Introduction
The significance of respiratory diseases as a major cause of morbidityand mortality has been reported [1]. Respiratory diseases like asthma, bronchitis, sinusitis and pneumonia may be inherent and sometimes cluster in families. It is sometimes well managed by medical personnel or the patients themselves. There could however be secondary complications as a result of microbial infections leading to frequent exacerbationwhich makes management of these conditions difficult [2]. The causes of these complications have often been associated with cigarette smoking [3, 4]. In spite of the strong relationship between cigarette smoking and the respiratory diseases only a minority of cigarette smokers develop these respiratory diseases [4]. Similarly not all the patients of these diseases smoke or have smoked in the past. Asthma is a chronic respiratory condition resulting in breathing difficulties caused by inflammation and narrowing of the major airways (the bronchi) in the lungs. Exacerbation of asthma symptoms usually requires an obvious trigger which irritates the smooth muscles of the bronchi causing them to constrict and produce more mucus which makes it hard to breath. Reports have it that the severity of asthma is increased by microbial infections when they line the bronchi.
Pneumonia is an inflammatory condition of the lungs which is characterized by inflammation of the parenchyma of the lungs and abnormal alveolar filling with fluid. Pneumonia can result from a variety of causes, including infection with bacteria, viruses, fungi or parasites, and chemical or physical injury to the lungs [1]. Its cause might also be described as unknown when infectious causes have been excluded using known routine screening methods but in most developing countries, Chlamydia pneumonia is not commonly screened for in routine laboratories [5, 6].
Sinusitis is the inflammation of the paranasal sinuses, which may be due to infection, allergy or autoimmune issues which like asthma may cluster in families. Most cases are due to viral infection and resolve in ten days.[1] Sinusitis can be acute, subacute or chronic. Microorganisms which have been implicated in sinusitis include Streptococcus pneumoniae, Haemophilusinfluenzae and Moraxella catarrhalis [4]. Reports involving C. pneumonia (Cp) in sinusitis are sparse. The presence of other microorganisms co-occurring has been reported with little reports on the involvement of Chlamydia pneumonia either in the acute or chronic infections.
Acute bronchitis is an inflammation of the large bronchi in the lungs that is usually caused by viruses or bacteria and may last several days or weeks [1]. Characteristics symptoms include cough, sputum (phlegm) production, and shortness of breath and wheezing related to obstruction of inflamed airways [4].
Though C. pneumonia has been identified as a possible cause of respiratory illness but it has only very few reports has implicated them as a secondary complication in inherent respiratory diseases like asthma, pneumonia, bronchitis and sinusitis in tropical developing countries. The aim of this study is to screen patients with respiratory diseases for C. pneumonia and establish an association between it and secondary complication (exacerbation), since there are no such reports in Nigeria. Reports have it that the significance of persistence or recurrent respiratory diseases in adult life is still to a large extent unknown or uncertain [2, 4, 7]. Hence more studies in this area will be essential in establishing whether the cause of secondary infection is recurrent or persistence. This justifies the screening of IgG and IgM in this study.
C. pneumonia which belongs to the family Chlamydiaceae, order Chlamydiales and genus Chlamydia is the third recognized human pathogen of the genus which has now been established as an important cause of respiratory infections such as pneumonia, bronchitis, pharyngitis [8-11]. Epidemiological survey have suggested that Chlamydia pneumonia is the commonest chlamydial infection in humans worldwide with antibodies to this organism found in 59% of adults in Pittsburg, 75% in Taiwan, 63% in Spain, 15% in Solomon’s Island and Jos Nigeria 66% [12-16]. Serological evidence has also established an association of Chlamydia pneumonia with other diseases including coronary heart diseases, myocardial infarction and scercoidosis [16-18]. This point to severity of Chlamydia pneumonia which cannot be overemphasized considering the sequelae of any of this disease. Gislason et al. [19] related Chlamydia pneumonia serology to decline in lung function in women but not in men, thereby suggesting a sexual distribution in Chlamydia pneumonia infections. This bacteriahas been reported to cause wheezing in people with asthma [1-2, 20-22] and an association between earlier Chlamydia pneumonia infection and a history of wheezing in young girls but not in males and that infection with Chlamydia pneumonia is an important risk factor for wheezing and possibly non-atopic asthma, predominantly in girls [1-2, 20-22]. Molin et al. [23] reported a prevalence rate related to age which is the reason why this study was stratified by age and sex. Microimmunoflourescence (MIF) assay among other common serological test for Chlamydia pneumonia is good to fair in the diagnosis of Chlamydia pneumonia which justify the use of MIF in this study [18].
Materials and Methods
Samples
Blood samples (n=2652) were collected from consenting individuals who show symptoms of respiratory diseases as observed by a clinician and reported to the clinics for complications relating to respiratory disease (Group 1). The diseases considered in this study included sinusitis, asthma, bronchitis and pneumonia as confirmed by a clinician and all the patients has an established diagnosis and history of the diseases according to the American Thoracic Society (ATS) criteria [21]. They had severe dyspnea, cough and sputum production at admission. Patients who had been treated with antibiotics 2 weeks previously were excluded from the study. Blood samples were collected at admission and 10 days after. Lung function test and the grade of dyspnea were measured by a clinician according to the Medical Research Council (MRC) modified scale [17]. Of the total number of patients sampled 306 were having bronchitis, 852 were asthma patients, and sinusitis had 897 patients and pneumonia with 564 patients. Another group of individuals (n=3070) (Group 2) who were confirmed to have a history of respiratory diseases mentioned but are not currently ill or having the above mentioned symptoms and there diagnostic criteria have been previously described [17]. However, some of the subjects in this group had mild to moderate disease according to ATS criteria. The control group (Group 3) consists of subjects (3711) who were selected randomly, had no symptoms of any respiratory diseases. The group 2 and the control groups were selected at random and were invited for sampling over a period of 2 years. They had single serum sample taken, lung function measured, and the rate of dyspnea measured according to the MRC standard. Clinical characteristics of both patients and control groups are shown in table 1. Different stopstations were set-up across the country with samples been collected by clinicians in each of these stop-stations. The stop-stations included Jos and Kaduna in the North Central zone of the country, Maiduguri in the North East zone of the country, Sokoto in the North West, Lagos in the South West, Nsukka in the South East and Porthacourt and Benin City in the South South.
