.
Table 1 displays the sonographic findings of the 4 foetuses with left isomerism. Structural heart disease, in combination with a complete atrioventricular septal defect, was prenatally observed in 3 and with an isolated ventricular septal defect in 1 of the cases. Persistent bradyarrhythmia and dysfunction at the level of ventriculoarterial connections – for example tetralogy of Fallot, common arterial trunk and aortic stenosis - were suspected in 3 and common atrium in 2 cases but dextrocardia in 1 case. Typical extracardiac prenatal findings were right sided abdominal viscera in 3 cases.
A summary of the autoptic findings is given in Table 2. The structural heart disease could be confirmed by post mortem in all 4 cases of left isomerism. As seen in the table, the majority of all segments of the foetal heart were affected by partial severe anomalies.
At the precardial venous level (S1) we diagnosed a lack of continuity of the IVC in all cases with a specific discontinuity below the liver (Figure 1B and 1C). As a consequence, its upper hepatic portion had a separate opening at the bottom of the atrium, and the lower portion drained into the enlarged hemiazygos or azygos vein (Figure 2A and 2B). The inferior portion of the IVC, therefore, could not be identified in its usual position but rather to the left of the spine. The venous drainage of the SVC was regularly found to be on the right side of the common atrium in 2 cases. In the other 2 cases, however, a persistent SVC was additionally (Figure 1B) observed on the left (Figure 1C). At venoatrial level (S2) a common atrium was confirmed in 2 cases. Moreover, bilateral sickle-shaped appendages and left configured atria were diagnosed (Figure 2A-2D). Both atrial appendages were elongated and attached by a narrow junction to the smooth-walled portion of the atrium, which presents as morphological left atrial appendages. There was absolutely no terminal crest. The pectinate muscles were confined to the appendages and not extended around the atrioventricular junction as in the morphologic right atrium. Within the atrium we found only a thin band of tissue, characteristic of a remnant of the atrial septum. The pulmonary veins joined the common atrium at the central part of the posterior atrial wall. Anomalies at the level of atrioventricular connection (S3) were also detected in all cases. Commonly, the undivided AV canal opened with a dysplastic AV valve into a bifid ventricle. At the ventricular level (S4) in the main functional anomalies have priority. Analysing the ventriculoarterial connection (S5) a double outlet right ventricle was diagnosed in 2 of 4 cases. With regard to the relationship of the great arteries to one another, the ascending aorta left the heart right and in front of the pulmonary trunk (Figure 2C and 2D). We diagnosed, therefore, a complete transposition of the great arteries with the aortic valve anterior and to the right of the pulmonary valve. Structural defects were also seen in the postcardial arterial segment (S6) in 3 of the 4 cases. It referred to hypoplasia of one of the great arteries and coarctation or malposition of the aortic arch as well as anomalies of the ductus arteriosus Botalli.
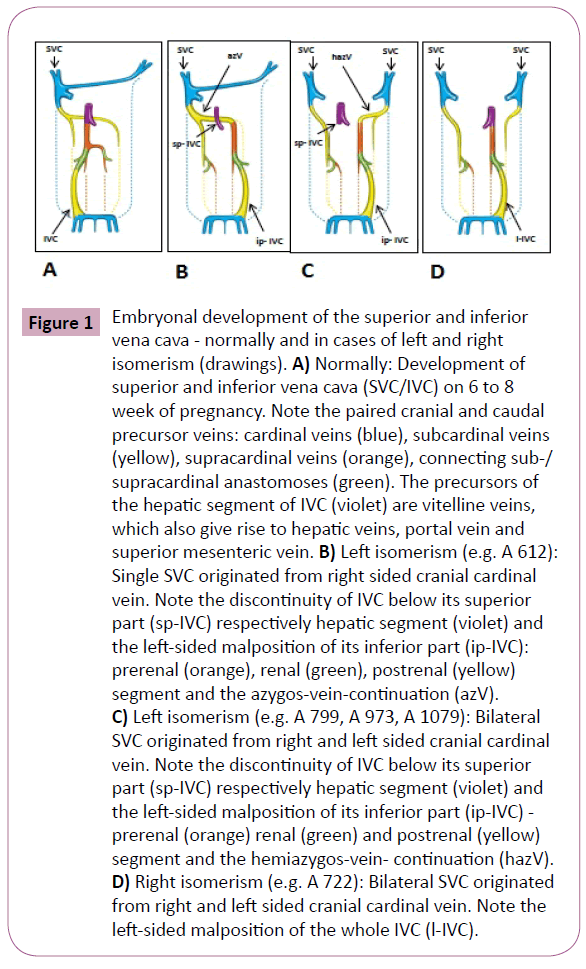
Figure 1: Embryonal development of the superior and inferior vena cava - normally and in cases of left and right isomerism (drawings). A) Normally: Development of superior and inferior vena cava (SVC/IVC) on 6 to 8 week of pregnancy. Note the paired cranial and caudal precursor veins: cardinal veins (blue), subcardinal veins (yellow), supracardinal veins (orange), connecting sub-/ supracardinal anastomoses (green). The precursors of the hepatic segment of IVC (violet) are vitelline veins, which also give rise to hepatic veins, portal vein and superior mesenteric vein. B) Left isomerism (e.g. A 612): Single SVC originated from right sided cranial cardinal vein. Note the discontinuity of IVC below its superior part (sp-IVC) respectively hepatic segment (violet) and the left-sided malposition of its inferior part (ip-IVC): prerenal (orange), renal (green), postrenal (yellow) segment and the azygos-vein-continuation (azV). C) Left isomerism (e.g. A 799, A 973, A 1079): Bilateral SVC originated from right and left sided cranial cardinal vein. Note the discontinuity of IVC below its superior part (sp-IVC) respectively hepatic segment (violet) and the left-sided malposition of its inferior part (ip-IVC) - prerenal (orange) renal (green) and postrenal (yellow) segment and the hemiazygos-vein- continuation (hazV). D) Right isomerism (e.g. A 722): Bilateral SVC originated from right and left sided cranial cardinal vein. Note the left-sided malposition of the whole IVC (l-IVC).
