Milovanovic M1*, Nilsson S2, Haakara PI3, Post C4 and Gerdle B5
1Department of Social and Welfare Studies, Linköping University, Linköping, Department of Internal Medicine, Vrinnevi Hospital, Norrköping,
Sweden
2Primary Health Care and Department of Medical and Health Sciences, Linköping University, Norrköping, Sweden
3Department of Neurobiology, Society and Caring Sciences, Karolinska Institutet, Stockholm, Sweden
4Department of Clinical and Experimental Medicine, Linköping University, Linköping, Sweden
5Division of Community Medicine, Department of Medical and Health Sciences, Faculty of Medicine and Health Sciences, Linköping University,
Pain and Rehabilitation Center, Anaesthetics, Operations and Specialty Surgery Center, Region Östergötland, Linköping, Sweden
- *Corresponding Author:
- Micha Milovanovic, PhD
Department of Welfare and Care, Faculty of Medicine and Health Sciences
Linköping
University SE-601 74 Norrköping, Sweden
Tel: +46700896320
E-mail: micha.milovanovic@liu.se
Received date: Mar 28, 2016; Accepted date: May 26, 2016; Published date: June 06, 2016
Citation: Milovanovic M, Nilsson S, Haakara PI, et al. High In vivo Platelet Activity in Female Fibromyalgia Patients. J Biomedical Sci. 2016, 5:3. doi:10.4172/2254-609X.100035
Copyright: © 2016, Milovanovic M. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Keywords
Fibromyalgia; Fibrinogen; Platelets; Platelet activity; Platelet heterogeneity
Introduction
Fibromyalgia syndrome (FMS) is a chronic pain syndrome [1] associated with several symptoms including; pain, fatigue, sleep problems, depression, digestive problems, intestinal disorders and anxiety [2]. In 1990, the American College of Rheumatology (ACR) established the now generally used criteria for FMS. ACR agreed that patients must have widespread pain in combination with widespread pain hypersensitivity for mechanical pressure, which is captured using manual palpation in at least 11 of 18 standardized anatomical locations (tender points) [1,3]. FMS is an independent diagnosis accepted by the World Health Organization (WHO). The population prevalence of FMS in the western world is 2-4% and it affects women more often than men. It generally starts as a local pain condition which spreads through the body over time; the risk factors for this spreading are mainly unknown [4].
Pain medicine lacks objective biomarkers to guide the diagnosis and choice of treatment. Hence, there is a lack of reliable and objective blood tests (biomarkers) that can be used as a part of clinical assessment of patients that fulfill the ACR criteria of FMS. As pain by definition is a subjective experience it has been pointed out that biomarkers for pain are an impossibility [5]. Bäckryd has proposed that “nocimarker” would be a better term than pain biomarker for denoting attempts to find objective, measurable correlates to the neurobiological processes involved in different pain conditions [6]. FMS is associated with central alterations (e.g. central hyperexcitability with disinhibition and possibly facilitation of nociceptive afferent activity) [7]. However, controversies exist regarding the role of peripheral factors e.g. peripheral nociceptive inputs, muscle and nociceptive C-fibre alterations and systematic alterations for maintaining central mechanisms [8].
Blood-including the subcomponents serum and plasma - reflects systemic aspects. Serum serotonin levels were significantly lower in FMS as compared to control individuals [9]. Behm and Associates investigated the immune role, specifically mononuclear cell cytokine production in FMS and reported lower levels of IL-5, IL-6, IL-8, IL-10 and IFN-γ [10]. Other studies have also reported alterations in the signature of cytokines in FMS [11,12]. Another study examined the peripheral benzodiazepine receptors on the leukocyte surface. There was an increased level of the receptors in monocytes [13]. Recently our group reported significantly increased plasma levels of lactate and glutamate in a cohort of chronic widespread pain (mainly FMS; 15 out of 17 subjects) [8]. There are very few studies regarding platelets features in FMS. Nevertheless, platelets collected from FMS patients had increased levels of magnesium and lower levels of adenosine triphosphate, in comparison with controls [14].
