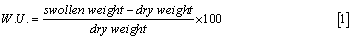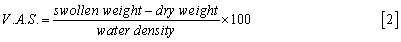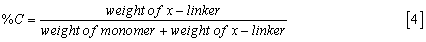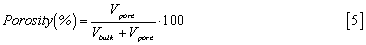Keywords
Hydrogel; Applications; Proteomics; Wound healing; Tissue engineering; Drug delivery; Vaccine; injectable hydrogel
Introduction
Hydrogels are polymeric matrixes that swell but don’t dissolve (in the short term) in water [1]. The swelling properties are due to the high thermodynamical affinity that this class of materials has for the solvent itself. In the past years this characteristic, coupled with a high versatility and a high tunability of material’s properties, lead to deep research and exploitation of hydrogels. These networks establish equilibrium with the liquid and temperature of their surroundings for shape and mechanical strength. Variations in the concentration, structure and/or functionality of the monomer and/or cross-linker used in such gels can change the structure. Indeed, many new gel-form materials, with a plethora of aims were developed and tested in different fields of engineering (e.g. environmental, electronics, biomedical), biotechnology and other disciplines.
This phenomenon can be appreciated by analyzing the number of articles on the topic: doing a quick query for the word “hydrogel” on PubMed it is easy to see the clear exponential tendency in the number of articles (Figure 1). This is a general trend shared by any kind of scientific publication that is, moreover, underestimated because of the inability of any database to capture the whole scientific literature. This phenomenon is considered not consistent by many academic personalities and, surely, generates concerns about the absence of a real correlation between publishing rates and knowledge [2]. Nevertheless, it is possible to assert that, even if this course of events is not evidence, it is at least a clue of the growing interest of the scientific community on the hydrogel topic.
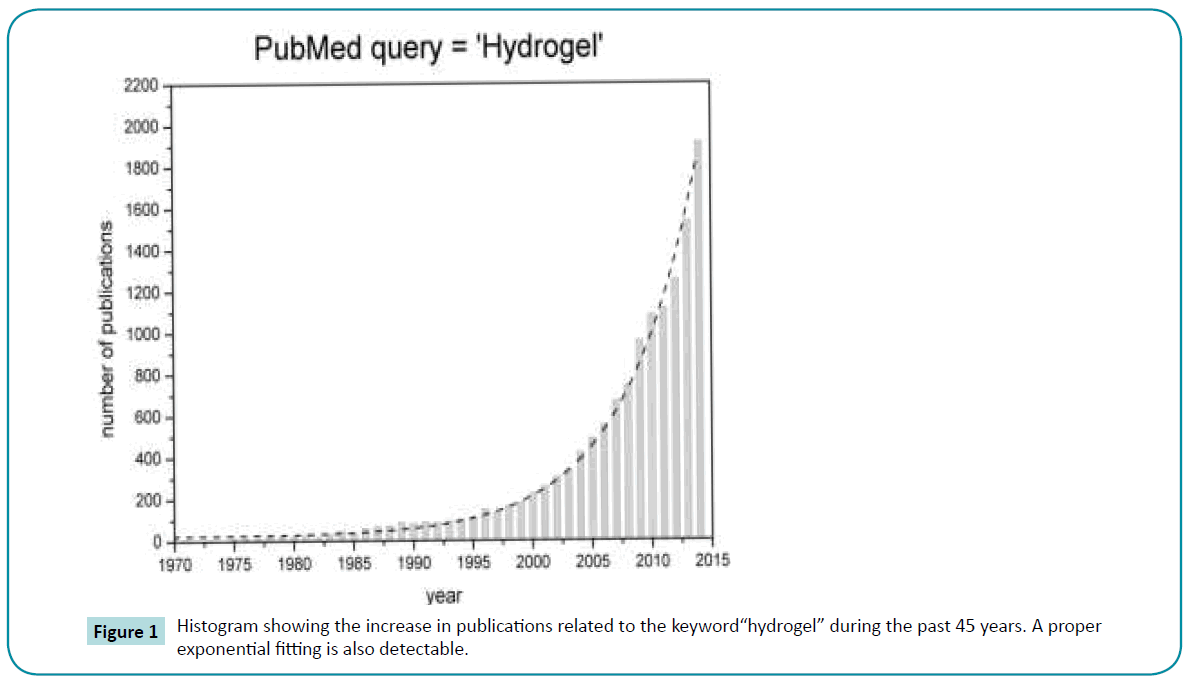
Figure 1: Histogram showing the increase in publications related to the keyword“hydrogel” during the past 45 years. A proper exponential fitting is also detectable.
Smart hydrogel systems with various chemically and structurally responsive moieties exhibit responsiveness to external stimuli including temperature, pH, ionic concentration, light, magnetic fields, electrical fields and chemicals. Polymers with multiple responsive properties have also been developed elegantly combining two or more stimuli-responsive mechanisms. Smart polymer hydrogels change their structural and volume phase transition as a response to external stimuli resulting in an enormous potential for scientific observations and for various advanced technological applications.
Hydrogels can be classified into two distinct categories, the natural and the synthetic hydrogels. Natural hydrogels include collagen, fibrin, hyaluronic acid, matrigel, and derivatives of natural materials such as chitosan, alginate and skill fibers. They remain the most physiological hydrogels as they are components of the extracellular matrix (ECM) in vivo. Two main drawbacks of natural hydrogels, however, make their final microstructures and properties difficult to control reproducibly between experiments. First, the fine details of their mechanical properties and their dependence on polymerization or gelation conditions are often poorly understood. Second, due to their natural origin (bovine fibrinogen, rat tail collagen… their composition may vary from one batch to another.
In contrast, synthetic hydrogels such as poly (ethylene glycol) diacrylate, poly(acryl amide), poly(vinyl alcohol) are more reproducible, although their final structure can also depend on polymerization conditions in a subtle way, so that a rigorous control of the preparation protocol, including temperature and environment control, may be necessary. Generally speaking, synthetic hydrogels offer more flexibility for tuning chemical composition and mechanical properties; users can, for example vary the concentration or molecular weight of the precursor, or alter the percentage of crosslinkers. They can also be selected or tuned to be hydrolysable or biodegradable over variable periods of time.
Hydrogels may be chemically stable or they may degrade and eventually disintegrate and dissolve. They are called «reversible» or «physical» gels when the networks are held together by molecular entanglements, and/or secondary forces including ionic, H-bonding or hydrophobic forces (Figure 2) [3]. Physical hydrogels are not homogeneous, since clusters of molecular entanglements, or hydrophobically-or ionically-associated domains, can create in homogeneities. Free chain ends or chain loops also represent transient network defects in physical gels.
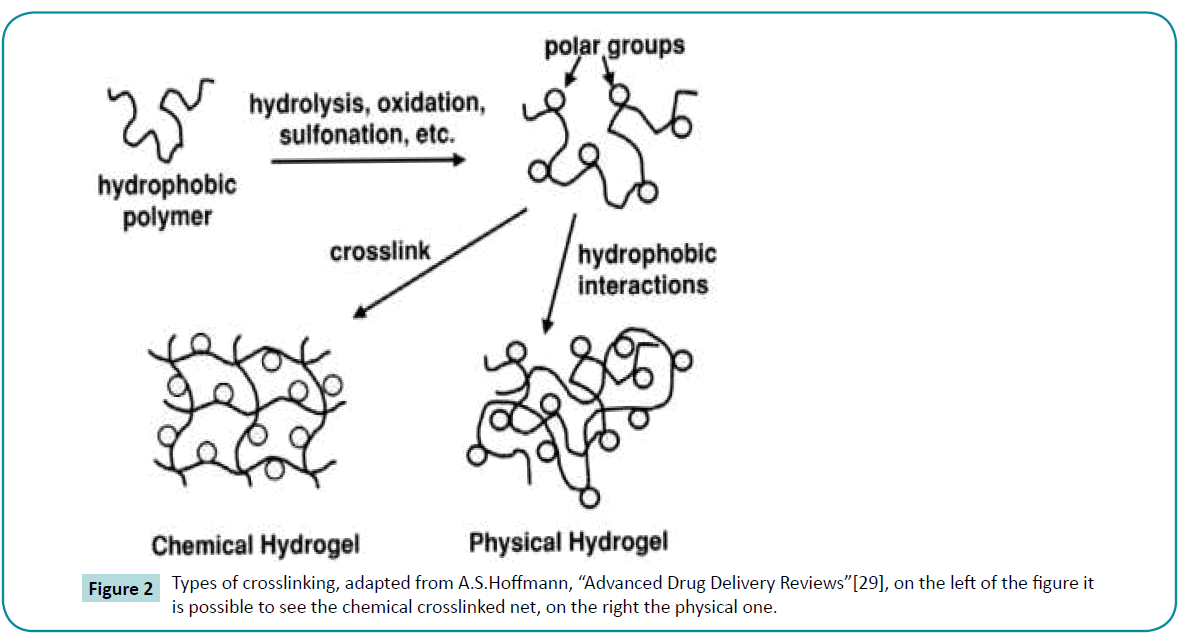
Figure 2: Types of crosslinking, adapted from A.S.Hoffmann, “Advanced Drug Delivery Reviews”[29], on the left of the figure it is possible to see the chemical crosslinked net, on the right the physical one.
When a polyelectrolyte is combined with a multivalent ion of the opposite charge, it may form a physical hydrogel known as an ionotropic hydrogel, calcium alginate is an example of this type of hydrogel. Hydrogels are called “permanent” or “chemical” gels when they are covalently-cross linked networks (Figure 3). The synthetic hydrogels of Wichterle and Lim were based on copolymerization of HEMA with the crosslinker EGDMA. Chemical hydrogels may also be generated by crosslinking of watersoluble polymers, or by conversion of hydrophobic polymers to hydrophilic polymers plus crosslinking is not necessary. In some cases, depending on the solvent composition, temperature and solids concentration during gel formation, phase separation can occur, and water-filled voids or macropores can form. In chemical gels, free chains ends represent gel network “defects” which do not contribute to the elasticity of the network. Other network defects are chain loops and entanglements, which also do not contribute to the permanent network elasticity.
Figure 3: SDS-PAGE migration for separation of proteins mixture.
In many applications, a functional additive is blended into a polymer matrix to enhance its properties. However, when the polymer and functional additive are applied to a surface, the functional molecule may be easily lost. In favorable cases, it may be possible to incorporate the additive directly into the polymer as a comonomer. In recent study, a functionalized polymer has been obtained through the combination of linking a photodynamic, antimicrobial dye, Rose Bengal, to vinyl benzyl chloride via etherification and then polymerizing this into a water-soluble polymer using chain growth copolymerization [4]. The amount of unincorporated dye was determined using dialysis in buffer solution at pH = 7,4. When Rose Bengal was first reacted with vinyl benzyl chloride before polymerization, it was found that it was nearly 100% incorporated into the copolymer.
Review
History
The word “hydrogel”, according to Lee, Kwon and Park, dates back toan article published in 1894. Anyway, the material described there was not a hydrogel as we describe it today; it was indeed a colloidal gel made with inorganic salts. Is yet remarkable to notice how the history of the term itself is consistently long [5]. Anyhow, the first crosslinked network material that appeared in literature and has been described by its typical hydrogel properties, one for all the high water affinity, was a polyhydroxyethylmethacrylate (pHEMA) hydrogel developed much later, in 1960, with the ambitious goal of using them in permanent contact applications with human tissues, hydrogels are in fact the first materials developed for uses inside the patient [6,7]. Since then the number of studies about hydrogels for biomedical applications began to rise, especially from the decade of 70's [5]. The aims and goals and the number of materials changed and enlarged constantly over the years. As suggested by Buwalda et al. [8], the history of hydrogels can be divided in three main blocks.
A hydrogel’s first generation that comprises a wide range of crosslinking procedures involving the chemical modifications of a monomer or polymer with an initiator. The general aim is to develop material with high swelling, good mechanical properties and relatively simple rationale.
Then, starting in the seventies, a different concept of hydrogel grew in importance: a second generation of materials capable of a response to specific stimuli, such as variations in temperature, pH or concentration of specific molecules in solution. These specific stimuli can be exploited to trigger likewise specific events, for example the polymerization of the material, a drug delivery or an in situ pore formation [9].