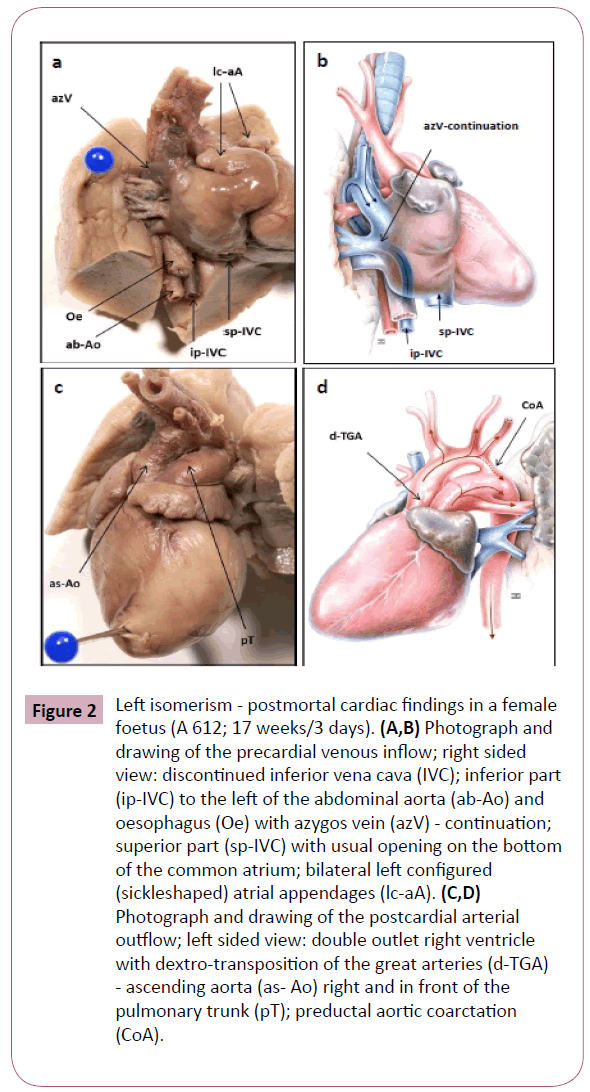
Figure 2: Left isomerism - postmortal cardiac findings in a female foetus (A 612; 17 weeks/3 days). (A,B) Photograph and drawing of the precardial venous inflow; right sided view: discontinued inferior vena cava (IVC); inferior part (ip-IVC) to the left of the abdominal aorta (ab-Ao) and oesophagus (Oe) with azygos vein (azV) - continuation; superior part (sp-IVC) with usual opening on the bottom of the common atrium; bilateral left configured (sickleshaped) atrial appendages (lc-aA). (C,D) Photograph and drawing of the postcardial arterial outflow; left sided view: double outlet right ventricle with dextro-transposition of the great arteries (d-TGA) - ascending aorta (as- Ao) right and in front of the pulmonary trunk (pT); preductal aortic coarctation (CoA).
With reference to extracardial post mortem findings in cases of left isomerism, the bronchial morphology corresponded to that of the bilateral left configured atria because both lungs were bilobed with two symmetrical hyparterial morphologic left bronchi. The liver was bilateral symmetrical, and in one case the extrahepatic biliary structures, such as the biliary bladder, were atretic. Multiple spleens (polysplenia) and the pancreatic tail were found in the right upper abdomen (Figure 3B). The right sided stomach continued in a distorted malpositioned duodenum on the left side and the duodeno-jejunal junction, as well as the ligament of Treitz, were found right of the spine. The portal vein was found below the superior part of duodenum and the superior mesenteric vessels behind its descending part. As a consequence the small bowel was located on the left and the whole large intestine on the right of the abdomen. With regard to this malpositioned colon we were not able to detect any mesenteric attachement (common mesenterium). Moreover, in 3 foetuses of left isomerism an incoherent laterality between the left sided heart axis (levocardia) and the partial situs inversus in the upper abdomen was diagnosed, also known as viscerocardiac heterotaxy or situs ambiguous.