In whole blood, platelets are the smallest corpuscular body. They differ in density within the span of 1.04–1.08 kg/l [15,16]. Platelet organelles are key determinants of density; highdensity cells have more α and dense granules [15]. In some studies it has been reported that platelet density increases as they get older [17,18], whereas other studies have come to an opposite view [19,20]. Furthermore, other scientists take the view that platelet density does not change in the circulation [21-23]. The clinical impact of platelet heterogeneity has been investigated for a long period of time. Platelet density is increased in conjunction with acute myocardial infarctions (AMI) [24]. ST-elevation AMI is further characterized by an inverse relationship between density and the inflammatory response [25]. The activity of inflammatory bowel disease is linked to small high-density platelets [26]. Low peak platelet density characterizes essential thrombocythemia [27] and preeclampsia severity is associated with large platelets having low peak density [28].
The study aims to investigate if platelet in vivo activity differ in FMS compared to a control group (CON) without FMS.
Material and Methods
Subjects
The study was approved by the local ethics committee of Linköping University, Sweden (reg. number: 2012/269-32). All participants gave informed consent. 24 female patients affected by FMS, aged 38 ± 9 years (mean ± SD) participated in the study. All FMS subjects were enrolled for this study from patients seeking care at the Pain and Rehabilitation Centre of the University Hospital, Linköping, Sweden. Patients fulfilling the criteria of the American College of Rheumatology criteria for FMs were included in the study [1]. The clinical diagnoses of FMS were retrospectively confirmed from the patient’s case histories and clinical examinations. 25 healthy females aged 50 ± 12 years (mean ± SD) were used as a CON. All of the CON group were enrolled in the study when visiting a nearby medical center for a normal routine check. Table 1 summarizes clinical data at the time of entry to the study.
| |
FMS |
CON |
p-value |
| Subjects (n) |
24 |
25 |
|
| Gender (male/female) |
0/24 |
0/25 |
|
| Age (years) |
38± 9 |
50±12 |
NS |
| |
|
|
|
| Body mass index (kg/m2) |
28±7 |
23±3 |
p < 0.05 |
| |
|
|
|
| Diabetes (n) |
0 |
0 |
NS |
| A2-blockers (n) |
0 |
2 |
NS |
| ACE-inhibitors (n) |
2 |
3 |
NS |
| Acetaminophen (n) |
18 |
0 |
p < 0.05 |
| Antidepressant (n) |
22 |
0 |
p < 0.05 |
| β-blockers (n) |
1 |
5 |
NS |
| Ca2+-blockers (n) |
0 |
6 |
NS |
| Diuretics (n) |
0 |
5 |
NS |
| NSAID (n) |
11 |
0 |
p < 0.05 |
| Statins (n) |
0 |
3 |
NS |
| Vitamin B12 (n) |
1 |
0 |
NS |
Table 1: Demographic data and pharmacological treatments at study inclusion for fibromyalgia syndrome patients (FMS) and controls (CON).
Laboratory investigations
All blood samples were collected in Vacutainer™ tubes (Becton and Dickinson, New Jersey, USA.). Venous blood (7.5 mL) was anticoagulated with 2.5 mL 0.129 M disodium citrate. In order to separate platelets according to density a linear Percoll™ (GE Healthcare Bio-Sciences AB, Sweden) gradient was used [29,30]. The following substances were mixed in order to provide the two Percoll™ solutions (1.09 and 1.04 kg/L) for the gradient (Table 2).
| PercollTM solutions |
1.09 kg/L |
1.04 kg/L |
| H2O |
11.42 g |
19.14 g |
| PercollTM |
32.84 g |
8.88 g |
| Platelet-inhibitory solution |
4.50 g |
3.00 g |
Table 2: List of Solutions.
To avoid in vitro platelet activity, the PercollTM solutions contained a platelet inhibitory solution (Na3EDTA and prostaglandin E1).
A two-chamber gradient maker was used to produce linear gradients. The gradients were manufactured in 50 mL test tubes covering the density span of 1.09 kg/L to 1.04 kg/L. 7.63 g of the 1.09 kg/L PercollTM mixture was layered in the bottom of the test tube. Then, 13.08 g of the 1.09 kg/L and 12.48 g of the 1.04 kg/L PercollTM solutions were employed into twochamber gradient makers to make the gradient. Subsequently, 10 mL citrate anticoagulated whole blood was carefully layered on top of a 50 mL test tube with the completely produced gradient. The tube was thereafter centrifuged at 2767 g for 1½ hours. After centrifugation, the underside of the test tube was punctured and the contents was separated by gravity into 17 different density fractions [30]. By this setting every fraction holds about 2 mL of the test tube content. Platelet counts were determined in all fractions using a CELL-DYN 4000 (Abbott Diagnostics, Illinois, USA). Platelet bound fibrinogen (%) were also measured in each fractions with a Beckman Coulter EPICS XL-MCLTM Flow Cytometer (Beckman Coulter, Inc., California, USA). Platelets were identified with a PEconjugated antibody against GPIb (Dako AS, Denmark). A FITCconjugated chicken antihuman fibrinogen polyclonal antibody (Biopool AB, Sweden) discriminated membrane bound fibrinogen [31]. As no agonist was added platelet bound fibrinogen in this setting mirrors in vivo platelet activity.