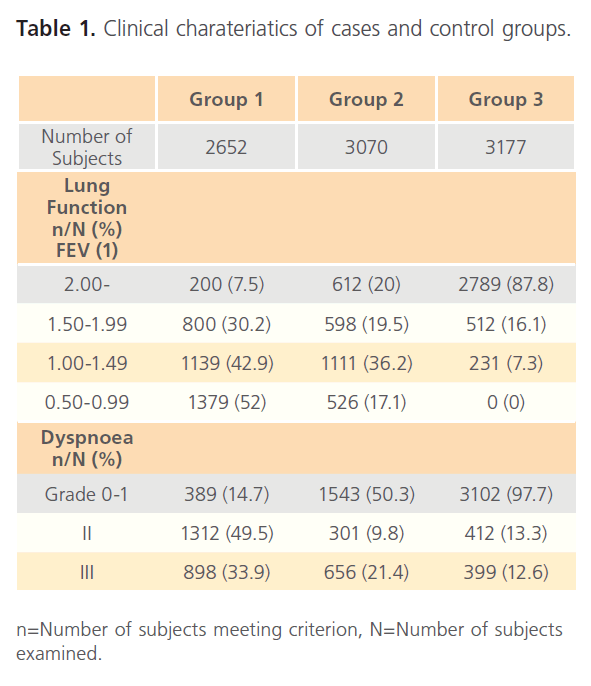
Table 1: Clinical charateriatics of cases and control groups.
Sample processing
The blood samples were collected aseptically from each of the subjects into sterile vacutaniers and were centrifuged (hetutich) at 3000 rpm, Sera were collected into sterile containers and stored at -20oC until analyzed.
Preparation of antigens
Antigens were prepared by culturing sputum and throat swabs into the yolk sac of chicken’s embryonated eggs. The eggs were harvested after 15 days of incubation and tested for positivity using the Romanowsky-Giemsa staining technique [28]. The slides were observed under the oil immersion microscope objective for specific Chlamydia pneumonia inclusions and elementary bodies. The positive results were harvested and used as antigens. The antigens were further confirmed positive by the use of Chlamydia pneumonia monoclonal antibodies slides. The antigens were titrated to the required concentration before been used for microimmunoflourescence assay (MIFA).
Microimmunoflourescence assay (MIFA)
The MIFA was carried out using the method of Hoysman et al. [18] using IgM and IgG slides (Sigma, USA). The test was carried out to detect IgM and IgG specific to Chlamydia pneumoniae. The IgG and IgM seropositivity, criteria were defined as titre ≥32 for IgG ≥16 for IgM (Hertz). Control test were done using 10 immunotypes of Chlamydia trachomatis (My bio Source) and a mixture of 2 Chlamydia psittaccistrains were used as control antigens in MIF titerations. A fourfold rise in titre of the cases was taken as diagnostic or the presence of IgM [5].
Statistical analysis
A one tailed variance analysis and linear regression analysis were applied to all the subjects to investigate the statistical significance of the difference in between males and females and from all the respiratory infections using the seropositivity to Cpas dependent variable and all other parameters as factorial variables. Step-wise logistic regression and analysis of covariance were performed using IgG and IgM seropositivity as dependent and group structure (asthma, bronchitis, pneumonia and sinusitis) as independent variables. The geometric mean (GMT) values relative to the group structures were compared using analysis of variance using age as covariate. All statistical analysis was done in SPSS version 13 and epidemiological analyses in epi-info using the Microsoft excel as platform.
Ethical Consideration
This study being an investigative one does not require approval by any ethical committee according to CDC guidelines. However oral consent was obtained from all the subjects who agreed to participate in this study on the agreement of anonymity and that sample will be for research purposes only. The Local Government Health authorities of all the stopstations approved the study.
Result
Examination of subjects for clinical characteristics of subjects shows that forced expiration volume (FEV1) shows that was highest the control group (Group3) with 87.8% measuring 2.00 and above while cases (Group1) have the highest number of subjects with very low FEV1 with 52% measuring 0.5- 0.99 while the Group2 though intermediate still has some regrettable number of patients with low FEV1 (17.1%) (table 1). The occurrence of Chlamydia pneumonia among hospitalized individuals with respiratory diseases (Group1) was very high, 2612 of 2652 (98%). Of the total number screened, 306 were patients with bronchitis of which 186 (60%) were positive while 852 patients had asthma with 57.4% positive to Chlamydia pneumoniae, 897 had sinusitis of which 58.9% were positive to Chlamydia pneumonia and of 564 pneumonia patients, 51.6% were positive. Age group distribution of CI=99%
positive cases relative to the different respiratory diseases in patients is shown in table 2. Sex distribution (table 3) shows that more males (162) have high proportion of Chlamydia pneumonia antibodies compared to females (144) (males t=0.73, p=0.947; females t=5.378, 0.013) among bronchitis patients, in asthma patients males were 414 while females positive to Chlamydia pneumonia antibodies was 549 patients (males t=2.125, p=0.124; females t=7.243, 0.005) for sinusitis proportion of positive males were 513 and females 381 (males t=6.607, p=0.007; females t=2.891, p=0.063) while in patients with pneumonia the proportion of positive patients to Chlamydia pneumonia antibodies were males 291 and females 273 (males t=1.370, p=0.264; females t=2.095, p= 0.127).
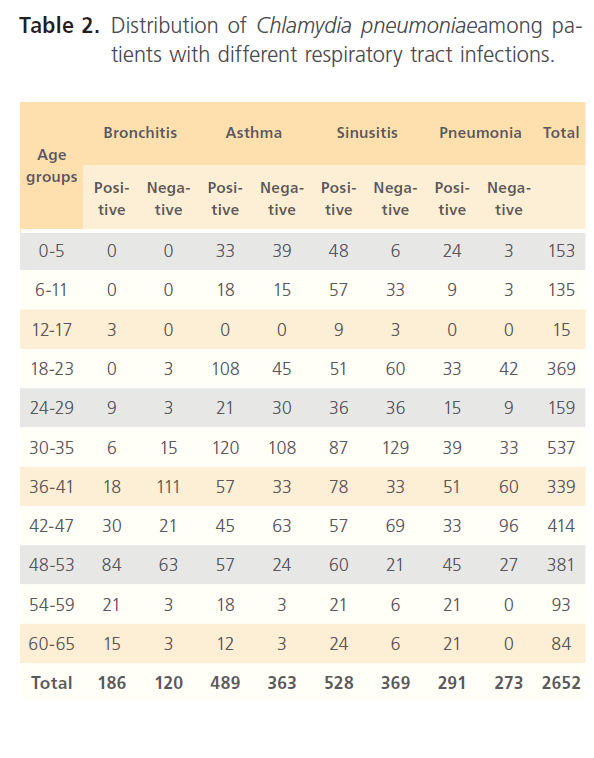
Table 2: Distribution of Chlamydia pneumoniaeamong patients with different respiratory tract infections.