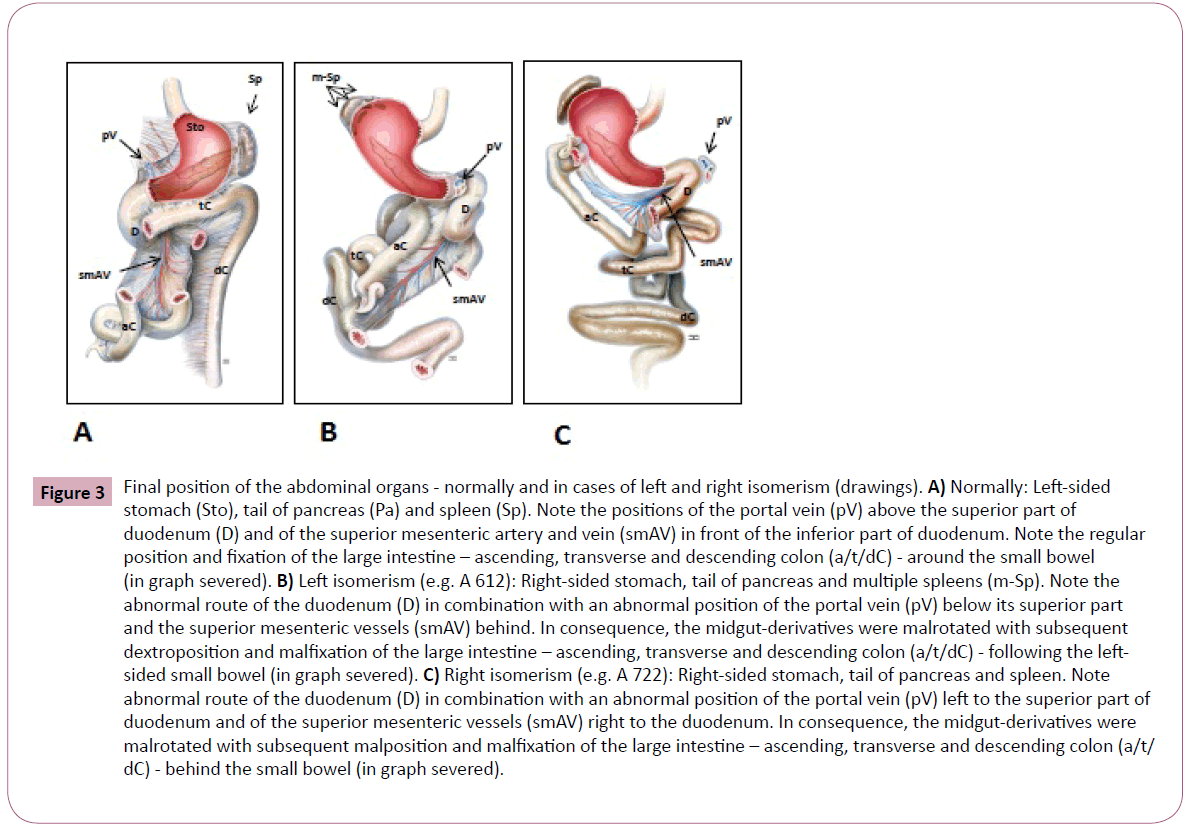
Figure 3: Final position of the abdominal organs - normally and in cases of left and right isomerism (drawings). A) Normally: Left-sided stomach (Sto), tail of pancreas (Pa) and spleen (Sp). Note the positions of the portal vein (pV) above the superior part of duodenum (D) and of the superior mesenteric artery and vein (smAV) in front of the inferior part of duodenum. Note the regular position and fixation of the large intestine – ascending, transverse and descending colon (a/t/dC) - around the small bowel (in graph severed). B) Left isomerism (e.g. A 612): Right-sided stomach, tail of pancreas and multiple spleens (m-Sp). Note the abnormal route of the duodenum (D) in combination with an abnormal position of the portal vein (pV) below its superior part and the superior mesenteric vessels (smAV) behind. In consequence, the midgut-derivatives were malrotated with subsequent dextroposition and malfixation of the large intestine – ascending, transverse and descending colon (a/t/dC) - following the leftsided small bowel (in graph severed). C) Right isomerism (e.g. A 722): Right-sided stomach, tail of pancreas and spleen. Note abnormal route of the duodenum (D) in combination with an abnormal position of the portal vein (pV) left to the superior part of duodenum and of the superior mesenteric vessels (smAV) right to the duodenum. In consequence, the midgut-derivatives were malrotated with subsequent malposition and malfixation of the large intestine – ascending, transverse and descending colon (a/t/ dC) - behind the small bowel (in graph severed).
A summary of the sonographic findings of the foetus with right isomerism is also given in Tables 1. Structural heart disease concerning complete atrioventricular septal defect and common arterial trunk was prenatally diagnosed. Other extracardiac findings were a right sided stomach and incomplete situs inversus.
Foetus
sex/gestational age |
Echocardiographic Findings |
Extracardiac Findings |
A 612 ?
17 weeks + 3 days |
• dextrocardia
• common atrium
• complete AVSD
• hyperplasia of both ventricles
• severe bradyarrhythmia |
• visceroatrial situs inversus
• right-sided stomach |
A 799 ?
22 weeks + 6 days |
• dextrocardia
• common atrium
• complete AVSD
• hyperplasia of both ventricles
• severe bradyarrhythmia |
• visceroatrial situs inversus
• right-sided stomach |
A 973 ?
16 weeks + 0 days |
• levocardia
• complete AVSD
• common arterial trunc
• sinusrhythm (158bpm) |
• abdominal situs inversus
• right-sided stomach |
A 1079 ?
14 weeks + 4 days |
• levocardia
• mitral valve atresia
• hypoplastic left ventricle
• VSD
• aortic stenosis
• bradyarrhythmia (44bpm) |
• visceroatrial situs solitus |
Left Isomerism
(sonographically suspected and post mortem confirmed) |
A 722 ?