Finally, a third generation of hydrogels focusing on the investigation and development ofstereo complexed materials (e.g. PEG-PLA interaction) [10,11] hydrogels crosslinkedby other physical interactions (e.g. cyclodextrines) [12,13].
This progress in hydrogel’s science is quickly leading to an increasing interest in the development of the so called “smart hydrogels”, polymeric matrixes with a wide spectrum of tunable properties and trigger stimuli. The topic is theoretically inexhaustible and the possible applications, the engineering and medical devices that can be obtained from it are above any imagination.
Since the pioneering work of Wichterle and Lim in 1960 on crosslinked hydrogels [7], and because of their hydrophilic character and potential to be biocompatible, hydrogels have been of great interest to biomaterial scientists for many years [14-16].
The important and influential work of Lim and Sun in 1980 [17] demonstrated the successful application of calcium alginate microcapsules for cell encapsulation. Later in the 1980s, Yannas and coworkers [18] incorporated natural polymers such as collagen and shark cartilage into hydrogels for use as artificial burn dressings. Hydrogels based on both natural and synthetic polymers have continued to be of interest for encapsulation of cells [19] and most recently such hydrogels have become especially attractive to the new field of “tissue engineering” as matrices for repairing and regenerating a wide variety of tissues and organs [20].
Physical and chemical properties
Despite much progress, a fundamental understanding of gel properties is not yet sufficient for a rational design of novel gel systems. For such designs, it is important to know how solute molecules interact with the gel, in particular, how they partition between the gel phase and the surrounding liquid phase. Partitioning depends on two major effects: size exclusion and molecular attraction/repulsion.
Swelling: Hydrogels are cross-linked polymer networks swollen in a liquid medium. The imbibed liquid serves as a selective filter to allow free diffusion of some solute molecules, while the polymer network serves as a matrix to hold the liquid together. Hydrogels may absorb from 10-20% (an arbitrary lower limit) up to thousands of times their dry weight in water.
The character of the water in a hydrogel can determine the overall permeation of nutrients into and cellular products out of the gel. When a dry hydrogel begins to absorb water, the first water molecules entering the matrix will hydrate the most polar, hydrophilic groups, leading to primary bound water. As the polar groups are hydrated, the network swells, and exposes hydrophobic groups, which also interact with water molecules, leading to hydrophobically-bound water, or secondary bound water. Primary and secondary bound water are often combined and simply called the total bound water. After the polar and hydrophobic sites have interacted with and bound water molecules, the network will imbibe additional water, due to the osmotic driving force of the network chains towards infinite dilution. This additional swelling is opposed by the covalent or physical crosslink, leading to an elastic network retraction force. Thus, the hydrogel will reach an equilibrium swelling level. The additional swelling water that is imbibed after the ionic, polar and hydrophobic groups become saturated with bound water is called free water or bulk water, and is assumed to fill the space between the network chains, and/or the center of larger pores, macropores or voids. As the network swells, if the network chains or crosslink are degradable, the gel will begin to disintegrate and dissolve, at a rate depending on its composition.
There are a number of methods used by researchers to estimate the relative amounts of free and bound water, as fractions of the total water content. All of them are controversial, since there is proton NMR evidence that the interchange of water molecules between the so-called bound and free states is extremely rapid, perhaps as fast as one H2O molecule every 10-9s. The three major methods used to characterize water in hydrogels are based on the use of small molecular probes, DSC and NMR. When probe molecules are used, the labeled probe solution is equilibrated with the hydrogel, and the concentration of the probe molecule in the gel at equilibrium is measured. Assuming that only the free water in the gel can dissolve the probe solute, one can calculate the free water content from the amount of the imbibed probe molecule and the known (measured) probe molecule concentration in the external solution. Then the bound water is obtained by difference of the measured total water content of the hydrogel and the calculated free water content.
The use of DSC is based on the assumption that only the free water may be frozen, so it is assumed that the endotherm measured when warming the frozen gel represents the melting of the free water, and that value will yield the amount of free water in the HG sample being tested. Then the bound water is obtained by difference of the measured total water content of the HG test specimen, and the calculated free water content.
In another formulation, swelling is the property to absorb water and retain it for a relative long time. It can be evaluated by measuring the dry weight and the swollen-state weight and computing either a ponderal variation (water uptake) or a volume of adsorbed solvent (both the quantities are considered as percentages).
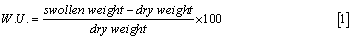
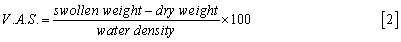
As simple as it may seem, the evaluation of swelling is the principal assay to be performed on hydrogel samples, as it can be a measure for many of their properties: crosslinking degree, mechanical properties, degradation rate and so on. For many gels, the evaluation of swelling and swollen state stability is the simplest, cheapest and surest way to discriminate between crosslinked gels and the not crosslinked original polymer [21].
Mechanical properties
The mechanical properties can vary and be tuned depending on the purpose of the material. It is possible to obtain a gel with higher stiffness increasing the crosslinking degree or lowering it by heating the material. The changes in mechanical properties link to a wide range of variables and causes and different analysis must be made according to the material, the conditions and the aim of the study. For example, while gelatin show a noticeable increase in Young Modulus through crosslinking [22], silk fibroin has a very high Young Modulus, but after the revitalization it will decrease [23]. These properties (young modulus, Poisson modulus, storage and loss moduli, tanδ) can be evaluated by a Dynamic Mechanical Analysis (DMA) device or a rheometer, according to the thousands of techniques available on the market that will be no further discussed here [24,25].
It’s important to note that in a hydrogel, the Young Modulus is the results of the union between water and gel matrix. If we have to seeds osteoblast cells we will need a more stiff material than if we culture adipocyte, the same rationale is valid for the development of a heterogeneous prosthetic device, for example substitute for the intervertebral disc.
Porosity and permeation: Pores may be formed in hydrogels by phase separation during synthesis, or they may exist as smaller pores within the network. The average pore size, the pore size distribution, and the pore interconnections are important factors of a hydrogel matrix that are often difficult to quantify, and are usually included together in the parameter called « tortuosity ». The effective diffusion path length across a HG film barrier is estimated by the film thickness times the ratio of the pore volume fraction divided by the tortuosity. These factors, in turn, are most influenced by the composition and crosslink density of the hydrogel polymer network.
Labeled molecular probes of a range of molecular weights (MWs) or molecular sizes are used to probe pore sizes in hydrogels [26].
Pore-size distributions of hydrogels are strongly affected by three factors:
• Concentration of the chemical cross-links of the polymer strands. That concentration is determined by the initial ratio of cross-linker to monomer.
• Concentration of the physical entanglements of the polymer strands. That concentration is determined by the initial concentration of all polymerizable monomers in the aqueous solution.
• Net charge of the polyelectrolyte hydrogel. That charge is determined by the initial concentration of the cationic and/or anionic monomer.
These three factors can be quantified using the composition of the hydrogel, that is, by the nominal concentrations of monomer and cross-linker:

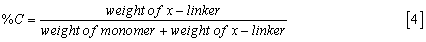
The porous structure of a hydrogel is also affected by the properties of the surrounding solution, especially by dissolved ionic solutes (Donnan effects) and by dissolved uncharged solutes which partition unevenly between the gel phase and the solution phase (Osmotic effects).
For rational design of hydrogels, it is useful to know the pore-size distribution depends on the hydrogel characterization commonly expressed by %C and %T.
Most techniques used to investigate the porosity of hydrogels are limited because they require the pore solvent and/or temperature to be altered, causing the gel to shrink, swell or require mathematical manipulation and assumption, which may introduce unwanted artifacts.
Porosity is a morphological feature of a material that can be simply described as the presence of void cavity inside the bulk. It is useful to control the porosity in many devices for a wide variety of applications, such as optimal cell migration in hydrogel-based scaffolds or tunable lode/release of macromolecules.
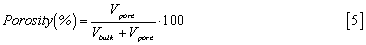
In a sample, pores can show different morphologies: they can be closed, open as a blind end or interconnected, again divided in cavities and throats. These porosities can had been studied and evaluated in papers in the past decades using a various spectrum of techniques. First of all, porosity can be evaluated by theoretical methods, such as unit cube analysis, mass technique, Archimedes method, liquid displacement method. These analysis are commonly coupled with optical and electronic microscopy.
Other interesting techniques are the mercury porosimetry, based on Washburn’s equation, with the inconvenience of being a destructive assay, the gas pycnometry, the gas adsorption (that can be issued using different procedures such as small quantity adsorption, monolayer and multilayer adsorption), liquid extrusion porosity, an assay that permits to evaluated sample’s permeability too, capillary flow porosity, again a test based on Washburn’s equation. Furthermore another important assay is the Micro-CT, also called X-ray Microtomography, a relative new imaging technique, simply described as a non-destructive highresolution radiography, capable of qualitative and quantitative assays on samples and evaluation of their pore interconnections. Between the quantitative assays that can be performed, micro-CT can give information on average pore size, pore size distribution, pore interconnection, struts/walls thickness and anisotropy/ isotropy of the sample (in the sense of presence/absence of preferential orientation of the pores). It is yet, nowadays, an expensive technique both in term of money and time [27,28].
Microscopy techniques can be used in thousands of different assays involving hydrogels. They are both involved in qualitative and quantitative tests, from simple morphological assessing of material’s properties to more complex biocompatibility assays.
Briefly, by microscopy techniques topography and surface morphology can be assessed. These techniques can be divided in many classes, by increasing magnification power: optical microscopy (OM), stereo microscopy (SM), electron microscopy (SEM and TEM), tunneling microscopy (STM), atomic force microscopy (AFM) [29].
Crosslinking: Crosslinking is not properly a property of hydrogels, while it is more of a cause of all the other properties of the material itself. The crosslinking degree can be correlated to basically every characteristic of a hydrogel. The nature of the crosslinking can vary a lot. Indeed, the hydrogel’s network can be obtained in many different ways. The processes can be divided into two big categories: first of all the so called physical crosslinking that occurs, for example thanks to hydrophobic interactions between chains, ionic interactions between a polyanionand a polycation (complex coacervation) or ionic interactions between a polyanion and multivalent cations (ionotropic hydrogel). The second category comprises the chemical bound gels. The crosslinking can occur by ultraviolet irradiation, heating or chemical crosslinking via crosslinker with a huge ensemble of reactions, such as Michael’s reaction, Michaelis-Arbuzov reaction, nucleophile addition and so on [30].
By controlling the degree of crosslinking is possible to tune the property of the material and optimize it for many different applications getting theoretically, in this way, a wide spectrum of applications starting from the same original polymer [33,34].
Applications: From the Lab to the Market
Hydrogels are widely used in the area of electrophoresis, bioseparations, proteomic, chromatography, tissue engineering… etc. They are well-known in foods and medicines as absorbents in disposable diapers, as filters for water purification and as separation materials for chromatography and electrophoresis. They are also of interest for controlled drug release and for concentration of dilute solutions of macromolecules [35].
Domestic uses
It’s not mandatory for life changing inventions to be high-tech devices with out-of-sight ambitions. Sometimes the simplest expedient can lead to a bigger progress than expensive and futuristic technologies. This is true for hydrogels, too. This class of materials has plenty of domestic usages that exploit their ability of loading, retaining and releasing fluids to significantly improve everyday life.
Diapers: An interesting application of hydrogel’s thermodynamical affinity for water, as not fancy as it can be, is the production of super-adsorbent diapers with the property of being dry even after a considerable adsorption of fluids. This is due to, as previously said, the nature of hydrogel’s water adsorption: these materials don’t act as sponges, unstably trapping liquids into their pores, but instead they retain water (or, sometimes, other solvents) in while if they are carrying considerable quantities of water at the same time. The development of hydrogel-containing diapers, most of them loaded with different formulations of sodium polyacrylate [36-38], in the past two decades cut down on a huge number of dermatological conditions related to a prolonged contact with wet tissues. Nevertheless many health concern aroseon the massive usage of disposable diapers: ≈95% of nappies in western countries are disposable ones and opinions about cloth nappies are still conflicting [39]. Indeed, many chemicals used in the production of such products, like scents, leak-proof materials and super-adsorbent polymers, seem to be key elements for the development of many conditions, from chronic diaper rash and asthma, to more serious problems such as male infertility or even testicular cancer. Furthermore, disposable diapers, since they are used in huge quantity, create a notable environmental issue since it’s not easy to dispose of them [40]. However, this is a topic that, as far as interesting and important, goes beyond the purpose of our review.