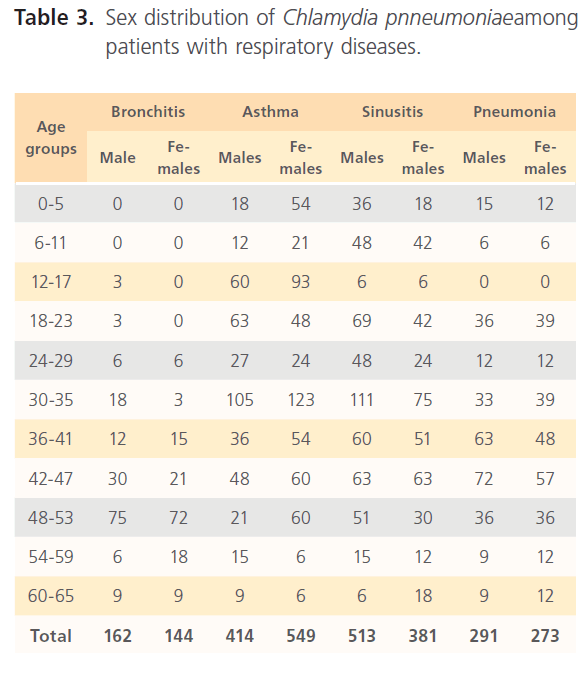
Table 3: Sex distribution of Chlamydia pnneumoniaeamong patients with respiratory diseases.
Of the hospitalized patients with bronchitis, samples with IgG to Chlamydia pneumonia was 120 (4.6%) of the total seropositivity while IgM was 186 (7.1%). Asthma had 369 (14.1%) samples positive to Chlamydia pneumonia IgG and 476 (18.2%) IgM, while sinusitis had 333 (12.7%) IgG and 574 (22%) for IgM. Pneumonia patients had 174 (6.7%) for IgG and 385 (14.7%) for IgM of the total seropositivity. Age group distribution of IgG and IgM for group1 is shown in table 4a. In non-hospitalized patients with respiratory diseases (group2) where 3070 subjects were screened with 1075(35%) seropositivity distribution of Chlamydia pneumonia IgG and IgM among patients with different respiratory diseases were as follows; bronchitis 75 (7%) for IgG and 72 (6.7%) for IgM; asthma 228 (21.3%) for IgG and 166 (15.4%) for IgM; sinusitis 333 (31%) for IgG and 88 (8.2%) for IgM; IgG for subjects with pneumonia was 174 (16.2%) while IgM was 80 (7.4%) (Table4b). In the control (group3), of the 3177 subjects that were screened, 836 (26.1%) were seropositive of which 442 (52.9%) were positive for IgG and 397 (47.5%) for IgM (Table4c).
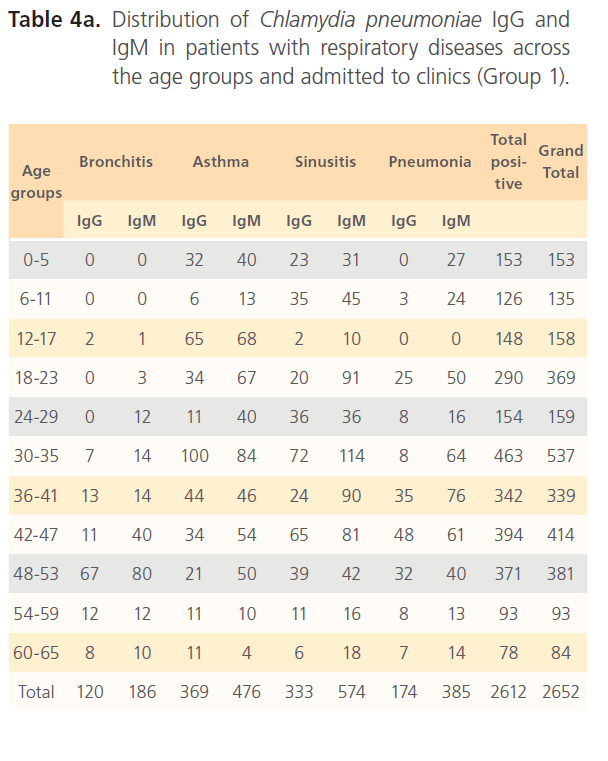
Table 4a: Distribution of Chlamydia pneumoniae IgG and IgM in patients with respiratory diseases across the age groups and admitted to clinics (Group 1).
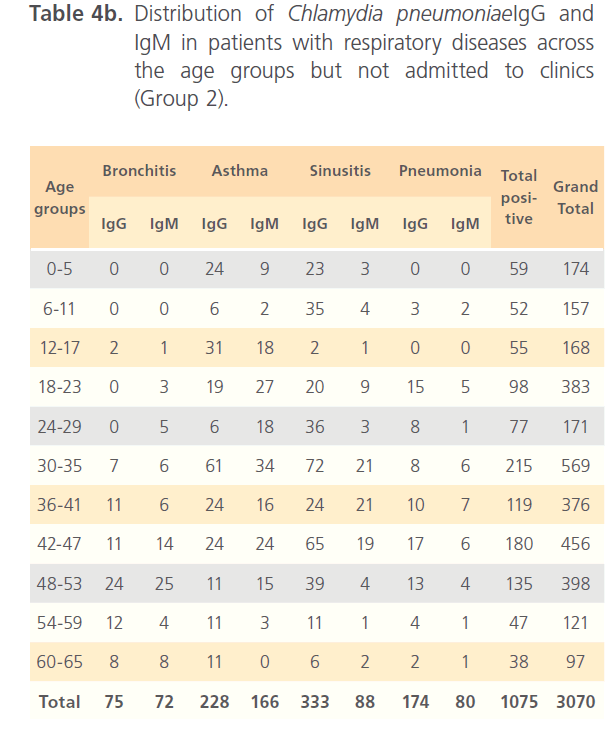
Table 4b: Distribution of Chlamydia pneumoniaeIgG and IgM in patients with respiratory diseases across the age groups but not admitted to clinics (Group 2).
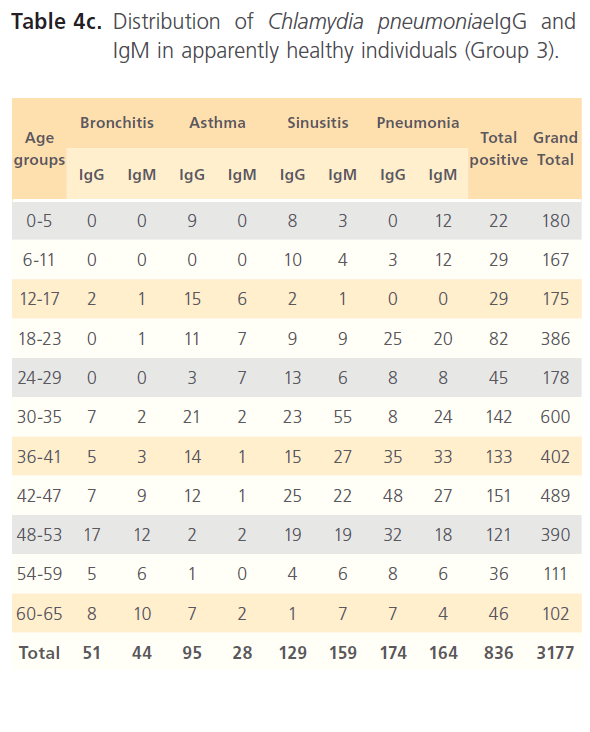
Table 4c: Distribution of Chlamydia pneumoniaeIgG and IgM in apparently healthy individuals (Group 3).