20 weeks + 2days |
• levocardia
• complete AVSD
• common arterial trunc
• sinusrhythm |
• incomplete situs inversus
• right-sided stomach |
Left Isomerism
(sonographically suspected but post mortem diagnosed right isomerism) |
AV = Atrio Ventricluar; AVSD = Atrio Ventricluar Septal Defect; VSD = Ventricluar Septal Defect; IVC = Inferior Vena Cava
Table 1: Sonographic findings in 5 foetuses with heterotaxy
The structural heart disease could be confirmed at post mortem. As seen in Table 2, all segments of the foetal heart were affected. At the precardial venous level (S1) we diagnosed a persistent left SVC and a leftsided IVC anterior to the abdominal aorta (Figure 1D). At venoatrial level (S2) the common atrium was characterized by inverse configured appendages and atria (Figure 4A-4D). That part of the atrial wall which is responsible for pulmonaryvenous return was hypoplastic. The pulmonary veins joined the common atrium at the central part of the posterior atrial wall between the both SVC and the coronary sinus was missed. At the level of atrioventricular connection (S3) the sonographically detected complete atrioventricular septal defect, with a commen dysplastic atrioventricular valve, could be confirmed. At the ventriculoarterial connection (S4 and S5) a single ventricle and a big semilunar valve with continuation into a common arterial trunk were diagnosed as seen in Figure 4A and 4B. Analysing the postcardial arterial segment (S6) we detected the route of the aortic arch to the right of the trachea and diagnosed mirrorinverted origins of its systemic branches followed by systemic pulmonary arteries.
| Foetus |
Cardiac Findings |
Extracardiac Findings |
| A 612 ? |
• levocardia
• S1: discontinued IVC with azygos continuation
• S2: common atrium with bilateral sickle-shaped appendages and atria
• S3: complete AVSD with common dysplastic AV valve
• S4: functional single ventricle
• S5: double outlet right ventricle (DORV) and d-transposition great arteries (d-TGA)
• S6: preductal aortic coarctation (CoA) |
• bilateral bilobar lungs and hyparterial bronchi
• bilateral symmetric liver; biliary atresia
• right-sided stomach, pancreatic cauda, and multiple spleens
• duodenal malposition
• malposition of portal vein and superior mesenteric vessels
• malposition of the small and large bowel, common
mesenterium |
| A 799 ? |
• dextrocardia
• S1: persistent left SVC; discontinued IVC with hemiazygos continuation
• S2: common atrium with bilateral sickle-shaped appendages and atria
• S3: complete AVSD with common dysplastic AV valve
• S4: functional single ventricle
• S5: ordinary
• S6: ordinary |
• bilateral bilobar lungs and hyparterial bronchi
• bilateral symmetric liver
• right-sided stomach, pancreatic cauda, and multiple spleens
• duodenal malposition
• malposition of portal vein and superior mesenteric vessels
• malposition of the small and large bowel, common mesenterium |
| A 973 ? |
• levocardia
• S1: persistent left SVC; discontinued IVC with hemiazygos continuation
• S2: bilateral sickle-shaped appendages and atria
• S3: dysplastic/atretic mitral valve
• S4: hypoplastic left ventricle (HLV)
• S5: double outlet right ventricle (DORV) and d-transposition great arteries (d-TGA)
• S6: hypoplastic pulmonary trunk, aplastic ductus arteriosus Botalli, right- sided aortic arch |
• bilateral bilobar lungs and hyparterial bronchi
• bilateral symmetric liver
• right-sided stomach, pancreatic cauda, and spleen
• duodenal malposition
• malposition of portal vein and superior mesenteric vessels
• malposition of the small and large bowel, common senterium
• uterus unicornis right, left-sided agenesia of uterus and uterine tube |
| A 1079 ? |
• levocardia
• S1: persistent left SVC; discontinued IVC with hemiazygos continuation
• S2: bilateral sickle-shaped appendages and dilatated atria
• S3: hypoplastic mitral valve
• S4: VSD, hypoplastic left ventricle
• S5: hypoplastic ascending aorta
• S6: hypoplastic aortic arch |