Watering beads for plants: Another simple application of hydrogels consists in rough powders of polyacrylamide or potassium polyacrylatematrix sold with a huge range of names (Plant-Gel, Super Crystals, Water-Gel Crystals) and used as long term reservoir of water for plant growth in gardening, domestic and sometimes industrial horticulture. On the opposite side as the one of diaper’s hydrogel, these materials are optimized for their ability of releasing water, instead of the ability of retaining it. The sustained release of many diverse species is, indeed, one of the main strength of hydrogels on the market, from gardening to genetic engineering. However, even if companies producing such crystals are promoting their practicality and versatility, in the last years the scientific community is questioning about their real utility. As Chalker-Scott from Washington State University pointed out in her publications on the topic, since the commonly used watering crystals are made out of non-renewable materials, whose monomers can be toxic (e.g. acrylamide), the potential risks of their usage are way higher than the benefits of water storage and controlled release that can, in addition, be obtained in many other ways with lower environmental impact [41].
Perfume delivery: During the nineties patents describing volatile species delivery technologies started to grow in number. In particular, the most significant patented inventions in the field seem to be issued by Procter&Gamble, processing the fragrances into cyclodextrin complexes [42-44].
The general aim was to develop devices capable of slowly dispense fragrances to the surroundings in the long-term and replace the classic salt-based (sodium dodecylbenzenesulphonate) tablets with new, more practical and, let’s say it, fancier house care solutions. The role of hydrogels in the process revolves around, once again, their swelling properties that can be exploited in materials “wherein release of a perfume smell is triggered by dynamic swelling force of the polymer when the polymer is wetted” [43]. These devices release volatile particles thanks to osmotic diffusion of the specie from the swollen hydrogel to new water in the environment.
Cosmetics: Cosmetic industry is a market constantly increasing in dimension and product offer. One of the reasons of this behavior is related to the long path to approval necessary for medical devices, medical procedures, drugs or biomolecules, in order to make allowed to be sold on the market. So, while on the other hand, the cosmetic market requires less time and money expenses for the approval of a product, it is common to test new inventions and devices in cosmetics before and in more risky clinical applications.
For a product to be approved in cosmetics, the most important parameter to be assessed is Primary Irritation Index (PII). This index is simple to obtained and exist both for skin and eyes, indeed for each level of PII corresponds a determinate effect [45,46]. Considering that the majority of hydrogels used in this field are suitable for cells culture and for other biomedical applications, is not surprising that their Irritation Index is among the lowest. Thus, with a relatively small investment, companies are able tolaunch on the market new cosmetic products based on hydrogels, such as so called “beauty masks”.
Usually made with engineered collagen (MasqueologyTM by SEPHORA USA Inc., BioCollagen Cosmeceuticals by NOVOSTRATA UK Ltd.), hyaluronic acid (SEPHORA USA Inc.), or polyvinylpyrrolidone (Pecogel®), these masks claim to hydrate the skin, restore its elasticity and promote anti-aging actions [47]. Pecogel by Phoenix Chemicals Inc., is a wide selection of hydrogels, based on polyvinylpyrrolidone, with differences in composition and/or crosslinking method. Pecogels are suitable for cosmetic purposes, such as sunscreen cream or mascara [48]. Furthermore, in some of the commercially available compounds such as Hydro Gel Face Masks by Fruit & Passion Boutiques Inc., the moisturizing action of these organic polymeric gels is coupled with more complex drug-delivery systems developed to release of biomolecules like vitamin C or B3.
The cosmetic industry is on the cutting edge of hydrogels, indeed a pH-Sensitive material P(MAA-co-EGMA) has been developed for release of cosmetics drugs like arbutin, adenosine, and niacinamide, well knowing molecules for wrinkle treatment and for skin-whitening [49]. This hydrogels change is permeability responding to the pH changes: At pH 4.0 it holds the pharmaceuticals inside the matrix, when in contact with skin, at pH 6 and above, the permeability rise and the drugs will delivered. The authors said that this behavior is due by the ionization/deionization of MAA carboxylic groups.
Plastic Surgery
From their first development into the scientific research field, hydrogel where seen as good materials for application in contact with the human body because of their extracellular matrix-like (ECM-like) properties [7,50]. This is the main reason why attempts were made to introduce hydrogels like new materials for plastic reconstruction.
On this path, for many years, Hyaluronic Acid (HA) was thought like the panacea for every pain [51]. Is not surprising, considered this, that HA has been studied to be applied in tissue filling applications. One notable company operating in the field is MacrolaneTM.
Starting in 2008, Macrolane’s treatments and products were specifically studied to enhance breast size and shape and offer a more biocompatible alternative to standard and aggressive silicone prosthesis. Anyhow, soon the scientific community started to point out the controversial behavior of these procedures in latter mammographies: briefly, HA worked as a shielding agent, appearing as denser tissue, and consequently ruining the outcome of the exam. Thus nowadays MacrolaneTM is used for diversely situated filling with the exception of breasts. The compound is injected inside the body with a syringe and let it gels restoring the volume. This procedure started to receive criticisms by the scientific community, but yet at the moment there is still not a sufficient amount of publications investigating the properties and possible risks of the gel, concerns started to rise in particular about the long term side effects of this treatment and more specifically about HA’s role in cancer control and development [52,53].
Another promising use of hydrogels is bulking agents for treatment of urinary incontinence: smart injectable gels can be involved in clinical procedures where these materials can be used to tighten the urethral channel and reduce patient’s incontinence. With such a simple solution it is possible to erase or at least reduce a consistent social handicap and help patients to hold a normal life [54]. An example of product used in this field is Bulkamid® by Contura International, a polyacrylamide hydrogel is a commercial product developed to help women with incontinence narrowing the conduct. There are few motive for the incontinence, and an exhaustive classification is made by Lose et al. where they subdivide each class, Bladder / Urethra incontinent, in overactivity / underactivity, in their work they wrote that bulk injectable systems are promising to improve urethral coaptation. Problematics like infections and body response have been evaluated. In a sample composed by 130 women researchers have found 10 patients with infections, 5 with injection site pain, 2 with incontinence and only 1 with injection site laceration.
Immunotherapy and vaccine
A nanovector composed of peptide-based nanofibrous hydrogel can condense DNA to result in strong immune responses against HIV. HIV infection is presently incurable and has led to more than 30 million deaths [55]. Traditional, vaccines such as live or attenuated viruses are ineffective and pose potential risks [56]. As a consequence, safe alternatives such as DNA vaccines have attracted great attention, they are easily degraded by DNases and lysosomes, and injected naked DNA plasmids are poorly distributed and inefficiently expressed; thus, DNA vaccines can only induce modest humoral and cellular immune reponses [57]. As such, DNA vaccines must be injected with a delivery system to enhance the immune responses. Different synthetic delivery systems have been constructed including polymers [58], liposomes [59] and nano- or microparticles [60]. Some disadvantages limit their further usage, such as toxicity, small amount of antigen loading and decreased biological activity of DNA during complex preparation.
Nanofibers provide an ideal platform [61]. Another platform suited for DNA delivery is based on hydrogel because of its high loading capacity, mild working conditions, and good biocompatibility [62]. Supramolecular hydrogel is a kind of hydrogel composed of nanofibers formed by the self-assembly of small molecules (molecular weight usually <2000) in aqueous solutions [63]. They can quickly respond to various external stimuli as: pH, temperature, ionic concentration or addition of enzymes.
Recently, peptide-based delivery system emerged as an alternative means to efficiently introduce genes or drugs into cells both in-vitro and in-vivo [64]. Some enzyme-triggered peptide based nanofibrous hydrogels are useful for the entrapment of drug molecules without reducing their activity because that they can work in mild conditions [65]. They are also biocompatible and have well-defined structures.
Nap-GFFY (Naphthalene acetic acid-Glycine phenylalanine tyrosine) was demonstrated as a short peptide to construct gelators of nanofibrous hydrogel. This nanovector can strongly activate both humoral and cellular immune responses to a balanced level rarely reported, which is crucial for HIV prevention and therapy. In addition, this hydrogel shows good biosafety in-vitro and in-vivo. Such hydrogel have a critical nanofibrous structure for the dramatically improved immune responses compared to existing materials. The reason of this high efficiency is that this nanovector can condense DNA, promote DNA transfection, and enhance gene expression in-vitro. Furthermore, it shows promising biocompatibility and no obvious toxicity. This peptide-based nanofibroushydrogel used as a HIV DNA nanovector can open the doors for effective vaccination based on peptide-based nanofibroushydrogels [66].
Environmental applications
Over past the years, nations gradually started to care about environmental issues and pollution. Many governments decided to opt for greener and safer for the environment policies. Water pollution is one of the biggest issues afflicting especially poor areas of Africa, Asia and South America. Thanks to their affinity for water, hydrogels might be used in two different ways to treat water source.
First the matrix can be used as a holder for purifying microorganism. Many interesting studies, on this particular path, were developed by encapsulating microorganisms inside diverse carrier materials [67]. Chlorella and Spirulina are the most used ones. These microorganisms are already used to remove pollutants chemicals from water resources. The idea is to keep the bacteria inside the network and consequently protect and control the bacteria-colturewhile cleaning the site of depuration. Both synthetic and natural hydrogels were been used. The best working hydrogels in literature appear to be Alginate derived [67] or alternatively carrageenan and agar [68].
A second interesting way to solve the problem of pollutants is to modify the hydrogels to let them seize and keep the pollutant inside the networks. Many authors have tried this way to seize metal ions: the group of Irani has sent to publish a paper in which they discuss a new composite hydrogel for PbI(II) removal. Briefly, they created a polyethylene-g-poly (acrylic acid)-co-starch/ OMMT (LLDPE-g-PAA-co-starch/OMMT) hydrogel composite, using it like an adsorbent pollutant (Pb(II)) remover. They put the hydrogel in a solution containing lead acetate and then measured the adsorption with an Atomic Absorption Spectrometer (AAS); after that they did a de-absorption phase and repeat for several cycles. The electrostatic attractions, ion exchange and chelation are possible explanation for the metal adsorption happened during the experiments. They reported that the equilibrium adsorption data of the hydrogel was consistent with Langmuir isotherm and the 430mg/g adsorption capacity was in line with other common adsorbents [69,70].
Another feasible way to achieve an interesting water filtering is explained in a paper by Yan et al., where the group performed etherification and consequent functionalization of chitosan beads in order to obtain carboxymethilated chitosan with an enhanced adsorption of metal ions. This has been proven to improve selective adsorption of specific ions like Cu(II), Pu(II) and Mg(II) [70]. It is interesting properties that can be exploited in dye removal application and that has been demonstrate to be possible by magnetical doping of hydrogel microspheres with interpenetrated network (IPN) structures [71].
Hydrogels could be either used like a probe to detect heavy metal ions like in the work by Wang et al. [72]. Moreover, other application of polyacrylamide gels is a flood control device called WATER GEL BAG® and produced by TaiHei Co., Ltd.
One of the biggest environmental problems currently hard to solve is for sure the loss of oil in seas and in other water sources. In the past years the quantity of oil substance dispersed into the hydrosphere around the world is risen dramatically. Food processing, hydrocarbons industry, refining process have increased the risk of pollution. It was discovered that effluents receiving wastewaters from industry have 40.000 mg/L oil concentration [73].
Different kind of depolluting system have been attempted like bentonite organically [74,75], palygorskite [76], a magnesium aluminum phyllosilicate, and activated carbon. In a study dated 2010 authors attempted to develop a hydrogel to retain water with oil-pollutant molecules [77]. They claim a very promising hydrogel is chitosan thanks to a high presence of amino groups and hydrogen that can react with vinyl monomers. Authors explained use of polyacrylamide grafted on the polysaccharides rises the capability to attract and hold solid particles which remain in suspension in water, called flocculants. The higher crosslinking degree, the lower the retaining capability. This is due to a smaller distance between two nodes in the matrix network. Reducing this distance, the swelling degree will decrease. Furthermore the initial concentration of wasted oil in the environment is another fundamental parameter because of its property of affecting the adsorption kinetic. In fact a higher concentration corresponds to faster swelling kinetics.