Antibody titration (IgG) (Fig. 1 and 2) for the group1 and group2 subjects reveals that antibody titer were high across the age groups with antibody titers higher in the group1 subjects with geometric mean titer (GMT) significantly from the group 2 subjects and also within the age groups. No antibody titration was done for IgM and for group3 subjects.
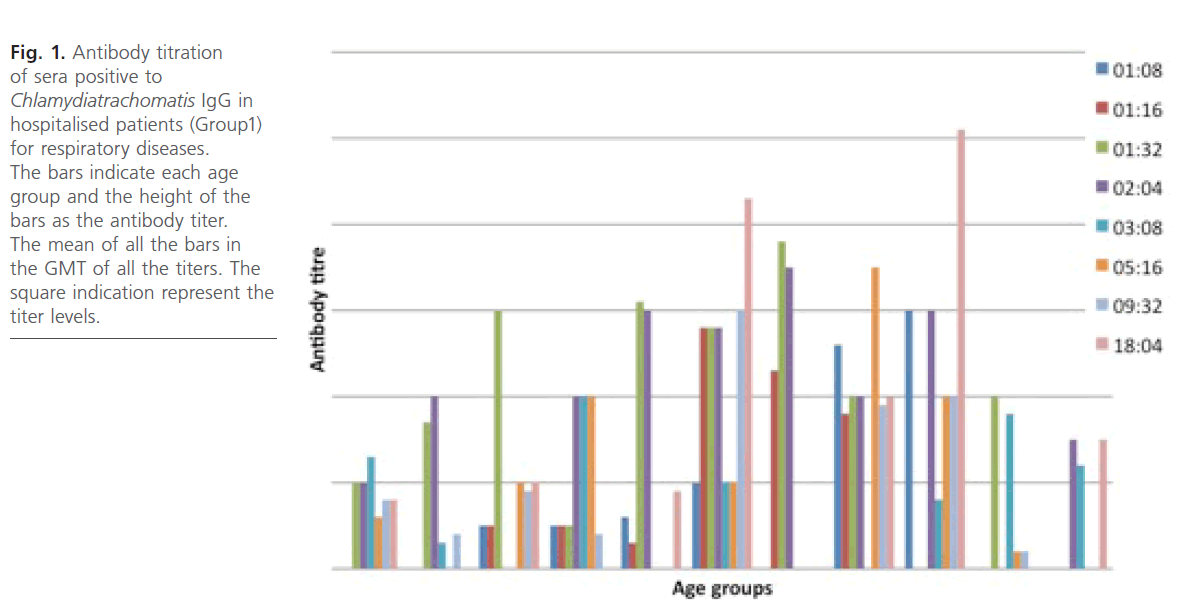
Fig 1: Antibody titration of sera positive to Chlamydiatrachomatis IgG in hospitalised patients (Group1) for respiratory diseases. The bars indicate each age group and the height of the bars as the antibody titer. The mean of all the bars in the GMT of all the titers. The square indication represent the titer levels.
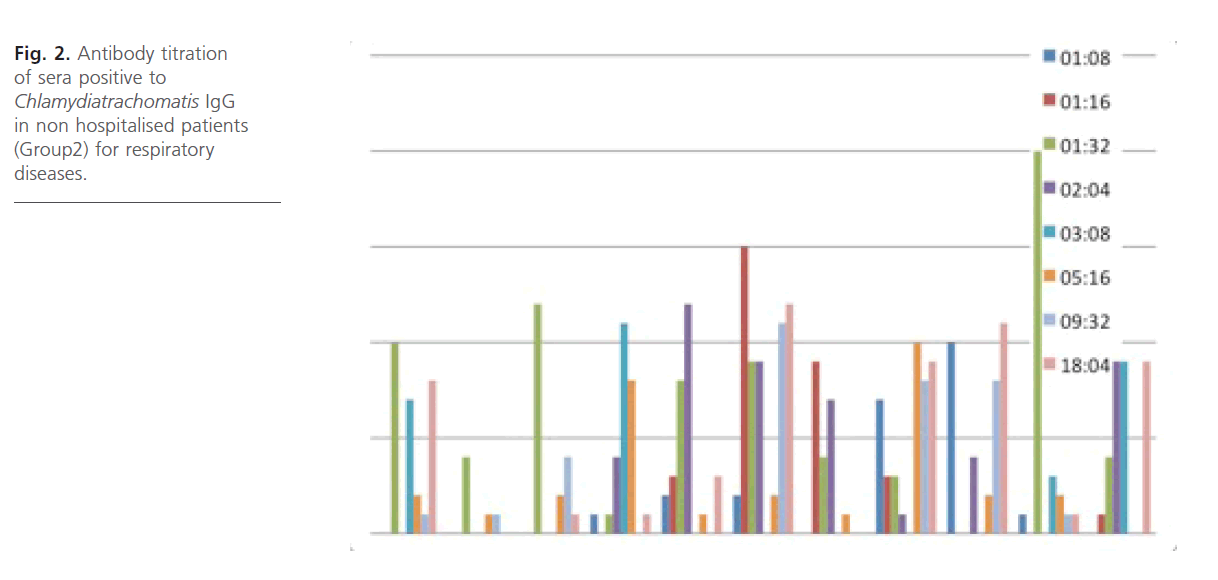
Fig 2: Antibody titration of sera positive to Chlamydiatrachomatis IgG in non hospitalised patients (Group2) for respiratory diseases.