• bilateral bilobar lungs and hyparterial bronchi
• bilateral symmetric liver
• right-sided stomach, pancreatic cauda, and multiple spleens
• duodenal malposition
• malposition of portal vein and superior mesenteric vessels
• malposition of the small and large bowel, common mesenterium |
| Left Isomerism (post mortem confirmed) |
| A 722 ? |
• levocardia
• S1: abnormal pulmonary venous return; persistent left SVC; left-sided IVC anterior to the aorta
• S2: common atrium with inverse configured appendages and atria
• S3: complete AVSD with common dysplastic AV valve
• S4: single ventricle and semilunar valve
• S5: common arterial trunc
• S6: right-sided aortic arch; mirror-inverted origin of its branches; systemic pulmonal perfusion |
• bilateral trilobar lungs and eparterial bronchi
• inverse liver
• right-sided stomach, pancreatic cauda, and spleen
• duodenal malposition
• malposition of portal vein and superior mesenteric vessels
• malposition of the small and large bowel, common mesenterium |
| Right Isomerism (post mortem detected) |
| S1: precardial venous, S2: venoatrial, S3: atrioventricular, S4: ventricular, S5: ventriculoarterial and S6: postcardial arterial segment (AV = atrioventricluar; VA = ventriculoarterial; AVSD = atrioventricluar septal defect; VSD = ventricluar septal defect; SVC/IVC = superior/inferior vena cava) |
Table 2: Postmortal cardiac and extracardiac findings in foetuses with heterotaxy; teratological evaluation according to the guidelines of the sequential segmental approach
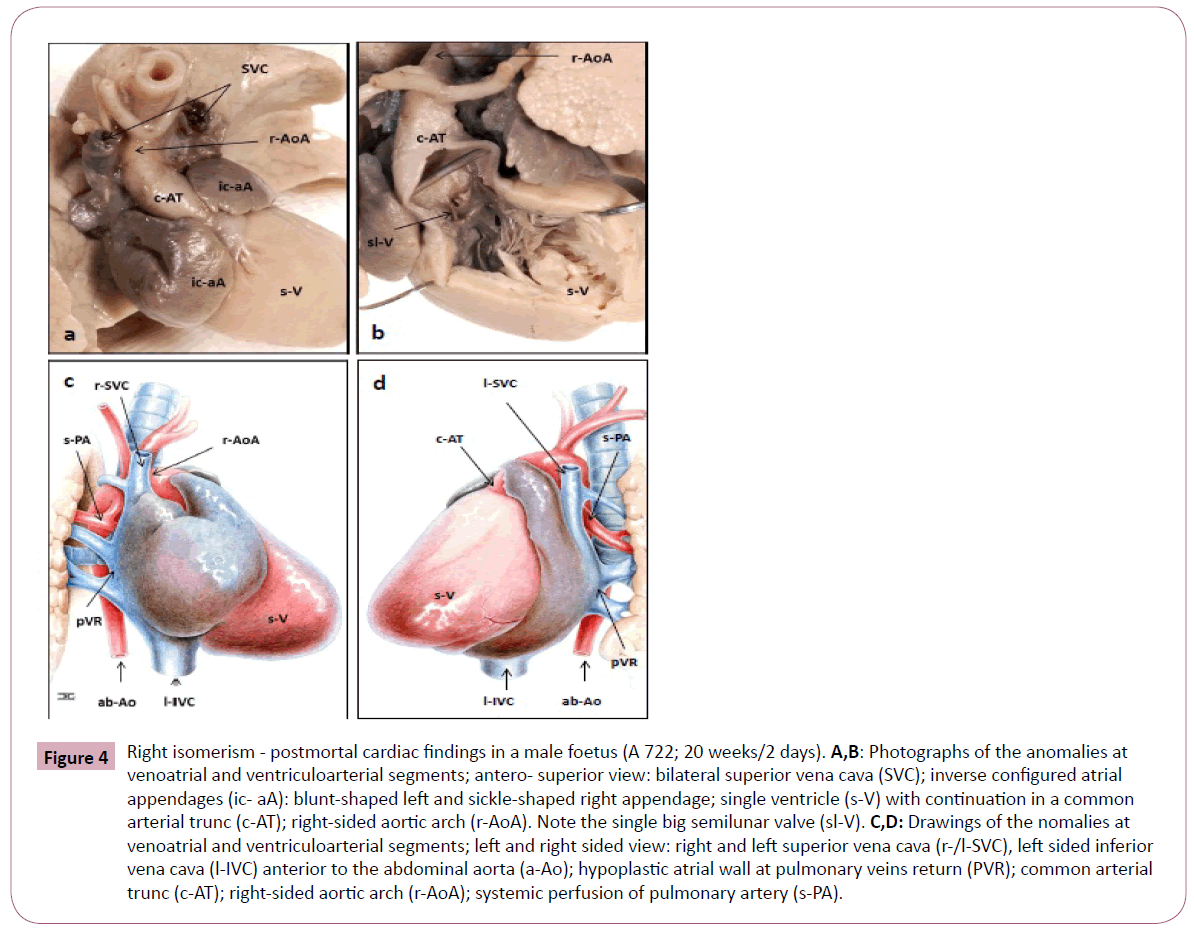
Figure 4: Right isomerism - postmortal cardiac findings in a male foetus (A 722; 20 weeks/2 days). A,B: Photographs of the anomalies at venoatrial and ventriculoarterial segments; antero- superior view: bilateral superior vena cava (SVC); inverse configured atrial appendages (ic- aA): blunt-shaped left and sickle-shaped right appendage; single ventricle (s-V) with continuation in a common arterial trunc (c-AT); right-sided aortic arch (r-AoA). Note the single big semilunar valve (sl-V). C,D: Drawings of the nomalies at venoatrial and ventriculoarterial segments; left and right sided view: right and left superior vena cava (r-/l-SVC), left sided inferior vena cava (l-IVC) anterior to the abdominal aorta (a-Ao); hypoplastic atrial wall at pulmonary veins return (PVR); common arterial trunc (c-AT); right-sided aortic arch (r-AoA); systemic perfusion of pulmonary artery (s-PA).
With reference to extracardial post mortem findings in the case of right isomerism, the lungs were trilobed with two symmetrical eparterial morphologic right bronchi. The liver was inverted and the stomach, the spleen as well as the tail of the pancreas were found in the right upper abdomen (Figure 3C). Moreover, there was an abnormal route of the duodenum in combination with an abnormal position of the portal vein on the left and abnormal located the superior mesenteric vessels right to the duodenum. As a consequence, the midgut derivatives were malrotated with the subsequent malposition and malfixation (common mesenterium) of the large intestine behind the small bowel. Finally, a viscerocardiac heterotaxy (situs ambiguous) was diagnosed due to the incoherent laterality between the left sided heart axis (levocardia) and the partial situs inversus in the upper abdomen.
Discussion
This study reports the complete spectrum of detected anomalies in 5 foetuses with heterotaxy-syndrome. Heterotaxy is a laterality disturbance associated with paired left or right sidedness viscera, the malpositioning of unpaired visceral organs and vessels as well as splenic anomalies. Congenital heart and gastrointestinal malformations are commonly associated with this syndrome. Some of the structural abnormalities can be recognised only during autopsy. The definition of heterotaxy, however, is fundamental for the evaluation of the visceroatrial arrangement, together with other characteristic signs of this syndrome.