Bacterial culture
As already discuss for the environmental applications section, hydrogels can hold inside their matrix a significant number of microorganism for purification of water, for production of biomolecules, or for simple culture of bacteria by themselves.
Indeed, agar is famous as the golden standard substrate for bacterial culture in biotechnological applications [78]. Since it is indigestible by a great number of bacteria and microorganism, it provides a perfect environment for their culture on a solid substrate [79]. Different kinds of agar are been studied, each with a potential use for likewise different kinds of bacterial. Between them brucella agar, columbi agar, schaedler agar, or trypicasesoy agar are the most common. None of these gels hassuperior results compared to the others, instead everyone is suitable for different applications in reason of their different pros and their cons [80,81].
Electrophoresis and proteomic
Gel electrophoresis currently represents one of the most standard techniques for protein separation. In addition to the most commonly employed polyacrylamide crosslinked hydrogels, acrylamide agarose copolymers have been proposed as promising systems for separation matrices in two-dimensional (2-D) electrophoresis, because of the good resolution of both high and low molecular mass proteins made possible by careful control and optimization of the hydrogel pore structure. As a matter of fact, a thorough understanding of the nature of the hydrogel pore structure as well as of the parameters by which it is influenced is crucial for the design of hydrogel systems with optimal sieving properties.
What is the proteomics? The term proteomics was first coined in 1995 [82], Proteomics is the study of the proteome, and is the large-scale study of proteins, particularly their structures and functions, in a given type of cell or organism, at a given time, under defined conditions, to make an analogy with genomics, the study of the genome. The proteome is the entire set of proteins, produced or modified by an organism or system. This varies with time and distinct requirements, or stresses, that a cell or organism undergoes. Proteomics is an interdisciplinary domain formed on the basis of the research and development of the Human Genome Project. While proteomics generally refers to the large-scale experimental analysis of proteins, it is often specifically used for protein purification and mass spectrometry.
In the field of proteomics, the ability to detect a large number of proteins in a single analysis represents a key issue to achieve fast and efficient operation [83]. In this context, the combined use of 2-D gel electrophoresis coupled with mass spectrometry has allowed enormous advances during last few decades and has become nowadays one of standard approaches for proteins separation and identification (Figure 3) [84,85].
Among the materials used for 2-D gel electrophoresis, polyacrylamide crosslinked hydrogels have been extensively investigated in the literature [86], because their tunable mesh size porosity appears to be ideal for separating proteins and DNA samples. Typically acrylamide concentrations higher than 5% are used to form the separation matrix, with the acrylamide concentration being selected to maximize resolution of the range of proteins of interest. Lower acrylamide concentrations are necessary when resolution of large high molecular mass (HMM) proteins (>500kDa) is sought, however at the expense of poor mechanical stability of the gel matrix that often yields difficulties in handling these media [74]. Other polymeric systems alternative to polyacrylamide gels have also been proposed as separation matrices for 2-D electrophoresis including agarose, modified polyacrylamide gels and acrylamide-agarose copolymers [87-89]. In particular, the advantages of the acrylamide-agarose system mainly lay in the possibility of improving the resolution of large HMM proteins without compromising the resolution of low molecular mass proteins, partly due to the optimal average pore size of these materials.
Indeed, the electrophoresis migration process through the polymeric gel matrix is driven by the interactions between the protein fragments and the porous network of the gel, causing the quality of protein resolution to be highly dependent on different structural parameters characteristic of the gel matrix [90]. Among these, mean gel pore size, pore size distribution and stiffness of the gel play a crucial role. In order to achieve improved separation performance in 2-D electrophoresis applications, it is therefore essential to understand the specific nature of the pore structure of the gel and the parameters through which this pore structure can be controlled and manipulated [91,92]. In particular, it is of great interest to investigate structure-property relation-ships of these hydrogels in the attempt to optimize their functional performance.
A wide large range of hydrogel chemical compositions was studied and their effect on structural and functional properties of the hydrogel was elucidated. By employing dynamic rheological tests and creep-recovery tests, a correlation was found between the rheological response of these hydrogels and their sieving properties.
More specifically, the evaluation of the crosslinking density by means of dynamic tests and the use of viscoelastic models for determining the resistance of non-permanent crosslinks to move in the network systems shed light on the pore structure of the hydrogel matrix and helped to clarify its influence on the electrophoretic separation performance. The mechanical stability of the crosslinked hydrogels was also investigated by means of tensile tests and correlated with the crosslinking density of the gel matrix.
Interpenetrating Polymer Networks (IPN)
Interpenetrating polymer networks (IPNs) hydrogels have gained great attention in the last decades, mainly due to their biomedical applications. IPNs are alloys of crosslinked polymers, at least one of them being synthetized and/or cross-linked within the immediate presence of the other, without any covalent bonds between them, which cannot be separated unless chemical bongs are broken [93]. The combination of the polymers must effectively produce an advanced multicomponent polymeric system, with a new profile [94]. According to the chemistry of preparation, IPN hydrogels can be classified in:
i- Simultaneous IPN: When the precursors of both networks are mixed and the two networks are synthesized at the same time by independent, non-interfering routs such as chain and stepwise polymerization [95].
ii- Sequential IPN: Typically performed by swelling of a singlenetwork hydrogel into a solution containing the mixture of monomer, initiator and activator, with or without a crosslinker. If a crosslinker is present, fully IPN result, while in the absence of a crosslinker, a network having linear polymers embedded within the first network is formed (semi-IPN) [96].
When a linear polymer, either synthetic or biopolymer, is entrapped in a matrix, forming thus a semi-IPN hydrogel, fully- IPN can be prepared after that by a selective crosslinking of the linear polymer chains [97,98].
Even if it is still a challenging task, the synthesis of ion imprinted IPN hydrogels constitutes a promising direction in increasing the selectivity of this novel types of sorbents, which is expected to receive much attention in the future.
Tissue Engineering (TE) Applications
Hydrogels are three dimensional polymer scaffolds used in several applications of tissue engineering. A particularly important group of techniques is the so called in-vivo tissue regeneration. In this case, a patient’s own cells are combined with the polymer, and held in-vitro until ready to be implanted. The hydrogel acts as a natural extra-cellular matrix that subsequently promotes cell proliferation and tissue re-growth. The pseudo-extra-cellular matrix, comprised of growth factors, metabolites and other materials, brings cells together and controls tissue structure with the ultimate goal of replacing the natural tissue that was lost or damaged.
When parts of the whole of certain tissues or organs fail, there are several options for treatment, including repair, replacement with a synthetic or natural substitute, or regeneration. The figure below shows how tissue or organ injury, disease or failure has evolved to reach the field of tissue engineering. Tissue repair or replacement with a synthetic substitute is limited to those situations where surgical methods and implants have achieved success. Although implants have been a reasonably successful option, tissue engineering holds out great promise for regeneration of the failed tissue. The first option of the diseased or injured organs is extracorporeal treatment, in which blood is circulated through polymeric membrane exchange devices. These devices are usually passive exchange systems, but more recently experimental systems may contain entrapped or encapsulated cells from other human or animal sources. Those latter systems are called bio-artificial or bio-hybrid organs. Total replacement of the diseased or malfunctioning organ or tissue with a natural substitute requires transplantation of an acceptable, healthy substitute, and there is a limited supply of such organs and tissues. Thus, tissue engineering holds out great promise for regeneration of organs (Figure 4). Hydrogels have become increasingly studied as matrices for tissue engineering [85].
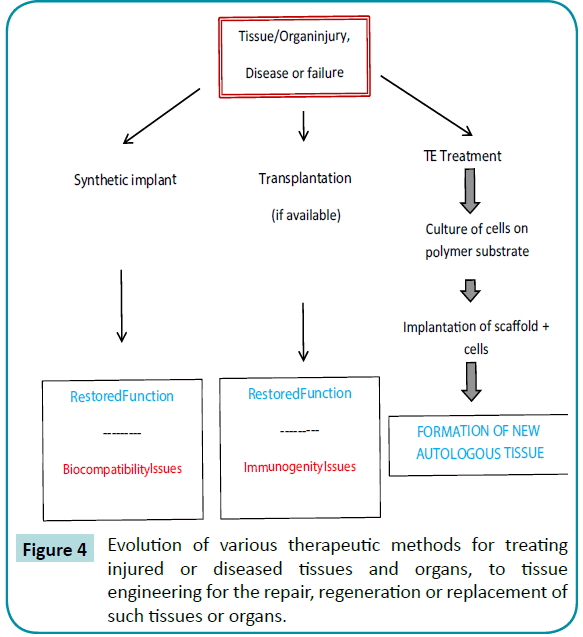
Figure 4: Evolution of various therapeutic methods for treating injured or diseased tissues and organs, to tissue engineering for the repair, regeneration or replacement of such tissues or organs.
Hydrogels designed for use as tissue engineering scaffolds may contain pores large enough to accommodate living cells, or they may be designed to dissolve or degrade away, releasing growth factors and creating pores into which living cells may penetrate and proliferate. Table 1 lists parameters and properties of hydrogels for these applications.
| Type of Hydrogel |
Molecular structures |
Composition of Hydrogel |
Important properties |
•Physical
•Chemical |
•Linearpolymers
•Block copolymers
•Graftcopolymers
•Interpenetrating networks (IPNs)
•Polyblends |
•Natural polymers and theirderivatives
•Syntheticpolymers
•Combinations of natural and syntheticpolymers |
•Degradability
•Injectability
•Mechanicalstrength
•Ease of handling
•Shape and surface/volume ratio (sheets, cylinders, spheres)
•Closed/open pores
•Water content and character
•Chemical modification (e.g. havingattachedcelladhesion ligands)
•Addition ofcells and/or drugs
•Sterilizability |
Table 1: Highlight on important properties and characteristics of hydrogels for tissue engineering.
One significant advantage of hydrogels as tissue engineering matrices vs. more hydrophobic alternatives such as PLGA is the ease with which one may covalently incorporate cell membrane receptor peptide ligands, in order to stimulate adhesion, spreading and growth of cells within the hydrogel matrix. However, a significant disadvantage of hydrogels is their low mechanical strength, posing significant difficulties in handling [99]. Sterilization issues are also very challenging. It is clear that there are both significant advantages and disadvantages to the use of hydrogels in tissue engineering, and the latter will need to be overcome before hydrogels will become practical and useful in this exciting field (Table 2).
| Advantages |
Disadvantages |
•Aqueous environment can protect cells and fragile drugs (peptides, proteins, oligo nucleotides, DNA)
•Good transport of nutrient to cells and productsfromcells
•May be easily modified with cell adhesion ligands
•Can be injected in-vivo as a liquid that gels at body temperature
•Usually biocompatible |
•Can be hard to handle
•Usually mechanically weak
•May be difficult to load drugs and cells and then cross link in-vitro as a prefabricated matrix
•May be difficult to sterilize |
Table 2: Important advantages and disadvantages of hydrogels as matrices for tissue engineering.
It should be noted that a gel used as a tissue engineering matrix may never be dried, but the total water in the gel is still comprised of bound and free water.
Langer has described tissue engineering as an interdisciplinary field that applies the principles of engineering and the life sciences to the development of biological substitutes that restore, maintain, or improve tissue function [100]. This field is currently advancing rapidly, combining progress in biology and technology, and has raised many hopes in several areas of biology and medicine. First and foremost it has a strong potential as an application in regenerative medicine, for developing lifelike replacement tissues and organs. But it also has an important role to play in fundamental biological research; it allows users to reproduce physiological micro-environments more closely in in-vitro settings than traditional culture methods, offering a way to bridge the gap between in-vivo experiments and conventional in-vitro studies. On a more operational side, the development of new in-vitro models based on human cells has raises the possibility of tackling many of the obstacles that currently hinder pharmaceutical research and drug development. Engineered tissues could reduce the limitations related to the transposition of findings from one organism to another, alleviate ethical problems related to animal testing, increase standardization, and allow more thorough studies of toxicity, metabolism and life cycle of putative drugs before entering clinical testing, this could, in turn, reduce the duration, cost failure rate and risk of clinical trials.