The attack rate of Chlamydia pneumonia in hospitalized patients was highest with sinusitis (36.15%) which was followed by asthma (31.97%), pneumonia was the third (21.40%) and bronchitis form the lowest (11.69%). However, attack rate was highest in older ages in bronchitis, for asthma it was highest in younger ages, although it was still high in older ages. For sinusitis, the attack rate was generally high with the extreme ages having highest. Patients with pneumonia had high attack rate all through, with the older ages having the highest (table 5). The RR for hospitalized patients positive to Chlamydia pneumonia was 3.54 with OR= 34.296 (1.603- 1.861) CI-99%. There was risk distribution across the age groups (table 6) with early age groups having the highest risks, though OR was also high in older age groups. The RR in non-hospitalized individuals with respiratory diseases was 1.34, OR was 1.528 (1.0857-1.878) CI=99%. Age group distribution in this group showed only slight variation with age group 0-5 having RR=2.77 while OR across the age groups did not also show significant variation across the age groups, though age 0-5 still had the highest OR (table 7). The RR of hospitalized patients positive to Chlamydia pneumonia IgG was 2.68, OR=3.661 (0.00-1.5831) CI=99%. The RR in the presence of Chlamydia pneumonia IgG varied significantly across the age groups with the age groups 0-5 having the highest RR as well as OR among the younger age groups. The older age of 60-65 has very high relative risk of 4.33 with very high OR of 140.8261 (table 8). The RR in the presence of Chlamydia pneumonia IgG in group2 individuals was 1.23, OR 1.324 (0.7883-1.4033) CI=99%. There were variations across the age groups with the extreme ages having the highest risks although the risks were low (table 9). Table 10 shows the relative risk of group1 patients to Chlamydia pneumonia in the presence of Chlamydia pneumonia IgM, RR=4.30, OR=9.1144 (1.8314-1.9379), CI=99%. There was also distribution across the age groups with the younger age groups higher than the older age groups. This was also the case for their odd ratios. The risk analysis of individuals with respiratory diseases in the presence of Chlamydia pneumonia IgM is shown in table 11. Further statistical analysis showed that individuals in group2 and group3 (controls) covaried with significance of 0.521, t=0.676 (-2.787-4.121) (table 12). Group1 (hospitalized patients) varied differently using the regression analysis.
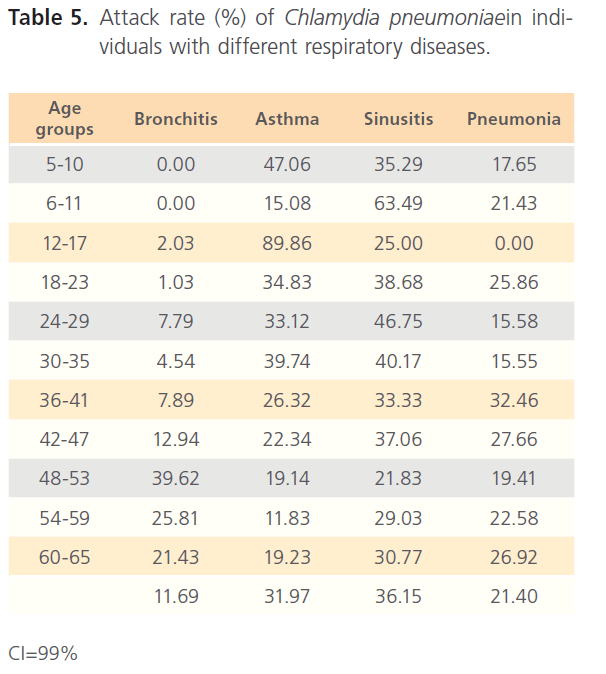
Table 5: Attack rate (%) of Chlamydia pneumoniaein individuals with different respiratory diseases.
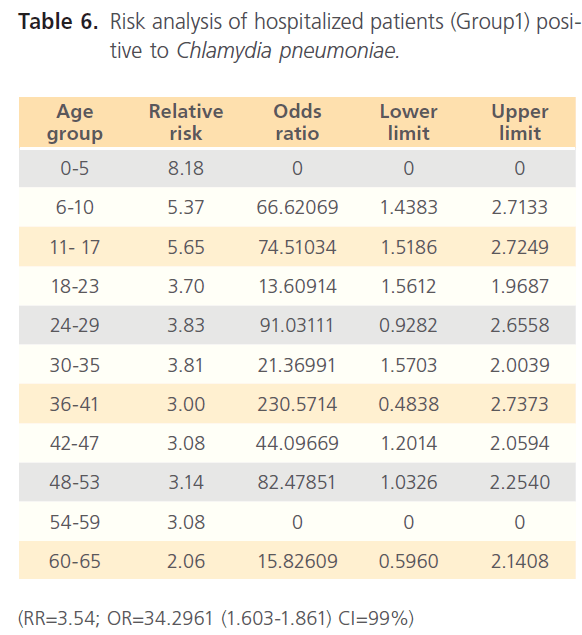
Table 6: Risk analysis of hospitalized patients (Group1) positive to Chlamydia pneumoniae.
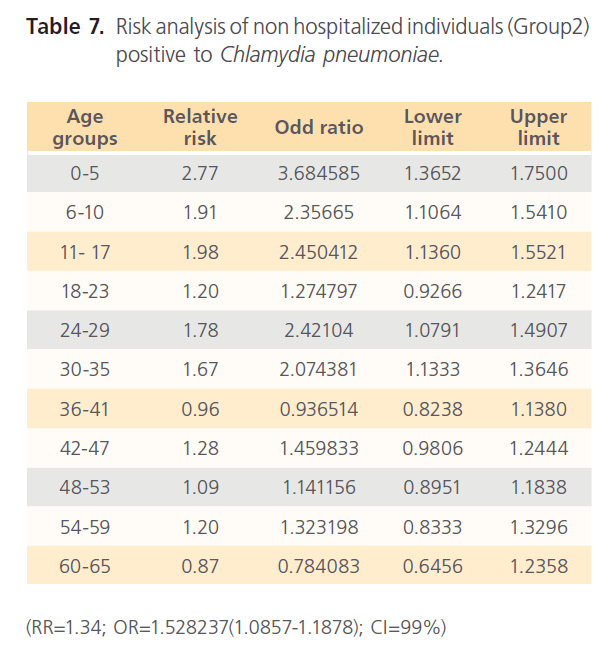
Table 7: Risk analysis of non hospitalized individuals (Group2) positive to Chlamydia pneumoniae.
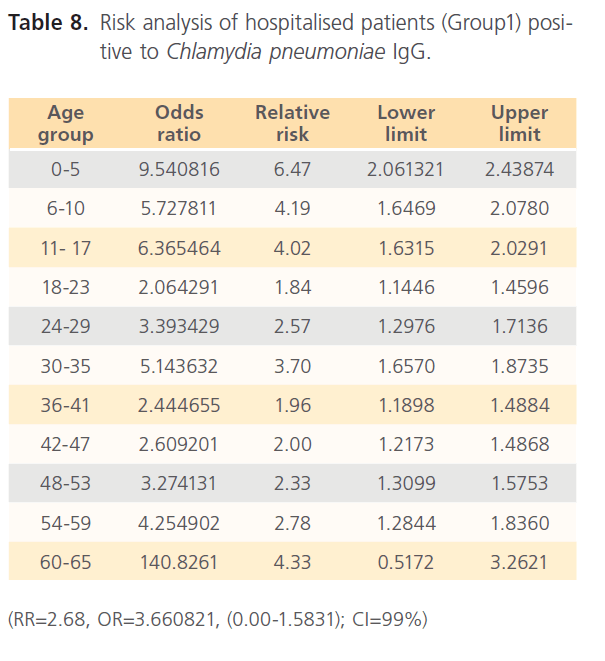
Table 8: Risk analysis of hospitalised patients (Group1) positive to Chlamydia pneumoniae IgG.