The diagnostic criteria of heterotaxy include the isomerism of the pulmonal lobes and the bronchial branching. Typical findings in the right isomerism are bilateral morphological right (trilobed) lungs with eparterial bronchi. In contrast, the left isomerism is characterised by bilateral left (bilobed) lungs with hyparterial bronchi. This otherwise very rare bronchopulmonar dysmorphism can be detected only during autopsy despite the progress made in prenatal diagnostic imaging.
Complex cardiac lesions are frequently diagnosed in patients with heterotaxy in both settings [1,5,8]. Using the segmental approach we detected distinct malformations at several cardiac levels. It concerns the veno-atrial, the atrio- ventricular, and the ventriculo-arterial segment, as well as the precardial veins and the great postcardial arteries.
The typical signs in cases of left isomerism include the bilateral sickle-shaped atrial appendages and the left configured atrial wall [2]. In most cases of the left isomerism the sinus node is absent resulting in a prenatal diagnosed heart block. We have been consistent, however, with Ware et al. [9], who indicated, that the absence of the sinus node in cases of left isomerism is not mandatory. The left isomerism is frequently combined with a dysplastic atrioventricular connection resulting from complete atrioventricular septal defects. Moreover, anomalies at the level of ventriculoarterial connection and the postcardial arteries, including transposition of the great arteries, left- or right-sided obstructions and malposition of the aortic arch, may also occur [2]. Preductal coarctation of the aortic arch may result from a decreased blood flow through the left side of the heart in combination with ventricular anomalies. Nevertheless, it is reported, that left isomerism may also coexist with a structural normal heart [10-12].
In contrast, right isomerism is frequently associated with bilateral blunt-shaped atrial appendages and a right configured atrial wall (right atrial isomerism). In our study we found an inversed atrial morphology in the foetus with right isomerism. The typical signs often include multiple severe anomalies at all cardiac levels. Commonly, there is a predominance of atrioventricular septal defects and anomalies at the level of ventriculoarterial connections including double outlet right ventricle, trans- or malpositioning of the great arteries, pulmonary atresia and common arterial trunk [1]. The postcardial arteries are frequently characterised by an aplastic ductus arteriosus Botalli and a rightsided aortic arch as seen in our study. Typical findings in cases of heterotaxy are disorders of the systemic and pulmonary venous return to the heart. The main features in cases of right isomerism include a duplicated SVC and a malpositioned IVC, which may be anterior or juxtaposed to the aorta. Commonly, there is also a predominance of anomalous pulmonary venous return [5] resulting from the hypoplasia of the corresponding atrial wall. Discontinuity of IVC is an excellent marker of left isomerism [1,5,12,13]. The reported prevalence ranges between 55 and 85%. In our study, all foetuses with left isomerism had a termination of the IVC below its hepatic segment. Systemic venous flow beyond this point was accommodated by dilated azygos or hemiazygos veins, which empty either into the single right-sided or an accessory left-sided SVC [14].
For a clearer understanding, it should be noted that the embryonic development of the caval veins is a very complex process, originating from paired left- and right-sided primitive veins [15]. As seen in Figure 1A, the IVC is composed of four different precursor segments: the hepatic, the suprarenal, the renal, and the infrarenal. The hepatic segment develops from the vitelline vein, and the three more caudally located segments evolve from the sub- and supracardinal veins as well as several connecting anastomosis. Normally, the suprarenal segment is formed by the right subcardinal vein and subcardinal-hepatic anastomosis. Discontinuity of the IVC in cases of left isomerism results from a failure of subcardinal-hepatic anastomosis with the consequent atrophy of the right-sided primitive veins and subsequent development of the left sided veins (Figure 1B and 1C). The suprarenal segment was formed by the left instead of the right subcardinal vein, and the renal segment by the left, instead of the right, sub-supracardinal anastomosis. Moreover, the infrarenal segment derived from the left instead of the right supracardinal vein. Only, the hepatic segment, therefore, drained separately into the right or the common atrium. In contrast, the malposition of the IVC in cases of right isomerism resulted from a laterally reversed development (Figure 1D). Congenital anomalies of the ICV in cases of heterotaxy syndrome without congenital cardiac defects are often discovered incidentally in adults, because they are usually asymptomatic. They may cause, however, diagnostic and therapeutic difficulties in cardiology, phlebology and surgery [12].
A left sided SVC is seen in 0.3 - 0.5% of the normal population and in 4.4% of those with congenital heart disease particularly in cases of heterotaxy. In the vast majority it is accompanied by a normal right sided SVC, termed SVC duplication. It results from failure of the embryonic left anterior cardiac vein to regress. Drainage is variable and can be to the right atrium via the oblique vein of Marshall, the coronary sinus or the left atrium. In the vast majority of cases (82 - 90%) a normal (but small) right sided SVC is also present, and a persistent bridging vein (left brachiocephalic vein) is seen in 25 - 35% of cases [15].
One of the important diagnostic indicators is the viscerocardiac heterotaxy. Mainly, the heart is left-sided with the apex orientated to the left. In all cases we found an inverted or symmetrical liver and a mirror-image stomach. Moreover, a high rate of congenital abnormalities of the intestine, particularly at the level of the foregut including the hepatobiliary and pancreatic tree, is reported in heterotaxy [6,13]. Foetuses with left isomerism are at risk of biliary atresia as shown in our study. The spleen and the pancreas were detected near the stomach in the right upper abdomen. It should be noted that both, the tail of pancreas and the spleen, develop in the dorsal mesogastrium. Occasionally, a high incidence of short or truncated pancreas is reported [12]. There were multiple splenules only in cases of left isomerism, in right isomerism, however, the spleen is sometimes absent. Although polysplenia is the most common finding in left isomerism, asplenia or a normal spleen may also be present [12]. As seen in Figure 3A, special attention should be also directed towards intestinal rotational disorders given the presumed risk of midgut volvulus [16]. The normal development of the human intestine involves two processes: the so called “rotation” of the midgut and the subsequent fixation of the colon and the mesentery.