In vivo, the formation of organs and tissues is based on the coordination in time and space of cell differentiation, polarity, shape, division and death. This coordination relies on the cellular integration of signals from microenvironment, mainly consisting of the extracellular matrix (ECM), and intercellular communication [101]. The transduction of a typical combination of these factors coupled to specific cytoplasmic components can induce the three progressive steps in differentiation. First, stem cells are specified towards a certain fate, then they shift from a specified state to a determined state, in which the cell fate cannot be reversed, and finally reach their differentiated state.
The main challenge of tissue engineering is to reconstitute in-vitro an environment that induces the differentiation of cells and their organization in an ordered functional tissue. The cell substrate is of particular importance, since in-vivo the extracellular space is occupied by the ECM. The chemical composition of the ECM and the resulting mechanical properties are both important aspects, as the transduction of both chemical and physical signals via cellular adhesion molecules affects cell shape, polarization, migration and differentiation [102]. Furthermore, the ECM topography orients tissue polarity and the morphogenesis of new organs.
Molecular hydrogels hold big potential for cells culture and tissue engineering; peptide-based molecular hydrogels, especially those of long peptides, could provide suitable environments for cell growth, division and differentiation. Zhang group and the Stupp group have demonstrated that peptide-based hydrogels could guide the differentiation of stem cells [103]. The challenge in this field was a method to separate cells from gels post-culture such as using short-peptide-based molecular hydrogels formed by biocompatible methods for 3D cell culture, stem cell controlled differentiation and cell delivery [104]. Responsive molecular hydrogels for the recovery of cells post-culture were also studied [105].
Collagen is the most abundant protein mammals, making up about 30% of the overall body protein content. In order to mimic collagen nanofibers, a serial of short peptides bearing collagen repeating tripeptide of Gly-Xaa-4-Hyp (GXO, X was Lys (K), Glu (E), Ser (S), Ala (A), or Pro (P)) was synthesized [106].
There are two main bottlenecks right now in tissue engineering. The first one is correlated with the possibility of obtaining, in-vitro, developed vascular tissue [107,108]. In second instance, once a pseudo-physiological angiogenesis is achieved, the ambitious aim is to create engineered whole organs [109]. First attempts in this direction where made by printing 3D organ-like structures with ECM and cells, or by decellularization and seeding.
Under these circumstances, hydrogels are usually studied as temporary substitutes of the extracellular matrix (ECM) because of comparable physico-chemical properties, such as stiffness and hydrophilicity [51]. Hydrogels used as scaffold in TE must be biocompatible and elicit the smallest response by the body. This is a very complicated problem, indeed, for the culture of eukaryotic cells implies a lot of precautions. Cells morphology, metabolism and overall phenotype are directly correlated with the signals they receive, first of all from the physical and chemicals properties of substrate.
The micro and nanostructure, the porosity and the stiffness of the surfaces are all important signals for the cells (topography and mechanical properties) [109]. A very important phenomenon, for instance, is the contact guidance, introduced by Weiss in 1934 [110], for which cells aligns to the shape and the microstructure of a given surface. Especially for some kind of cell culture (e.g. myocytes), this phenomena is crucial for the creation of new working tissue [111,112]. Hydrogels for TE must be developed attempting to fit this necessity.
For the purpose, different materials have been investigated. Actually research is focused mainly on degradable scaffold to allow cell migration while the matrix degenerates. Researches demonstrated the importance of engineered degradation on non-degradable scaffolds by viability test [113,114]. These hydrogels can be divided by the origin of the polymer they are made of: either if it is natural or synthetic. In the present article the attention will be focused on natural derived hydrogels
Dextran: Dextran is natural polysaccharide obtained from the digestion of amylopectin. Low molecular weight dextran is been used like a plasma expander thanks to the relatively inert and nontoxic behavior of this polysaccharide [115]. The most interesting characteristic of dextranareits protein rejection properties, the so called non-fouling [99], coupled with great biocompatibility due to its glycocalyx mimic behavior [100]. This property is useful to create an ECM-like hydrogels for tissue engineering, for instance, Yunxiao and collaborators created a copolymer between methacrylate-aldehyde-bifunctionalized dextran (DEXMA- AD) and gelatin B. This material was obtained by ultraviolet (UV)-crosslinking between a methacrylate groups on Dex-MA-AD and the aldehyde groups, allowing the inclusion of gelatin in the matrix that granting enzymatically degradation and cell adhesive properties. Researchers demonstrate that this kind of hydrogels could promote adhesion of vascular endothelial cells [116,117].
Gelatin: Gelatin is the denatured form of collagen, one of the major component of ECM. Collagen however carries immunogenicity problems due to the presence of antigens from the original tissue. Gelatin is a protein materials with a long α helix with a high content in glycine (≈25%) [118]. Gelatin exist in two different form, processed in acid solution (type A, usually porcine) or in alkali solution (type B, usually bovine) [119]. In its natural form is quickly dissolved in water, but it can be crosslinked to obtain a hydrogel with higher mechanical properties and degradation rate.
The crosslinking can be performed in many different ways, from the physical side we could use UV reticulation or the chain polarity. From the chemical one is very common to polymerize, with enzymes too, the chains with a bound on the side groups of amino acids. Lysine and Glutamic Acid are the most employed for it.
Thanks to his similarity to the natural ECM, many compounds were been developed with gelatin-coating or included element. Chitosan-gelatin, fibroin-gelatin, alginate-gelatin, dextrangelatin are very common. Das and collaborators attempted to create a fibroin-gelatin biomaterial for bioprinting cells-laden in a 3D tissue constructs. They developed two kinds of hydrogels. One crosslinked by sonication, the other crosslinked using tyrosinase enzyme. The results showed that sonication-gelatinfibroin hydrogel shown better osteogenic differentiation while tyrosinased-gelatin-fibroin supported better chondrogenic and adipogenic differentiation [120].
Chitosan: Chitosan is a polysaccharides from chitin of the crustacean skeleton. It is composed by the repetition of N-glucosamine units. A crucial index to assess chitosan’s properties is the degree of acetylation, defined as the number of amine in the material. For instance, it is proved that chitosan could decrease the adsorption of protein and the binding of bacteria [121]. Actually it is yet not clear how this materials could been attached by cells. Some studies report a first period in which chitosan repelled cell adhesion, and a second in which cells starts to bind to it. There is no a uniformity in results about this refractory-period, anyhow it can be exploited to seed different kind of cells. For example, Tao Jang et al. published a study in which they used photo polymerization on chitosan in order to deposit cells between the chitosan patterns. They suggest that after the refractory-period, while cells are disposing in nochitosan coated regions, it is possible to seeds another type of cells for the development of a more complex system [122].
In another study chitosan has been coupled with gelatin to create an in-situ gel for cell seeding and/or drug delivery. In particular the group evaluated the difference in crosslinking by two different enzyme [123].
Hyaluronic acid: Hyaluronic Acid – HA is a Glycosaminoglycan GAG enclosed in the natural ECM, core of the material is a polysaccharide with high affinity for water. Usually, to increase the mechanical properties of this biomaterial, a covalent crosslinking between chains is done. Tezel& Fredrickson have reported that a too high degree of modification and cross-linking could influence the biocompatibility property of the material [124].
In vivo HA can have different molecular weights. Low and high molecular weight HA cause an opposite cells behavior [125]. HA macromolecules shown an anti-inflammatory, immunosuppressive properties and blocks angiogenesis, while cleaved small fragments induce the opposite behavior, enabling endothelial cells migration and angiogenesis [126,127]. Indeed, low molecular weight HA have been correlated with some cancers, like prostatic one [125,128]
HA hydrogels were obtained in order to exploit the angiogenic power of the molecule during the degradation of a material. Kisiel’s group, for example, crosslinked HA with proteasedegradable peptides and added cell adhesion ligands to improve the cell spreading on the material [129].
On another hand, Shu et Al. tried to mimic the ECM copolymerizing HA and gelatin. They added a thiol group to the Hyaluronan, in this way they were able to crosslinked modified-Hyaluronan with modified-Gelatin by disulfide bonds [130].
Pectin: Another polysaccharides used in tissue engineering hydrogels is pectin. It is obtained from cells walls after a low pH, high temperature processing. Unfortunately, until now, researchers have not reached the goal to standardize this product in an economically sustainable way [131].
Based on its esterification degree pectin is classified from low methoxyl to high methoxyl. Tuning this property changes the mechanical behavior of the material. Reticulation can occur by lowering pH to obtain physical gel, or using divalent or trivalent ions to obtain water-insoluble gel [35,132].
In the tissue engineering field, pectin seems very interesting because of its promotion of nucleation of mineral phase when immersed in a specific biological solution [131,133].
Alginate: Derived from brown algae, alginate is a polysaccharide composed of beta-D-mannuronic acid and alfa-L-gluronic acid. Its reticulation can alsooccurby divalent cations (Ca2+, Fe2+, Ba2+) [134].
In tissue engineering alginate can be used as an immunoisolation barrier [117]. Alginate scaffold for the regeneration of annulus fibrosus are also been developed. This hydrogels have shapememory capability, are cytocompatible and supports proliferation and metabolic activity [135]. Moreover, alginate hydrogels were used to reduce liver cells death [136] and with silk-fibroin as a substrate for stem cells culture [137].
Culture of organs-on-chips
Recent trends towards the development of in-vitro multicellular systems with definite architectures, or “organs on chips” are studied. First, the chemical composition and mechanical properties of the scaffold have to be consistent with the anatomical environment in-vivo. In this perspective, the flourishing interest in hydrogels as cellular substrates has highlighted the main parameters directing cell differentiation that need to be recapitulated in artificial matrix. Another scaffold requirement is to act as a template to guide tissue morphogenesis. Therefore specific micro-fabrication techniques are required to spatially pattern the environment at microscale. 2D patterning is particularly efficient for organizing planar polarized cell types such as endothelial cells or neurons. However, most organs are characterized by specific sub units organized in three dimensions at the cellular level. The production of such 3D patterns invitro is necessary for cells to fully differentiate, assemble and coordinate to form a coherent micro-tissue. These physiological microstructures are often integrated in micro-fluidic devices whose controlled environments provide the cell culture with more like-life conditions that traditional cell culture method. Such systems have a wide range of applications, for fundamental research, as tools to accelerate drug development and testing, and finally, for regenerative medicine.
The in-vivo extra-cellular matrix (ECM) consists mainly of collagen fibers, elastin fibers, glycoproteins and polysaccharides. It acts as a mechanical support to the cells it surrounds and plays an important role in cell shape, cell polarity, cell migration, resistance to external forces, and signal transduction. The primary focus of tissue engineering is to achieve an in-vivo-like cellular environment, by developing in-vitro artificial ECM. In regenerative medicine applications, such scaffolds could either be directly introduced into an injured organ in order to induce in-vivo genesis, or first seeded with cells in-vitro and then transplanted once those cells have differentiated. For in-vitro research and testing applications, they are generally directly used as a substrate. In all cases, the artificial structures need to recapitulate the in-vivo environment signals responsible for cell differentiation into the desired tissue.
Biomaterial scaffolds are essentially made of hydrogels. Based on their ability to retain water by swelling, they mimic the high water content of the extracellular matrix [138]. Cell adhesion ligands must be present in hydrogels to allow cells to adhere, spread, migrate and proliferate. There is a large variety of adhesion molecules, such as laminin and its derivatives [138], fibronectin [139] and collagen [140]. It is therefore crucial to select the adhesion molecules for which the seeded cell type has the largest affinity to make adhesion effective. Natural hydrogels are bioactive and usually provide native adhesion sites. Conversely, synthetic hydrogels are inert, since their carbon skeleton presents no adhesion molecules or endogenous factors inducing proliferation and cell differentiation.