Statistics
Microsoft Excel® was used for the statistical evaluations. In text and tables are reported mean values ± one standard deviation (SD). The unpaired Student’s t test were employed for evaluating quantitative data. p-values ≤ 0.05 were regarded to indicate significance.
Results
Back-ground data
No significant difference in age was found (Table 1). The Body Mass Index (BMI) was higher in FMS than in CON (p < 0.05) (Table 1). Except demographic data, Table 1 also shows as expected differences in medication where the most notable was the differences in consumption regarding Acetaminophen, Antidepressant and NSAID medicines (p < 0.05).
Laboratory data
The distribution of platelets in the 17 density fractions is demonstrated in Figure 1. There was no difference between the groups. Figure 2 shows in vivo platelet activity i.e. % fibrinogen bound platelets in 17 different density fractions. FMS compared to the CON, showed significantly more fibrinogen bound platelets in most of the platelet density fractions. Especially, numbers 2-14 and 16 displayed significantly higher platelet in vivo activity (p < 0.05). In contrast, the platelet from fractions (numbers 1, 15 and 17) did not circulate activated in FMS (Figure 2).
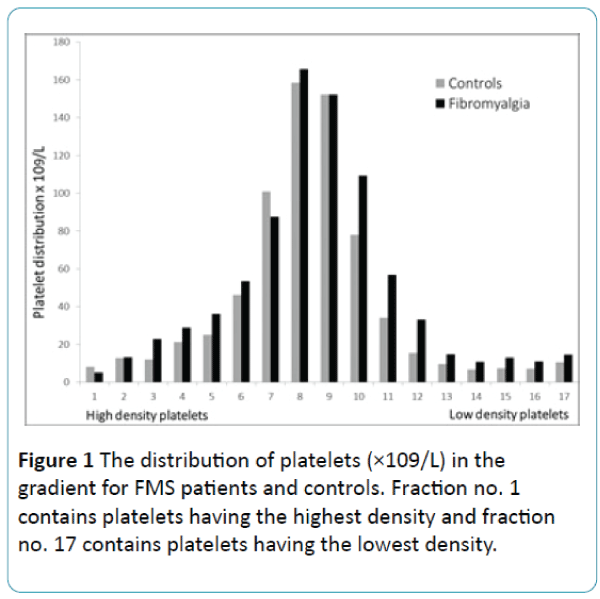
Figure 1: The distribution of platelets (×109/L) in the gradient for FMS patients and controls. Fraction no. 1 contains platelets having the highest density and fraction no. 17 contains platelets having the lowest density.
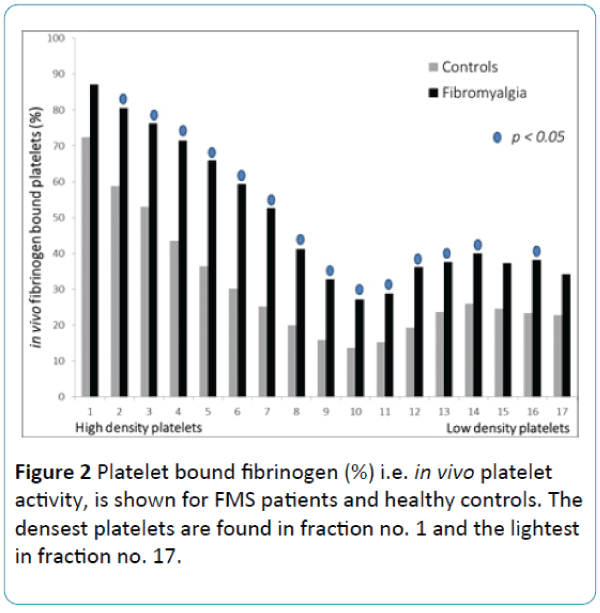
Figure 2: Platelet bound fibrinogen (%) i.e. in vivo platelet activity, is shown for FMS patients and healthy controls. The densest platelets are found in fraction no. 1 and the lightest in fraction no. 17.