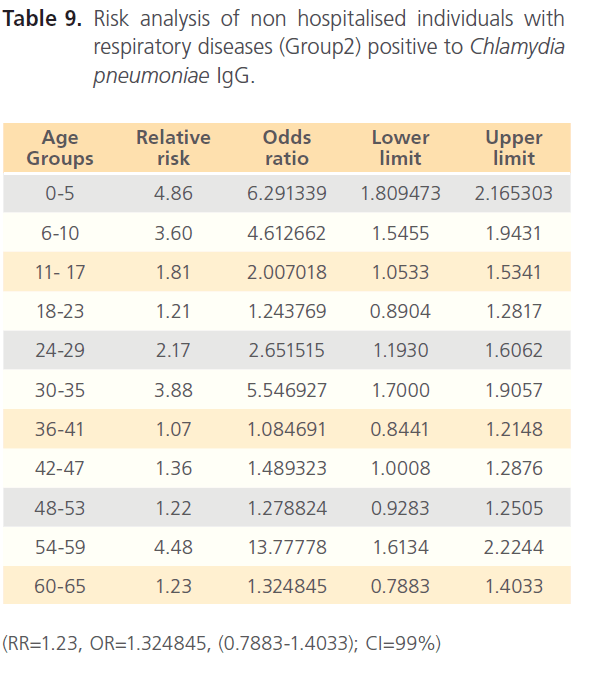
Table 9: Risk analysis of non hospitalised individuals with respiratory diseases (Group2) positive to Chlamydia pneumoniae IgG.
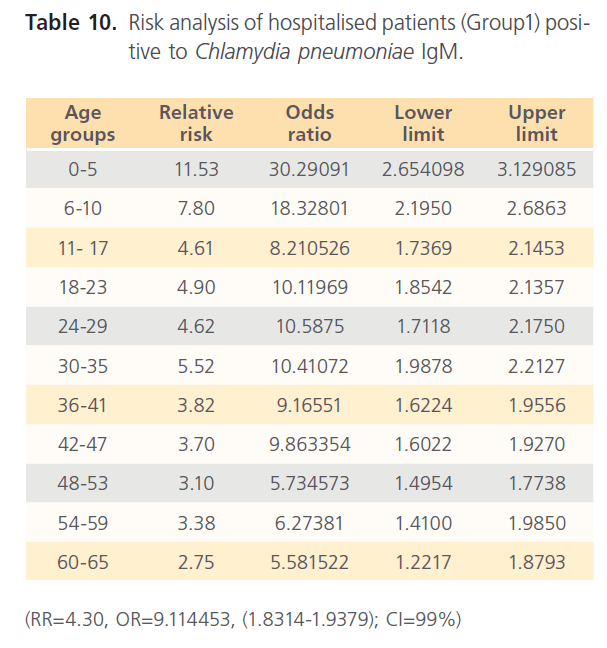
Table 10: Risk analysis of hospitalised patients (Group1) positive to Chlamydia pneumoniae IgM.
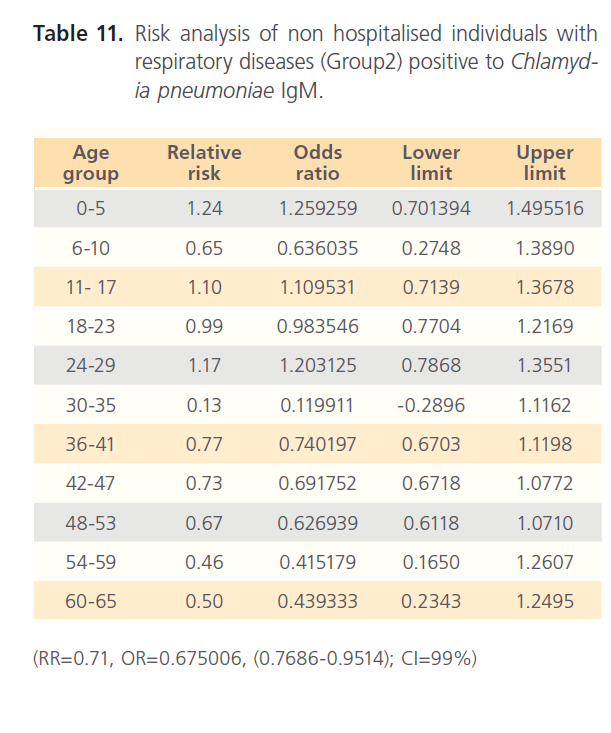
Table 11: Risk analysis of non hospitalised individuals with respiratory diseases (Group2) positive to Chlamydia pneumoniae IgM.
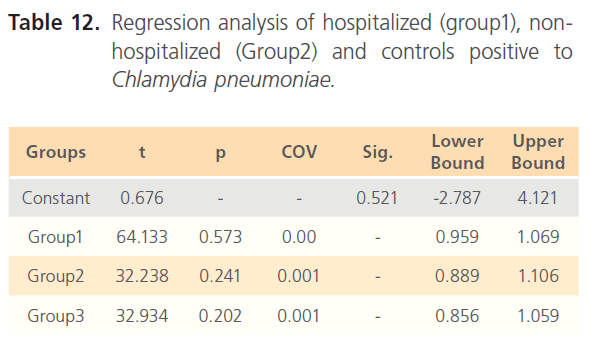
Table 12: Regression analysis of hospitalized (group1), nonhospitalized (Group2) and controls positive to Chlamydia pneumoniae
Discussion
This prospective study on the prevalence of Chlamydia pneumonia in persons with respiratory diseases presents data that are representative of a defined geographic area with little bias even with strict selection criteria for sampling since there was large enough samples using the sample size calculator (Sample survey online calculator) which gave credence to the statistical and epidemiological power in this study. This study became necessary since it is one of the few studies in any tropical environment and about the first report of C. pneumonia regards secondary complications of respiratory diseases or even the first reports in Nigeria. Secondary complications in this study are defined with the clinical symptoms produced by patients who already have these diseases which can include exacerbation in the case of those whose diseases are pulmonary.