To enrich their potential as bioactive materials, synthetic hydrogels are generally supplemented with adhesion molecules [141], either by covalent grafting, adsorption or electrostatic interaction. Adhesion molecules can be grafted after hydrogel polymerization, or added to the pre-polymerized mixture and either physically trapped or chemically incorporated during polymerization. Finally, in the case of photo-activated materials such as PEGDA, adhesion molecules can be chemically modified to covalently attach to the hydrogel backbone. The grafting of PEG polymer has been thoroughly described in the review of Zhu et al. [142].
Culture of cells is very demanding area. It deals with living cells that are highly sensitive to their surrounding environment. It also depends strongly on differentiation, a delicate, complex and still only partly understood phenomenon. It thus requires the development of increasingly sophisticated technologies. Although the use of hydrogels as physiological substrates to replace the in-vivo ECM is globally accepted, all parameters must be carefully manipulated to avoid additional unwanted signals. The search for optimal artificial matrices has led to the invention of dynamic hydrogels that can be tuned to reproduce the in-vivo micro-environment, which is permanently renewed and crossed by various signals.
Despite these major improvements, challenges remain. Until now, a physiological substrate alone is, in most cases, insufficient to induce cells to assemble into a multicellular entity and coordinate their activity to form a functional tissue. Additional hydrogel structuring at micrometer scale is necessary. In many cases, a unique association of physiological substrate coupled to a 3D structure replicating the physical constraints perceived by the cells in-vivo will be needed to induce in-vitro the differentiation of cells into a functional tissue.
Nonetheless, the vast majority of these ?organs on chips ?are mostly static, while in-vivo cells are often subjected to mechanical deformations associated with the organ’s function. Furthermore, the biochemical environment can vary in time depending on the physiology of the entire organism. These physical and chemical stimuli generally affect the differentiation and self-organization of cells into functional tissues and are thus crucial to mimic. Tissue engineering therefore needs to move beyond 3D culture to achieve “4 dimensional structures“ that encompass the temporal dimension of organ function as well as it spatial ones. Combining the 3D micro-structured tissues and the dynamic stimuli appears to be the next to reach in micro-organ engineering.
Bone regeneration
This work gives a general view on the use of hydrogels as scaffold for bone regeneration, with special emphasis on injectable systems, membranes for guided bone regeneration, biofunctionalization and biomimetic mineralization.
Alginate hydrogel has been shown to be a useful tool for producing bone and cartilage tissues [142]. It is very biocompatible in humans and peptides can also be covalently coupled to the molecules. Alginate is a naturally occurring polymer found in kelp (seaweed) that is commonly used in gel formation.
The regeneration of large bone defects caused by trauma or disease remains a significant clinical problem. Although osteoinductive growth factors such as bone morphogenetic proteins have entered clinics, transplantation or autologous bone remains the gold standard to treat bone defects. The effective treatment of bone defects by protein therapeutics in humans requires quantities that exceed the physiological doses by several orders of magnitude. This not only results in very high treatment costs but also bears considerable risks for adverse side effects. There issues have motivated the development of biomaterials technologies allowing to better control bimolecular delivery from the solid phase. Importantly, a relatively narrow therapeutic window of growth factor dose is expected to lead to regeneration, whereas too low or too high growth factor doses will most likely lead to severe side effects, as shown in animal models [143]. Since for the generation of constant growth factor levels, multiple injections or even continuous infusion of high doses would be necessary, alternative routes involving biologically inspired growth factor delivery are sought. In an ideal situation, the localized delivery of growth factors should present physiologically relevant doses and preserve its activity for prolonged periods of time. Moreover, carrier materials should facilitate the communication with cells of the host so as to be actively involved in the process of regeneration. Synthetic hydrogels, highly swollen three-dimensional (3D) networks of macromolecules, have emerged as powerful candidate biomaterials to fulfill these requirements [144]. Hydrogels for growth factor delivery applications have either been generated from natural derived biomolecules such as alginate, collagen, and fibrin or from synthetic polymers employing chemical or physical crosslinking reactions [145].
For growth factor administration, the growth factor can either be freely embedded in the hydrogel or bound to it. In the former approach growth factor release is driven by passive diffusion or coupled to material degradation. Release kinetics can be varied by altering material degradation rate or by changing growth factor quantity. To allow covalent tethering to synthetic or biologically derived hydrogels, GF were chemically modified or genetically engineered to contain functional groups such as thiols, acrylates, azides, … An example of growth factor delivery from fibrin hydrogels to regenerate bone tissue was reported by Hubbell and co-workers [146].
Recently, a fibrin scaffold possessing integrin-binding sites adjacent to growth factor-binding sites to obtain synergistic effects was used to regenerate bone [147]. For this purpose a trifunctional peptide was created consisting of an N-terminal Gln sequence for covalent incorporation into fibrin matrices, the major integrin binding domain of fibronectin (FN III9-10) and at the C-terminus another domain of fibronectin (FN III12-14) to bind various growth factors. Since FN III12-14 binds growth factors from different families it allows for straightforward delivery of multiple growth factors. By delivering BMP-2 and PDGF-BB, two growth factors known to induce bone formation and previously shown to bind to FN III12-14, the authors tested for improved growth factor efficiency in ectopic positions in nude mice and calvarial critical size defects in rats. By using growth factor concentrations that didn’t show any in-vivo effects when delivered in empty fibrin matrices, they were able to demonstrate that the FN III9-10/12- 14 functionalized matrices enhanced growth factor-induced bone formation and recruitment of bone forming progenitor cells. Due to the enhanced growth factor signaling, concentrations could be dramatically reduced and bone tissue deposition was observed with much lower doses than elsewhere reported, showing promise for the use of such implants in clinical applications.
In another study a novel photo-cross-linkable chitosan–lactidefibrinogen (CLF) hydrogel was developed and characterized and they also evaluate the efficacy of bone morphogenetic protein-2 (BMP-2) containing a CLF hydrogel for osteogenesis in-vitro and in-vivo. Radiography, microcomputed tomography and histology confirmed that the BMP-2 containing CLF hydrogels prompted neo-osteogenesis and accelerated healing of the defects in a dose-dependent manner. Thus the CLF hydrogel is a promising delivery system of growth factors for bone regeneration [148].
A photo-cured hyaluronic acid (HA) hydrogels were designed and prepared containing an osteogenesis-inducting growth factor, GDF-5 (growth and differentiation factors 5). Those hydrogels were prepared and confirmed to have controlled GDF-5 release profiles. Cytotoxicity and cell viability suggest that GDF-5loaded HA hydrogel has proper biocompatibility for use as a scaffold which can induce osteogenesis. Moreover, the HA hydrogel showed improved osteogenesis in both in-vitro tests, the results from these tests show that the HA hydrogel can be used as a scaffold for bone tissue regeneration [149]. Gelatin hydrogels were also used for bone regeneration at both the critical-sized bone defect.
Cardiac applications
In the last decade, advancements have been made towards developing injectable hydrogels for the purpose of cardiac repair. Hydrogel injections alone have been shown to attenuate the decline in cardiac function and left ventricular remodeling typically seen after myocardial infarction in both large and small animal models. Furthermore, hydrogels have also been shown to improve cell retention when co-injected for cellular cardiomyoplasty and to prolong release of therapeutics when used as a delivery vehicle. This chapter will review the basics of hydrogel properties and discuss recent studies of hydrogels alone and in conjunction with cells or therapeutics for cardiac repair.
Dental applications
Pulp regeneration therapy is important to overcome the limitations of conventional therapy to induce reparative dentinogenesis. Presently, dentists have no choice but to remove the whole dental pulp with an endodontic procedure when a dentin defect with pulp exposure reaches a critical size resulting in an irreversible pulp condition. To overcome this limitation, it is considered important to develop pulp regeneration therapy as well as clarify the mechanisms of pulp wound healing. Pulp wound healing and regeneration have common processes, and results of a number of studies have indicated that pulp wound healing consists of initial inductions of apoptosis of damaged pulp cells [134],followed by reactionary dentinogenesis by surviving odontoblasts and reparative dentinogenesis by odontoblast-like cells [150,151]. Reactionary or reparative dentin is formed toward the residual dental pulp, however, not in the area in which the dentin-pulp complex has been lost. To achieve the regeneration of the dentinpulp complex, induction of appropriate pulp wound healing and formation of new dentin in dentin defects are essential, and a few studies have reported vital pulp therapies to form new dentin in defects [152].
Fibroblast growth factor-2 (FGF-2), which is normally stored in the extracellular matrix and released by enzymatic degradation of extracellular matrix molecules, plays a role in physiologic conditions such as enamel and dentin formation of the tooth germ [153], as well as pathologic conditions [154]. It was previously demonstrated that a gradual and continual release of biologically active FGF-2 was achieved by in-vivo biodegradation of gelatin hydrogels that incorporated FGF-2 [155]. Furthermore, a controlled release of FGF-2 from gelatin hydrogels induced neovascularization and regeneration of several tissues, including bone [156], periodontal tissues [157], and others [158].
Wound healing applications
Wound healing is the promise of a new way to heal damaged skin tissue with high biocompatible and bioactive materials. Skin burned, diabetic ulcer, are problems that at the state of the art are very expensive to treat. Prosthetic-tissue engineered skin are been made, unfortunately they are not ready-to-use; they are expensive and have many needs that are not always matched by patients. Theoretically, in wound healing applications a crucial parameter to assess is the wound contraction that can be evaluated in this way, remembering that A0 is the original burn wound area, and At is the burn wound area at the time of biopsy:

Many systems has been studied, with or without chemicals to aid the skin regeneration. Hyaluronic-acid and gelatin are both two promising materials for the aim because of their natural presence inside human ECM of the skin tissues. Moreover, in literature can be found healing systems made from cellulose [159], alginatechitosan copolymers [160], chitosan-gelatin-honey copolymers [161] and new biphasic gelatin-silk [162]. Most of the products already on the market use a combination of selected materials and proper seeding of cells from various origins (allogenic or autogenic), for instance we cite to applications of HYAFFTM esterified hyaluronic acid [163], both produced by FIDIA Ltd.: LaserskinAutograft®, made of a HA-membrane with keratinocytes, and Hyalo-graft 3D®, made with HA, but with fibroblasts added.
Drug delivery applications
By drug delivery we intend the whole ensemble of procedures, devices and techniques to avoid the problems of standard delivery of medicals, first of all the burst release and quick decay of the effects of the drug overtime, especially for short half-life pharmaceuticals [164].
Hydrogels, and smart hydrogels in particular, can be a very interesting solution in reaching a sustained and targeted release of pharmaceuticals, both increasing the effect of the drug itself and lowering side effects at the same time [165].
Silk Fibroin, protein-based biomaterial with high Young Modulus (1-10GPa) obtained processing the silk from various insects, like spider or silk worm (Bombyx Mori). The silk fiber is composed by fibroin and sericine. This second protein is correlated with inflammatory reactions when coupled with fibroin. To obtain a pure fibroin wire, silk is boiled in alkaline solution at 120° C for at least 1 hour. Solubilization and regeneration is necessary for use this biomaterial for tissue engineering, like scaffolding. Usually the fibroin is processed in a solution of LiBr [166,167], in a ternary solvent composed by Calcium Chloride / Ethanol / Water to break fiber to fiber bond [168] or by SDS [168,169]. This allows the casting of the polymer and the consequent creation of different structure for geometrical complexity.
In 2011 Chinmoy et Al. used silk fibroin from Bombyx Mori and from NathereaMylitta to create a 3D scaffold for cardiac tissue engineering. Results showed that metabolic cell response, cardiomyocytes growth and the number of junction between cells for AntheraeaMylitta fibroin are very similar to fibronectin ones. They concluded that silk fibroin from AM enables an efficient attachment of cells without affecting their response to extracellular stimuli.
Hydrogels as soft contact lenses
Contact lenses are one of the reasons why hydrogels where developed in the first place. They come with many different features and procedures of usage. In particular, soft contact lenses with a short turnover are the ones for which hydrogel are mostly useful. The most used polymer exploited to produce soft-contact lenses is silicone, under hydrogel form, thanks to its permeability for oxygen.