Discussion
The current study shows that FMS is characterized by substantial alterations in heterogeneity of in vivo circulating platelets. Indeed, compared with controls FMS patients showed a higher in vivo platelet activity i.e. platelet bound fibrinogen. To the best of our knowledge, this is the first report investigating FMS with respect to platelet bound fibrinogen in different platelet density subpopulations. One can assume that the current method has several weaknesses. One of these are the preanalytical effect on platelet activation. It can probably be considered that if platelets are treated as described it is impossible to avoid an activation of the platelets, which will affect the results obtained. However our opinion is that the effect is comparable in the two groups, i.e. we have dealt with and performed all analyzes in exactly the same way on every individual in both groups. If the activation of the platelets had occurred in the preanalytical process, it had happened in both groups. In any case, the result shows a significant difference whether a preanalytical activation has appeared or not. A cautious conclusion that we can draw from our findings is that the disease is connected to in vivo activated platelets.
It is also well known that platelet function is affected by anti-platelet drugs in the body. However, some individuals exhibit strong reactions while others only have weak platelet inhibition. Earlier studies have shown that individual variation exists when this drug is given [32]. In the current study none of the controls received any anti-platelet drugs, still, it is proposed that NSAID drugs affect platelet aggregation to varying degrees [33]. In the current study 11 of the FMs did receive NSAID. It is possible that the drug influenced the results of platelet fibrinogen binding (Figure 2). One can postulate that without NSAID FMS would have an even higher percentage of platelet fibrinogen binding.
Also, it is well-known that platelet activity measurements can depend on the platelet number, nevertheless, platelet distribution (Figure 1) was similar in both groups, i.e., no differences were found between the groups. For this reason we do not see the platelet count as an important factor that could have affected our obtained results.
The main criticism of this study is probably that we have not selected an age-matched control group. Obviously there is a difference in age between both groups, but not significant. The control group consists of some older individuals. It is however very difficult to comment on whether the age of patients alter platelet activity. There are no studies that support the latter. We have assumed that the age difference between the groups should not affect platelets. Moreover, for obvious reasons we only selected women in the control group because FMS group consisted of women.
The present method used in this work is relatively simple to perform, for that reason, it should be possible to use the method as a supportive diagnostic tool in the clinical examination of patients with widespread pain. However, it is important to verify the present results in other cohorts of FMS. It is also important to investigate if the finding of activated platelets is specific for FMS or could be connected to chronic pain of different etiologies.
The clinical significance of the observed platelet heterogeneity i.e. activated in vivo platelets remains unclear; including what may be the origin and consequence of disparities. One cannot judge the clinical relevance of our findings based on the present study. For this reason, it is important to make further attempts to investigate why the platelets are activated in FMS and in such studies include assessments of co-morbidities.
Acknowledgement
Funding from the Östergötland County Council made this work possible.
Conflict of Interest Statement
We declare that no economic relationships exists that can be construed as a conflict of interest.
9491
References
- Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, et al. (1990) The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Report of the Multicenter Criteria Committee.Arthritis Rheum 33: 160-172.
- Hudson JI, Goldenberg DL, Pope HG Jr, Keck PE Jr, Schlesinger L (1992) Comorbidity of fibromyalgia with medical and psychiatric disorders.Am J Med 92: 363-367.
- Mease P (2005) Fibromyalgia syndrome: review of clinical presentation, pathogenesis, outcome measures, and treatment.J RheumatolSuppl 75: 6-21.
- Larsson B, Björk J, Börsbo B, Gerdle B (2012) A systematic review of risk factors associated with transitioning from regional musculoskeletal pain to chronic widespread pain.Eur J Pain 16: 1084-1093.
- Bäckryd E (2012) Pain and consciousness mocks philosophers and scientists.Lakartidningen 109: 1039-1040.
- Cagnie B, Coppieters I, Denecker S, Six J, Danneels L, et al. (2014) Central sensitization in fibromyalgia? A systematic review on structural and functional brain MRI.Semin Arthritis Rheum 44: 68-75.
- Gerdle B, Larsson B, Forsberg F, Ghafouri N, Karlsson L, et al. (2014) Chronic widespread pain: increased glutamate and lactate concentrations in the trapezius muscle and plasma.Clin J Pain 30: 409-420.
- Wolfe F, Russel IJ, Vipraio G, Ross K, Anderson J (1997) Serotonin levels, pain threshold, and fibromyalgia symptoms in the general population. J Rheumatol 24: 555-559.