Anomalies of midgut derivatives are usually termed “malrotation”. It includes “nonrotation”, “incomplete rotation” and the rare “reversed complete or incomplete rotation” [17]. While the risk of “malrotation” in the general population is less than 1%, it may be as high as 70% in cases of heterotaxy although the exact incidence is not precisely known. For patients with situs ambiguous various anatomical configurations of the small bowel and the colon have been reported [13]. Our findings are consistent with those of Kluth et al. [18], who indicated that the normal position of the bowel is depended on the normal development of the duodenal loop. As such, this loop emerges via localized growth and lengthening of the duodenum in the early period of embryonic development. Further growth pushes the tip of the duodenojejunal loop beneath the mesenteric root, which then reaches its normal position left of the spine. Based on our study, we suggest that the improper formation of the duodenal loop seems to be the crucial factor for different forms of malrotation in cases of heterotaxy. In all investigated foetuses we found the duodenal loop with an abnormal route left of the midline and continuation in an abnormal right-sided duodenojejunal junction. The abnormal route of the duodenum was associated with different malpositions of the portal vein. In right isomerism the vein was found to the left of the upper border of the duodenum. In left isomerism, though, it had an abnormal infrapyloric origin. Following its subsequent ascension in front of the duodenum the vein enters the malpositioned midline liver. The preduodenal ascension of the portal vein is one of the radiological sign diagnosed in cases of left isomerism [13]. Embryonically, the portal venous system is derived from the vitelline veins, which drain the primitive gastrointestinal tract. The two veins are connected by three interconnecting veins, a cranial (in the liver), a middle (behind the duodenum), and a caudal (in front of the duodenum). Normally, the portal vein is formed by selective obliteration in early development. Atrophy of the cranial and caudal inter-connecting veins along with the caudal segment of the right and cranial segment of the left vitelline veins results in a normal S-shaped portal vein. An abnormal position of the portal vein is based on left- and right-sided variations in this process.
Moreover, the abnormal route of the duodenum was in all investigated foetuses combined with a malposition of the superior mesenteric artery and vein. These vessels provide the central axis of the midgut loop which temporarily projects into the remains of the extraembryonic coelom in the proximal part of the umbilical cord. The malposition of the superior mesenteric vessels is the prerequisite for the abnormal arrangement of the gut following its return into the abdominal cavity. Moreover, all variants of malrotation are accompanied by a subsequent malfixation of the ascending and descending colon.
With reference to the overlap of cardiac and visceral anomalies in the two forms of heterotaxy syndrome, the differentiation of left and right isomerism mainly relies on the bronchopulmonal morphology and the anomalies of the IVC. Discontinuity of the IVC represents an excellent marker for left isomerism, as this anomaly is rare under other circumstances. In our study the IVC had a lack of continuity in all foetuses with left isomerism. The reported incidence of left isomerism in cases of heterotaxy ranges between 55% and 85% and includes both infants and foetuses who were the subject of a post-mortem examination. The prenatal diagnosis of right isomerism remains a difficult task. An important marker is the juxta-/anteroposition of IVC to the abdominal aorta in combination with viscerocardiac heterotaxy and complex cardiac malformations. Special attention should be paid to the anomalies of the pulmonary venous return as has been demonstrated in our study.
Autopsy studies have shown that recognition of the morphological characteristics of the isomeric atrial appendages is the best guide to the existence of the 2 entities in heterotaxy syndromes [9]. It remains unclear, however, whether the morphology of the atria and their appendages is genetically determined by the type of isomerism or mechanically modulated by the disturbed haemodynamic in these hearts. According Berg et al. [5], neither the presence of an atrioventricular septal defect nor other cardiac anomalies were significantly correlated to atrial morphology, suggesting that the atrial morphology is independent from the major cardiac malformations in heterotaxy syndromes.
Foetuses with complex cardiac anomalies and an abnormal visceral situs are strongly predictive of a normal karyotype. Significant progress, however, was recently achieved in the understanding of the genetic pathways ultimately responsible for left/right asymmetry in animal models [3]. Briefly, an initial break in symmetry occurs at the primitive node as a leftward “nodal flow”. It provokes asymmetric signals that are transmitted toward the left lateral plate mesoderm. Transcription factors, Nodal, Lefty2, and Pitx2c, regulate genetic programmes on the left side of the body and create asymmetric organ morphogenesis. Moreover, several genes, including ZIC3, NODAL, LEFTY2, CFC1, SHROOM3, seem to be responsible for left-right laterality and its disturbance in cases of heterotaxy [3,13,14]. Nonetheless, the mechanism of consistent left/right asymmetry during embryogenesis remains a major area that is open to be research.
Conclusions
The detailed segmental analysis of the foetuses with heterotaxy by a highly skilled expert in teratology is of extreme importance for the advancement of the prenatal investigation. In spite of being uncommon, congenital anomalies, particularly in the cardiovascular, bronchopulmonal and gastrointestinal system, may cause difficulties in the interpretation of prenatal findings. A basic knowledge of embryology is required for the correct interpretation and description of the real extent of complex malformation pattern by sonographers and radiologists. With patience and attention to detail the specific congenital morphology in both settings of heterotaxy may be made easier to understand. This is essential in order to effectively counsel parents on further diagnostic steps that may be taken in addition to likely postnatal outcomes [6].