Though the reliability of MIF method specific C. pneumonia antibodies has been questioned (cited in 5) some other reports have also stated its importance as a diagnosis tool for C. pneumoniae [18]. Hoymans et al. [18] reported the importance of methodology for serological screening of C. pneumonia especially in coronary heart diseases. However, for the avoidance of possible cross reactivity between different Chlamydia species since they have the group reactive antigen, this study screened for MIF IgG and IgM, as well as taking into consideration the original criteria for antibody positivity. Completeness in fluorescence was taken as positive while partial fluorescence no matter how bright was taken as negative. And then carry out statistical comparison of both the methods hence one acting as quality control for the other which showed that results from both methods were not significantly different. The representativeness of the patients with uniform sampling per geographical zone of Nigeria, coupled with reliable laboratory analysis allowed for meaningful statistical and epidemiological comparison. In contrast to many previous studies, both culture and serological methods were used in this study to identify C. pneumonia as a possible cause of secondary complication in respiratory diseases while most studies used both IgG and IgA or one of them alone, this study used IgG and IgM. Despite these differences, this study confirm many features of C. pneumonia been a possible etiology of secondary complication (exacerbation); in particular the high proportion of patients with C. pneumonia infection in respiratory diseases like asthma, bronchitis, sinusitis and pneumonia whether or not community acquired. Most of the patients positive for C. pneumoniae were the older age groups for bronchitis, for asthma both the extreme ages were high, sinusitis cut across all ages groups while pneumonia patients also cut across all ages with the extreme ages having higher proportion positive to C. pneumoniae. Thereby, suggesting age distribution. The high attack rate in the cases (Group 1) and a decrease in the attack rate of unhospitalised individuals (Group 2) and the very low attack rate in those individuals without respiratory disease is an epidemiological confirmation of the involvement of Chlamydia pneumoniae in the causation of secondary complication in the hospitalized patients and that Chlamydia pneumoniae is responsible for their being hospitalised. The high RR and OR in IgG antibodies is indicative of recurrent infection and the high RR and OR for IgM is indicative of recent infection, but when patients have high RR and OR for both IgG and IgM, it indicates both recurrent and persistence infection in this study and this has been reported in other studies [3]. This also confirms a report elsewhere with particular emphasis on bronchitis and asthma where high proportion of Chlamydia pneumoniae has been blamed for exacerbation [24]. The high proportion of C. pneumoniae in this study is also in agreement with Miyashita [25] who reported C. pneumoniae as a significant cause of both lower and upper respiratory tract infection which included pneumonia, bronchitis, pharyngitis and sinusitis where they could be mild or asymptomatic, it was also suggested that there could be a possible association with acute exacerbation of asthma and obstructive pulmonary diseases (COPD) which was also confirmed in this study with their high attack rate especially in the IgM antibody being a pointer to recent infection [25]. Miyashita [25] also reported that seroepidemiological studies reveals antibody prevalence rate of 50 to 70% and that everybody have been infected at one time or the other and this could be responsible for the positive results in the two controls used in this study especially the high IgG antibodies in both cases and controls which is a pointer that the subjects at one point in time came in contact or were infected with the organism. Another factor could be that C. pneumoniae infections are usually persistence and sometimes could be chronic. In the comparison of group of subject’s especially clinical patients, the greatest practical importance lies in their similarities rather than their differences. This is exemplified by the proportion of patients with C. pneumoniae infection which increased with age in patients with bronchitis; asthma remained high in the mid age groups, while sinusitis cut across the age groups with pneumonia having very high attack rate in older age groups. Age group distribution in this study reveals that the extreme ages have a higher risk as seen in their stratified relative risk and odd ratio in the different age groups and this cut across the 2 groups of subjects. Earlier studies have also reported the C. pneumoniae exacerbation to be dependent on age [5, 19]. This also confirms earlier result in this study as well as earlier reports. However, differences between patients in group1 and other groups were statistically significant which implied that Chlamydia pneumoniae is responsible for the secondary complication in hospitalized patients and this is in agreement with earlier reports implicating C. pneumoniae as a possible cause of secondary complications or exacer bations in respiratory diseases since non-hospitalised individuals with respiratory diseases have low proportion of positive individuals and very low proportion of seropositivity in healthy individuals as seen in attack rates as well as their relative risk [5, 26-27]. In this study Chlamydia trachmatis and Chlamydia psittacci was not screened for, Hertzen et al. [5] reported the occasional appearance of these two especially in the IgA class and hence did not have any significant effect in the outcome of this study. This study however, reveals proportion of IgG and IgM in the cases (group1) which is higher than those of the group2 and group 2 higher those of the group3 with a statistical difference between the group1 subjects and subjects in other groups. The result also shows that some subjects were positive for both IgG and IgM, with a similar study showing the co-occurrence of IgA and IgG in patients with C. pneumoniae [27]. And this also infer that the patients may have also come in contact with this agent at some time later than the time of this research, this also explains why individuals in group2 though not having the complication also has high proportion of IgG, and also the presence of some number of IgG positive individuals in control in group3. This has earlier been reported that at any point in life nearly all individuals have come in contact with C. pneumonia [25] whether or not the individual is having respiratory disease. The fact that most of the high proportion of the cases were seropositive to C. pneumoniae IgM infer that the patients would have been recently infected and such may be the cause for the secondary complication (exacerbation) been experienced by these patients. While the presence of IgG is indicative of the fact that the individuals have contacted the infection at one point or the other, this does not say that C. pneumoniae is responsible for persistent infection which could be determined by the presence of IgA and this was the weakness in this study. However, this study was able to show the presence of IgG and IgM in the same individual in patients with respiratory diseases which point to the fact that such patients could be having persistence or recurrent infection with high attack rates as well as their risk ratio. A confirmation that C. pneumoniae could be the cause of secondary complication or exacerbation in patients with different respiratory diseases is evident in the gradual decreases in the relative risk and odd ratio which decreased from group1 (hospitalized) to group2 and to group3 respectively in their risk analysis. The titeration results also confirms the infection as a cause of exacerbation in patients with respiratory diseases since most of the hospitalized patients have antibody titre higher than 1:16 which has been reported to be diagnostic which also decreased from subjects in group1 to subjects in group3 [28]. Hertzen et al. [5] while examining C. pneumoniae in COPD exacerbation in Finland, using MIF IgG and IgA found an association in 7% of the patients which is in accordance with this study which has a higher percentage. However, the drawback in their study lies in the fact that they used relatively small number of samples and examined only a certain age group unlike this study where all the age groups meeting our selection criteria was included in the study. This study also reported a sexual distribution of secondary complication caused by C. pneumoniae in patients with respiratory diseases which is also in accordance with earlier reports. More of the sexual distribution was in asthmatic patients with shows a significant difference between males and females with females having higher proportion of positive individuals, there was also sexual distribution for patients with bronchitis with significant difference between males and females with males having higher proportion of seropositivity. Sinusitis was also distributed in this manner while there was no significant difference between males and females for pneumonia patients and hence was not sexually distributed. This distribution may also be a function of the diseases themselves and not Chlamydia pneumoniae infection in particular. Gislason et al. [19] reported a decline in lung function in women but not in men as a result of C. pneumoniae persistent infection of the lungs which they suggested could be as a result of hormonal differences, the influence of sex and C. pneumoniae antibodies was also reported in children with wheezing [22]. This study agrees that hormonal changes could be responsible for the sexual distribution but the question to be asked is at what stage of the female life do these hormonal changes come to influence infection due to Chlamydia trachomatis? It is also suggested that there could be a decline in immunity as one approach the very old age which could explain why subjects in that category had higher proportion of C. pneumoniae infection. Though these reports were few and more researches are needed in this area to confirm the influence of sex and age in C. pneumoniae infection in general and its implication in secondary complications in patients with respiratory diseases.