Despite the high microbial keratitis risk for a prolonged wear time, which subsists for most of ophthalmic applications in this field, extended wear time of silicone hydrogel lenses are five times safer than other conventional hydrogels for what concern infections [170,171]. Only about 5% of drugs administrated by eye drops are bioavailable, and currently eye drops account for more than 90% of all ophthalmic formulations. The bioavailability of ophthalmic drugs can be improved by a soft contact lens-based ophthalmic drug delivery system. Several polymeric hydrogels have been investigated for soft contact lens-based ophthalmic drug delivery systems: (i) Polymeric hydrogels for conventional contact lens to absorb and release ophthalmic drugs; (ii) Polymeric hydrogels for piggyback contact lens combining with a drug plate or drug or drug solution; (iii) Surface-modified polymeric hydrogels to immobilize drugs on the surface of contact lenses; (iv)Polymeric hydrogels for inclusion of drugs in a colloidal structure dispersed in the lens; (v) Ion ligand-containing polymeric hydrogels; (vi) molecularly imprinted polymeric hydrogels which provide the contact lens with a high affinity and selectivity for a given drug.
The success of the therapy of eye ailments with ophthalmic drugs strongly depends on achieving sufficient drug concentration on the cornea for a sufficient period of time, but the typical delivery of drugs by eye drops which currently account for more than 90% of all ophthalmic formulations is very inefficient and in some instances leads to serious side effects [172]. Upon instillation in the eye, the drug mixes with the fluid present in the tear film and has a short residence time of approximately 2 minutes in the film. Only about 5% of the drug is absorbed into the cornea and the remaining either gets absorbed in the conjunctiva or flows through the upper and the lower canaliculi into the lacrimal sac [173]. The drug containing tear fluid is carried from the lacrimal sac into the nasolacrimal duct. The nasolacrimal duct empties into the nasal cavity, where the drug is then absorbed into the bloodstream. This absorption leads to drug wastage, and more importantly, the presence of certain drugs in the bloodstream leads to undesirable side effects. Furthermore, the application of ophthalmic drugs by eye drops results in a rapid variation in the drug delivery rates to the cornea and this limits the efficacy of the therapeutic system [174]. In addition, dosage through eye drops is inconsistent and difficult to regulate, as most of the drug is released in an initial burst of concentration [175]. To increase the residence time of the drug in the eye, thereby reducing wastage and minimizing side effects, a number of researchers have proposed using contact lenses for ophthalmic drug delivery. It has been shown that in the presence of a lens, ophthalmic drugs have a much longer residence time in the post-lens tear film, compared with 2 minutes in the case of topical application as eye drops [176]. The longer residence time will result in higher drug flux through the cornea and reduce the drug inflow into the nasolacrimal sac, thus reducing drug absorption into the bloodstream. Several methods have been proposed to make ophthalmic drugs deliverable by soft contact lens.
The silicone hydrogel lenses can be designed to release ophthalmic drugs for an extended period varying from 10 days to a few months. The transport of timolol and DX in the silicone gels is diffusion limited, but the release profiles are complex particularly for dexamethasone which is the evidence of complex microstructure of the gels. The silicone hydrogels may also be suitable for other drug delivery applications such as puncta plugs, ophthacoils, retinal implants, transdermal patches, wound healing patches, etc.
Injectable hydrogels
The desire and need to minimize traditional open surgeries and in light of the challenges along with the traditional intravenous administration of chemotherapeutics for example [177], injectable hydrogel-drug system emerges as a powerful tool for noninvasive and in-situ controlled-release of drugs. Such application could also reduce the healthcare expenses and improve the recovery time for the patients. Minimal invasive procedures using endoscopes, catheters and needles have been developed considerably in the last few decades. In the field of tissue engineering and regenerative medicine, there is a need for advancement over the conventional scaffolds and pre-formed hydrogels. In this scenario, injectable hydrogels have gained wider appreciation among the researches, as they can be used in minimally invasive surgical procedures. Injectable gels with their ease of handling, complete filling the defect area and good permeability have emerged as promising biomaterials (Figure 5).
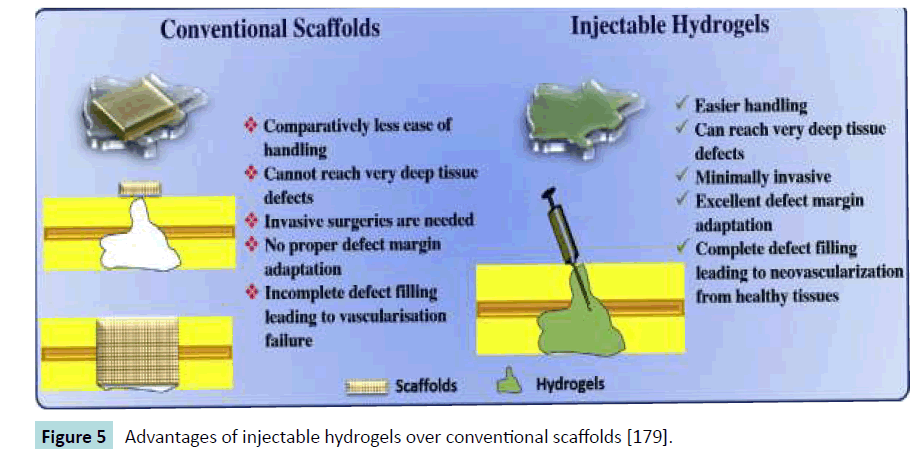
Figure 5: Advantages of injectable hydrogels over conventional scaffolds [179].
Basing on their origin, two kinds of biodegradable polymers are used for preparation of injectable hydrogels: naturally derived and synthetic polymers. Naturally derived polymers such as polysaccharides (cellulose, chitosan, hyaluronic acid, chondroitin sulfate, dextran sulfate, alginate, pectin, etc.), proteins (collagen, gelatin, heparin, fibrin, etc.) and synthetic polymers like polypeptides, polyesters, polyphosphazenes, etc. are widely used [178,179]. In comparison with synthetic polymers, most but not all naturally derived polymers are expected to have better interaction with cells along with increased cell proliferation and differentiation [180]. On the other hand, synthetic polymers possess tunable mechanical properties and degradation profile.
Mutually, exclusive advantages of these polymers have motivated the researchers to investigate combinatorial systems of synthetic and naturally derived polymers, thereby improvising the properties of injectable hydrogels [181]. Injectable hydrogels are prepared using various physical and chemical crosslinking methods as shown in Figure 6 [182].
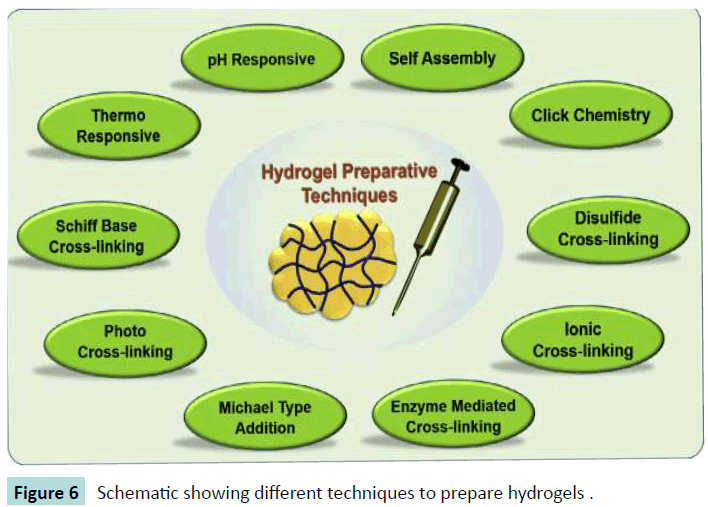
Figure 6: Schematic showing different techniques to prepare hydrogels .
Particle migration, poor shape definition and/or rapid resorption limit the success of current urethral bulking agents. The use of injectable agents to augment urethral tissue function is now common practice because they facilitate minimally invasive delivery of the bulking agent and provide the advantage of low cost and low patient morbidity. The procedure can be performed in an outpatient setting with the patient under local anesthesia. Current therapies using cross-linked collagen, autologous fat or carbon coated beads have provided acceptable cure rates [183]. However, complications or limitations of the treatments include the need for repeat injections, allergic reactions, material resorption and particle migration. Several newly applied materials, including calcium hydroxyapatite and materials consisting of natural or synthetic polymers appear to be efficacious as current therapies but extensive long-term data are still unavailable [184].
Recent clinical trials as well as previous animal studies demonstrated that transplantation of autologous chondrocytes suspended in a polymeric alginate hydrogel produced encouraging results and suggested that injectable alginate hydrogels may have potential use as bulking agents [185]. Covalently cross-linked alginate hydrogels provide a versatile system with potential as an injectable bulking agent. The shape memory characteristics of these materials facilitated the minimally invasive delivery of a bulking construct in a predefined three-dimensional macroporous form, which minimized migration or leakage of the material from the puncture site and ensured integration with host tissue. The hydrogels are easy to prepare and can be stored dehydrated at room temperature, required no specialized injection equipment and can be delivered by a single injection. Such hydrogels have the potential to fulfill many requirements for an optimal urethral or other soft tissue bulking agent.
Injectable hyaluronicacid MRI provides the possibility to monitor the hydrogel and shape of the materials correctly by only H-MRI. There are many very promising studies involving hydrogels and imaging techniques. In one of them, by Yang X. and collaborators, an injectable hyaluronic acid hydrogel has been modified with fluorine-19 and subsequently tested. The authors claim its high potential in many clinical applications since, besides it being biocompatible and easily injectable, it can moreover be tracked with minimal invasive manners [186].
Applications in electronics
The use of hydrogels as matrixes in electronics is very promising considering the high tunability and precision of capacitors with hydrogel dielectrics. By changing the polymer and the solution carried it is possible to build high-performant cheap capacitors.
These Hydrogel electrolytes can be composed by organic polymers. According to Choudhury et al. [187], poly(ethylene oxide), potassium poly(acrylate), poly(vinyl alcohol) and gelatin are among the most promising materials for the purpose. However, organic polymer seem to show lower properties compared to the ones of inorganic hydrogels. Many authors affirm the more interesting characteristic of silica hydrogel compared to standard polymer hydrogels, nevertheless, opinions are still divided on the subject.
Conclusions
In this review we provide a historical overview of the developments in hydrogel research from simple networks to smart materials. Hydrogels are widely present in everyday products though their potential has not been fully explored yet. These materials already have a well-established role in contact lenses, hygiene products and wound dressing markets but commercial hydrogel products in tissue engineering and drug delivery are still limited. Many hydrogel-based drug delivery devices and scaffold shave been designed, studied and in some cases even patented, however not many have reached the market. More progress is expected in these two areas. Limited commercial products with hydrogels in drug delivery and tissue engineering are related to some extent to their high production costs.
There are numerous patents on hydrogels but only a few reached the market, hydrogels are used for producing contact lenses, hygiene products and wound dressings. Other commercial uses of hydrogels are in drug delivery and tissue engineering.
More developments are expected in drug delivery and tissue engineering, high production costs of hydrogels are limiting their further commercialization.
Over the past decades, significant progress has been made in the field of hydrogels as functional biomaterials. Biomedical application of hydrogels was initially hindered by the toxicity of crosslinking agents and limitations of hydrogel formation under physiological conditions. Emerging knowledge in polymer chemistry and increased understanding of biological processes resulted in the design of versatile materials and minimally invasive therapies. Hydrogel matrices comprise a wide range of natural and synthetic polymers held together by a variety of physical or chemical crosslinks. With their capacity to embed pharmaceutical agents in their hydrophilic crosslinked network, hydrogels form promising materials for controlled drug release and tissue engineering. Despite all their beneficial properties, there are still several challenges to overcome for clinical translation.
Acknowledgments
The authors gratefully acknowledge MITACS program of Québec and Sarko Holdings for financial support of this work.
7218
References
- Kopecek J (2002) Polymer chemistry: swell gels.Nature 417: 388-389, 391.
- ISSN International Centre: The ISSN register. https://www.issn.org (2006). Accessed 20 Feb 2007.
- Van Noorden R (2014) Global scientific output doubles every nine years. Nature News Blog.
- Campoccia D, Doherty P, Radice M, Brun P, Abatangelo G, et al. (1998) Semisynthetic resorbable materials from hyaluronan esterification. Biomaterials 19: 2101-2127.
- Wei Yang Seow, Charlotte A.E. Hauser(2014)Short to ultrashort peptide hydrogels for biomedical uses. Materials Today 17: 381-388.