- Behm FG, Gavin IM, Karpenko O, Lindgren V, Gaitonde S, et al. (2012) Unique immunologic patterns in fibromyalgia.BMC ClinPathol 12: 25.
- Uçeyler N, Häuser W, Sommer C (2011) Systematic review with meta-analysis: cytokines in fibromyalgia syndrome.BMC MusculoskeletDisord 12: 245.
- Sturgill J, McGee E, Menzies V (2014) Unique cytokine signature in the plasma of patients with fibromyalgia.J Immunol Res 2014: 5.
- Faggioli P, Giani L, Chianese R, Cusa C, Mazzone A (2004) Increase in peripheral benzodiazepine receptors on monocytes in fibromyalgia.Rheumatology (Oxford) 43: 1224-1225.
- Bazzichi L, Giannaccini G, Betti L, Fabbrini L, Schmid L, et al. (2008) ATP, calcium and magnesium levels in platelets of patients with primary fibromyalgia.ClinBiochem 41: 1084-1090.
- Chamberlain KG, Froebel M, Macpherson J, Penington DG (1988) Morphometric analysis of density subpopulations of normal human platelets.ThrombHaemost 60: 44-49.
- Chamberlain KG, Penington DG (1988) Monoamine oxidase and other mitochondrial enzymes in density subpopulations of human platelets.ThrombHaemost 59: 29-33.
- Mezzano D, Hwang KL, Catalano P, Aster RH (1981) Evidence that platelet buoyant density, but not size, correlates with platelet age in man. Am J Hematol 11: 61-76.
- Boneu B, Vigoni F, Boneu A, Caranobe C, Sie P (1982) Further studies on the relationship between platelet buoyant density and platelet age.Am J Hematol 13: 239-246.
- Karpatkin S (1969) Heterogeneity of human platelets. II. Functional evidence suggestive of young and old platelets.J Clin Invest 48: 1083-1087.
- Corash L, Tan H, Gralnick HR, Shafer B (1977) Heterogeneity of human whole blood platelet subpopulations: I. Relationship between buoyant density, cell volume, and ultrastructure. Blood 49: 71-87.
- Penington DG, Lee NL, Roxburgh AE, McGready JR (1976) Platelet density and size: the interpretation of heterogeneity.Br J Haematol 34: 365-376.
- Caranobe C, Sie P, Boneu B (1982) Serotonin uptake and storage in human platelet density subpopulations.Br J Haematol 52: 253-258.
- Martin JF, Shaw T, Heggie J, Penington DG (1983) Measurement of the density of human platelets and its relationship to volume.Br J Haematol 54: 337-352.
- Martin JF, Plumb J, Kilbey RS, Kishk YT (1983) Changes in volume and density of platelets in myocardial infarction.Br Med J (Clin Res Ed) 287: 456-459.
- Järemo P, Hansson G, Nilsson O (2000) Elevated inflammatory parameters are associated with lower platelet density in acute myocardial infarctions with ST-elevation. Thromb Res 100: 471-478.
- Järemo P, Sandberg-Gertzen H (1996) Platelet density and size in inflammatory bowel disease.ThrombHaemost 75: 560-561.
- Järemo P (1999) Platelet density in essential thrombocythemia and polycythemia vera.Platelets 10: 61-63.
- Järemo P, Lindahl TL, Lennmarken C, Forsgren H (2000) The use of platelet density and volume measurements to estimate the severity of pre-eclampsia.Eur J Clin Invest 30: 1113-1118.
- Järemo P1 (1995) Computerised method for recording platelet density distribution.Eur J Haematol 54: 304-309.
- Milovanovic M, Lotfi K, Lindahl T, Hallert C, Järemo P (2010) Platelet density distribution in essential thrombocythemia.PathophysiolHaemostThromb 37: 35-42.
- Lindahl TL, Festin R, Larsson A (1992) Studies of fibrinogen binding to platelets by flow cytometry: an improved method for studies of platelet activation. Thromb Haemost68: 221-225.
- Järemo P, Lindahl TL, Fransson SG, Richter A (2002) Individual variations of platelet inhibition after loading doses of clopidogrel.J Intern Med 252: 233-238.
- Cronberg S, Wallmark E, Söderberg I (1984) Effect on platelet aggregation of oral administration of 10 non-steroidal analgesics to humans.Scand J Haematol 33: 155-159.