In conclusion, the findings in this study, the high attack rate as occasioned by the relative risk and odd ration, the presence of IgM and IgG in high proportion of the subjects, the high antibody titres in the patients. And a comparison to the relative attack rate as occasioned by the RR and OR, low antibody titre in the controls as compared to the cases, may indicate C. pneumoniae as a cause of secondary complication (exacerbation) in patients with respiratory diseases.
68
References
- Cohen, BH., Ball, WC Jr., Brashears, S. Risk factors in chronic obstructive pulmonary diseases (COPD). Am. J. Epidemiol. 1977; 105: 223-231.
- Jokinen, C., Heiskanen Juvonen, H., Kallinen, S., Kleemola, M., Koskela, M., Leinonen, M., Runnberg, P. et al. Microbial Etiology of Community-Aquired pneumonia in the adult population of 4 municipal in Eastern Finland. Clin. Infect. Dis. 2011; 32: 1141-1154.
- Higgins, MW., Keller, JB., Landis, JR. Risk of chronicobstructive pulmonary disease. Collaborative assessmentof the validity of the Tecumseh Index of Risk. Am Rev Respir Dis. 1984; 1130: 380-385.
- Davis, RM., Novotny, TE. The epidemiology of cigareue smokingand its impact on chronic obstructive pulmonary disease. Am Rev Respir Dis. 1989; 140: S82-S84.
- von Hertzen, L., Isoaho, R., Leinonen, M., Koskinen, R., Laippala, P., TOyrla, M. Chlamydia pneumoniae antibodies in chronic obstructive pulmonary disease. Int J Epidemiol. 1996; 25 (3): 658-664.
- Sullivan, RJ Jr., Dowdle, WR., Marine, WM., Hierholzer, JC. Adult pneumoniain a general hospital. Arch Intern Med. 1972; 129: 935-942.
- Berntsson, E., Blomberg, J., Lagerga°rd, T., Trollfors, B. Etiology of community. acquired pneumonia in patients requiring hospitalization. Eur J Clin Microbiol. 1985; 4: 268-272.
- Woodhead, MA., Macfarlane, JT., McCracken, JS., Rose, DH., Finch, RG. Prospective study of the aetiology and outcome of pneumonia in the community. Lancet 1987; 1: 671-674.
- Fang, GD., Fine, M., Orloff, J. New and emerging etiologies forcommunity-acquired pneumonia with implications for therapy: a prospective multicenter study of 359 cases. Med. 1990; 69: 307-316.
- Mundy, LM., Auwaerter, PG., Oldach, DO. Community-acquiredpneumonia: Impact of immune status. Am J Respir Crit Care Med. 1995; 152: 1309-1315.
- Macfarlane, JT., Finch, RG., Ward, MJ., Macrae, AD. Hospital study ofadult community-acquired pneumonia. Lancet 1982; 2: 255-258.
- Fletcher, C., Peto, R., Tinker, C., Speizer, F. The Natural History of Chronic Bronchitis and Emphysema. Oxford: Oxford University Press, 1976.
- Tager, I., Speizer, F. Role of infection in chronic bronchitis. N Engl J Med. 1975; 292: 563-571.
- Gump, D., Phillips, B., Forsyth, B., Mclntosh, K., Lamborn, K., Stouch, W. Role of infection in chronic bronchitis. Am Rev Respir Dis. 1979; 113: 465-474.
- Niewoehner, D. The role of chronic bronchitis in the pathogenesis of chronic obstructive pulmonary disease. Semin Respir Infect. 1988; 3: 14-26.
- Grayston, JT., Campbell, LA., Kuo, CC. A new respiratorytract pathogen: Chlamydia pneumoniae strain TWAR. J Infect Dis 1990; 161: 618-625.
- vonHertzen, L., Alakarppa, H., Koskinen, R. Chlamydia pneumoniae infection in patients with chronic obstructive pulmonary disease. Epidemiol Infect 1997; 118: 155-164.
- Hoysman, VY., Bosmans, JM., Van Renterghem, L., Mak, R., Ursi, D., Wuyts, F., Vrints, CJ., Ieven, M. Importance of methodology in determination of Chlamydia pneumoniae seropositivity in healthy subjects and in patients with coronary artherosclerosis. J Clin Microbiol 2003; 14 (9): 4049-4053.
- Gislason, T., Guonason, V., Benediktsdottir, B., Olafsson, I., Aspelund, T., Thjodleiffson, B., Janson, C. Persistent Chlamydia pneumoniae serology is related to decline in lung function in women but not in men. Effect of persistent Chlamydia pneumoniae infection on lung function. BMC Pulmonary Medicine 2010; 10 (44): 1-6.
- Daian, CM., Wolff, AH., Bielory, L. The role of atypical organisms in asthma. Allergy Asthma Proc. 2000; 21: 107-111.
- Martin, RJ., Kraft, M,. Chu, HW. A link between chronic asthma and chronicinfection J Allergy Clin Immunol. 2001; 107: 595-601.
- Normann, E., Gnarpe, J., Wettergren, B., Janson, C., Wickman, M., Nordvall, L. Association between Chlamydia pneumoniae antibodies and wheezing in young children and the influence of sex. Thorax 2006; 61: 1054-1058.
- Molin, GD., Longo, B., Not, T., Poli, A., Campello, C. A population based seroepidemiological survey of Chlamydia pneumoniae infections in school children. J Clin Pathol 2005; 58: 617-620.
- Blasi, F., Tassia, P., Alberti, S.Chlamydia pneumoniae.Clin Infect Dis.2009; 15 (1): 29-35.
- Miyashita, N. Chlamydia pneumoniae and respiratory infections. Kekkaku 2006; 81 (9): 581-588.
- Nabipour, I., Vahdat, K., Jafari, SM., Pazoki, R., Sanjdideh, Z. The association of metabolic syndrome and Chlamydia pneumoniae,Helicobacter pylori, Cytomegalovirus and Herpes simplex type I: ThePersian Gulf Healthy heart study. BMC Cardiovascular diabetology 2006; 5: 25.
- Jha, HC., Vardhan, H., Gupta, R., Varma, R., Prasad, J., Mittal, A. Higher incidence of persistent chronic infection of Chlamydiapneumoniae among coronary artery disease patients in India is a causefor concern. BMC Infect Dis. 2007; 7: 48.
- Okoror, LE. Geographical/Seasonal Distribution and Characterization of Chlamydia in Nigeria. A tropical study 2007; 19.





