Left and right isomerism may have both biventricluar hearts with large atrioventricular defects. Patients with right isomerism tend to have complex cardiac lesions more frequently, whereas in left isomerism only ventricular septal defects or no cardiac anomalies may occur [19]. In general, the mortality in foetuses and neonates is high in the presence of heart block and hydrops or pulmonary atresia and anomalous pulmonary venous return, and the morbidity is mainly determined by the cardiac defects. Death in the first year of life is common in right isomerism. In contrast, the prognosis of left isomerism may be quite good in the absence of heart block, as it tends to present with less severe cardiac anomalies or even with normal intracardiac anatomy.
Although neonatal morbidity and mortality are determined mainly by the cardiac abnormalities, the visceral anomalies may strongly affect the long-term outcome. With the improvement in long-term outlook for these patients with modern cardiac surgery the intra-abdominal anomalies have become increasingly significant. A detailed prenatal examination to enable proper counselling in an interdisciplinary approach (i.e. obstetricians, neonatologists, cardiologists and cardiac surgeons, and visceral surgeons) is thereby crucial.
Acknowledgement
The authors wish to express their thanks to all parents involved for giving permission to collect the presented data. Many thanks also to Sabine Müller for her technical assistance throughout the study and to Jens Geiling for his excellent drawings.
8868
References
- Berg C, Geipel A, Smrcek J, Krapp M, Germer U, et al. (2003) Prenatal diagnosis of cardiosplenic syndromes: a 10-year experience. Ultrasound Obstet Gynecol 22: 451-459.
- Pepes S, Zidere V, Allan LD (2009) Prenatal diagnosis of left atrial isomerism. Heart 95: 1974-1977.
- Shiraishi I, Ichikawa H (2012) Human heterotaxy syndrome from molecular genetics to clinical features, management, and prognosis. Circ J 76: 2066-2075.
- Jacobs JP, Anderson RH, Weinberg PM, Walters HL III, Tchervenkov CI, et al. (2007) The nomenclature, definition and classification of cardiac structures in the setting of heterotaxy. Cardiol Young 2: 1-28.
- Berg C, Geipel A, Kohl T, Smrcek J, Germer U, et al. (2005) Fetal echocardiographic evaluation of atrial morphology and the prediction of laterality in cases of heterotaxy syndromes. Ultrasound Obstet Gynecol 26: 538-545.
- Gottschalk I, Berg C, Heller R (2012) Disorders of laterality and heterotaxy in the foetus. Z GeburtshilfeNeonatol 216: 122-131.
- Anderson RH, Shirali G (2009) Sequential segmental analysis. Ann Pediatr Cardiol 2: 24-35.
- Berg C, Geipel A, Kamil D, Krapp M, Breuer J, et al. (2006) The syndrome of right isomerism: prenatal diagnosis and outcome. Ultraschall Med 27: 225-233.
- Ware AL, Miller DV, Porter CB, Edwards WD (2012) Characterization of atrial morphology and sinus node morphology in heterotaxy syndrome: an autopsy-based study of 41 cases (1950-2008). Cardiovasc Pathol 21: 421-427.
- De Paola N, Ermito S, Nahom A, Dinatale A, Pappalardo EM, et al. (2009) Prenatal diagnosis of left isomerism with normal heart: a case report. J Prenat Med 3: 37-38.
- Dilli A, Gultekin SS, Ayaz UY, Kaplanoglu H, Hekimoglu B (2012) A rare variation of the heterotaxy syndrome. Case Rep Med 2012: 840453.
- Hildebrand H, Gunzenhauser D, Weber K, Frese W, Fröber R, et al. (2007) Heterotaxia syndrome without congenital cardiac defects in dilated cardiomyopathy. Dtsch Med Wochenschr 132: 931-937.
- Tawfik AM, Batouty NM, Zaky MM, Eladalany MA, Elmokadem AH (2013) Polysplenia syndrome: a review of the relationship with viscero-atrial situs and the spectrum of extra-cardiac anomalies. Surg RadiolAnat 35: 647-653.
- Saito T, Watanabe M, Kojima T, Matsumura T, Fujita H, et al. (2011) Successful blood sampling through azygos continuation with interrupted inferior vena cava. A case report and review of the literature. Int Heart J 52: 327-230.
- Kellman GM, Alpern MB, Sandler MA, Craig BM (1988) Computed tomography of vena caval anomalies with embryologic correlation. Radiographics 8: 533-556.
- Papillon S, Goodhue CJ, Zmora O, sharma SS, Wells WJ, et al. (2013) Congenital heart disease and heterotaxy: upper gastrointestinal fluoroscopy can be misleading and surgery in an asymptomatic patient is not beneficial. J Pediatr Surg 48: 164-169.
- Pepes S, Zidere V, Allan LD (2009) Prenatal diagnosis of left atrial isomerism. Heart 95: 1974-1977.
- Kluth D, Jaeschke-Melli S, Fiegel H (2003) The embryology of gut rotation. Semin Pediatr Surg 12: 275-279.
- Lee SE, Kim HY, Jung SE, Lee SC, Park KW, et al.(2006) Situs anomalies and gastrointestinal abnormalities. J Pediatr Surg 41:1237-1242.