- Lee SC, Kwon IK, Park K (2013) Hydrogels for delivery of bioactive agents: a historical perspective.Adv Drug Deliv Rev 65: 17-20.
- Kopecek J (2007) Hydrogel biomaterials: a smart future? Biomaterials 28: 5185-5192.
- Wichterle O, Lìm D (1960) Hydrophilic Gels for Biological Use. Nature 185: 117-118.
- Buwalda SJ, Boere KW, Dijkstra PJ, Feijen J, Vermonden T1, et al. (2014) Hydrogels in a historical perspective: from simple networks to smart materials. J Control Release 190: 254-273.
- Yom-Tov O, Neufeld L, Seliktar D, Bianco-Peled H (2014) A novel design of injectable porous hydrogels with in situ pore formation. ActaBiomater 10: 4236-4246.
- Abebe DG, Fujiwara T (2012) Controlled thermoresponsive hydrogels by stereocomplexed PLA-PEG-PLA prepared via hybrid micelles of pre-mixed copolymers with different PEG lengths. Biomacromolecules 13: 1828-1836.
- Chung HJ, Lee Y, Park TG (2008) Thermo-sensitive and biodegradable hydrogels based on stereocomplexedPluronic multi-block copolymers for controlled protein delivery. J Control Release 127: 22-30.
- Kirakci K, Å Ãcha V, Holub J, Kubà P, Lang K (2014) Luminescent hydrogel particles prepared by self-assembly of β-cyclodextrin polymer and octahedral molybdenum cluster complexes.InorgChem 53: 13012-13018.
- Kono H, Teshirogi T (2015) Cyclodextrin-grafted chitosan hydrogels for controlled drug delivery. Int J BiolMacromol 72: 299-308.
- Park K, Shalaby WSW, Park H (1993). Biodegradable Hydrogels for Drug Delivery. Technomic.
- RS. Harland, RK. Prud’homme (1992) Polyelectrolyte Gels: Properties, Preparation and Application. American Chemical Society.
- Lim F, Sun AM (1980) Microencapsulated islets as bioartificial endocrine pancreas.Science 210: 908-910.
- Yannas IV, Lee E, Orgill DP, Skrabut EM, Murphy GF (1989) Synthesis and characterization of a model extracellular matrix that induces partial regeneration of adult mammalian skin.ProcNatlAcadSci U S A 86: 933-937.
- Sefton MV, May MH, Lahooti S, Babensee JE (2000) Making microencapsulation work: conformal coating, immobilization gels and in vivo performance.J Control Release 65: 173-186.
- O Okay (2010) General Properties of Hydrogels. In: Gerlach G, Arndt KF. Hydrogel Sensors and Actuators, Engineering and Technology. Springer Series on Chemical Sensors and Biosensors. editor: Springer
- Byju AG, Kulkarni A, Gundiah N (2013) Mechanics of Gelatin and Elastin based hydrogels as Tissue Engineered Constructs. International Conference on Fracture
- MondalM, Trivedy K, Nirmal Kumar S (2007)The silk proteins, serin and fibroin in silkworm Bombyxmori. Caspian J EnvSci5: 63-76.
- Menard KP (2008) Dynamic Mechanical Analysis: A Practical Introduction. 2nd ed. CRC Press
- Dong LC, Hoffman AS, Yan Q (1994) Dextran permeation through poly(N-isopropylacrylamide) hydrogels.J BiomaterSciPolym Ed 5: 473-484.
- ASTM International. F2540. Standard Practice Standard Guide for Assessing Microstructure of Polymeric Scaffolds for Use in Tissue Engineered Medical Products. 2004
- ASTM International. F2603 standard practice Standard Guide for Interpreting Images of Polymeric Tissue Scaffolds. 2006
- Gulrez SKH, Al-Assaf S, Phillips GO (2011) Hydrogels: Methods of Preparation, Characterisation and Applications, Progress in Molecular and Environmental Bioengineering. In: Carpi A. Analysis and Modeling to Technology Applications
- Bian L, Hou C, Tous E, Rai R, Mauck RL, et al. (2013) The influence of hyaluronic acid hydrogel crosslinking density and macromolecular diffusivity on human MSC chondrogenesis and hypertrophy.Biomaterials 34: 413-421.
- Sung HW, Huang DM, Chang WH, Huang RN, Hsu JC (1999) Evaluation of gelatin hydrogel crosslinked with various crosslinking agents as bioadhesives: in vitro study.J Biomed Mater Res 46: 520-530.
- Weber LM, Lopez CG, Anseth KS (2009) Effects of PEG hydrogel crosslinking density on protein diffusion and encapsulated islet survival and function.J Biomed Mater Res A 90: 720-729.
- Wellner N, Kacuráková M, Malovi´ková A, Wilson RH, Belton PS (1998) FT-IR study of pectate and pectinate gels formed by divalent cations. Carbohydr Res308: 123–131.
- Tanaka T (1986) In: Encylopedia of Polymer Science and Engineering. 2nd ed. Vol 6: 514-536. Wiley.
- Brandt KA, Goldman SA, Inglin TA. US Patent RE32649 E. Hydrogel-forming polymer compositions for use in absorbent structures. 1988
- Assarsson PG, King PA, Yen SN (1975) US Patent 3901236 A. Disposable absorbent articles containing hydrogel composites having improved fluid absorption efficiencies and processes for preparation
- Gross JR, Mcfadden RT (1975) US Patent 3926891 A Method for making a crosslinkable aqueous solution which is useful to form soft, water-swellablepolyacrylate articles
- Statista, The Statistics Portal. https://www.statista.com. Accessed Feb 2015.
- Umachitra G, Bhaarathidhurai (2012) Disposable baby diaper--a threat to the health and environment.J Environ SciEng 54: 447-452.
- Trinh T, Gardlik JM (1990) European Patent EP 0392608 A2. Solid consumer product compositions containing small particle cyclodextrin complexes
- Subkowski T, Bollschweiler C, Wittenberg J, Siegel W, Pelzer R (2013) International Patent WO 1999004830 A1. Low molecular weight modulators of the cold-menthol receptor trpm8 and use thereof.
- Peppas NA, Peppas LB (1997) Controlled release of fragrances from polymers. J ApplPolym Sci. 66: 509–513.
- NFPA Guidelines & Definitions. Oklahoma State University (2008). Accessed Feb 2015.
- An SM1, Ham H, Choi EJ, Shin MK, et al. (2014) Primary irritation index and safety zone of cosmetics: retrospective analysis of skin patch tests in 7440 Korean women during 12 years.Int J CosmetSci 36: 62-67.
- Lorenz DH (1994) US patent 5306504 A Skin adhesive hydrogel, its preparation and uses
- E. Lee and B. Kim (2011) Smart delivery system for cosmetic ingredients using pH-sensitive polymer hydrogel particles. Korean J ChemEng 28: 1347-1350
- Slaughter BV, Khurshid SS, Fisher OZ, Khademhosseini A, Peppas NA (2009) Hydrogels in regenerative medicine.Adv Mater 21: 3307-3329.
- Rhee SM, You HJ, Han SK (2014) Injectable tissue-engineered soft tissue for tissue augmentation.J Korean Med Sci 29 Suppl 3: S170-175.
- Pienaar WE, McWilliams S, Wilding LJ, Perera IT (2011) The imaging features of MACROLANEâ„¢ in breast augmentation.ClinRadiol 66: 977-983.
- Yamaguchi S, Nagumo Y, Niwa K (2013) Efficacy and safety of Macrolane(â„¢) for breast enhancement: a 12-month follow-up study in Asian women.J PlastSurg Hand Surg 47: 191-195.
- Joint United Nations Programme on HIV/AIDS. UNAIDS report on the global AIDS epidemic;https://www.unaids.org/globalreport,2010
- Wen Y, Collier JH2 (2015) Supramolecular peptide vaccines: tuning adaptive immunity.CurrOpinImmunol 35: 73-79.
- Wijesundara DK, Jackson RJ, Ramshaw IA, Ranasinghe C (2011) Human immunodeficiency virus-1 vaccine design: where do we go now?Immunol Cell Biol 89: 367-374.
- Qiao Y, Huang Y, Qiu C, Yue X, Deng L, et al. (2010) The use of PEGylated poly [2-(N,N-dimethylamino) ethyl methacrylate] as a mucosal DNA delivery vector and the activation of innate immunity and improvement of HIV-1-specific immune responses.Biomaterials 31: 115-123.
- Greenland JR, Letvin NL (2007) Chemical adjuvants for plasmid DNA vaccines.Vaccine 25: 3731-3741.
- Jiang L, Qian F, He X, Wang F, Ren D, et al. (2007) Novel chitosan derivative nanoparticles enhance the immunogenicity of a DNA vaccine encoding hepatitis B virus core antigen in mice.J Gene Med 9: 253-264.
- Liu Y, Sun Y, Yan H, Liu X, Zhang W, et al. (2012) Electrospun fiber template for replica molding of microtopographical neural growth guidance.Small 8: 676-681.
- Mu X, Zheng W, Xiao L, Zhang W, Jiang X (2013) Engineering a 3D vascular network in hydrogel for mimicking a nephron.Lab Chip 13: 1612-1618.
- Aida T, Meijer EW, Stupp SI (2012) Functional supramolecular polymers.Science 335: 813-817.
- Schwartz JJ, Zhang S (2000) Peptide-mediated cellular delivery.CurrOpinMolTher 2: 162-167.
- Williams RJ, Smith AM, Collins R, Hodson N, Das AK, et al. (2009) Enzyme-assisted self-assembly under thermodynamic control.Nat Nanotechnol 4: 19-24.
- Zhang X, Chu X, Wang L, Wang H, Liang G, et al. (2012) Rational design of a tetrameric protein to enhance interactions between self-assembled fibers gives molecular hydrogels.AngewChemInt Ed Engl 51: 4388-4392.
- Reynolds WS, Dmochowski RR (2012) Urethral bulking: a urology perspective.UrolClin North Am 39: 279-287.
- Moreno-Garrido I (2008) Microalgae immobilization: current techniques and uses.BioresourTechnol 99: 3949-3964.
- Joint I, Mühling M, Querellou J (2010) Culturing marine bacteria - an essential prerequisite for biodiscovery.MicrobBiotechnol 3: 564-575.
- IraniM, Ismail H, Ahmad Z, Fan M (2015) Synthesis of linear low-density polyethylene-g-poly (acrylic acid)-co-starch/organo-montmorillonite hydrogel composite as an adsorbent for removal of Pb(??) from aqueous solutions. J. Environ. Sci. 27: 9-20.
- Yan H, Dai J, Yang Z, Yang H, and Cheng R (2011) Enhanced and selective adsorption of copper(II) ions on surface carboxymethylated chitosan hydrogel beads. ChemEng J174: 586–594.
- Ahmad H, Nurunnabi M, Rahman MM, Kumar K, et al. (2014) Magnetically doped multi stimuli-responsive hydrogel microspheres with IPN structure and application in dye removal. Colloids Surf PhysicochemEng Asp. 459: 39-47.
- Wang X, Ye G, Wang X (2014) Hydrogel diffraction gratings functionalized with crown ether for heavy metal ion detection. Sens Actuators B Chem 193: 413–419
- Arcadio PS, Gregoria AS (2003) Physical–Chemical Treatment of Water and Wastewater. IWA Publishing, CRC Press
- Panpanit S, Visvanathan C (2001)The role of bentonite in UF flux enhancement mechanisms for oil/water emulsion. J MembrSci 184: 59-68.
- Hadi M, Viraraghavan T(1999) Removal of oil from water by bentoniteorgano-clay. In: Hazardous & Industrial Wastes - Proceedings of the Mid-Atlantic Industrial & Hazardous Waste 187–196.
- Qiu Z, Zhang Y, Fang Y (1995) Removal of oil from concentrated wastewater by attapulgite and coagulant. Water Qual Res J 30: 89-99.
- Sokker HH, El-Sawy NM, Hassan MA, El-Anadouli BE (2011) Adsorption of crude oil from aqueous solution by hydrogel of chitosan based polyacrylamide prepared by radiation induced graft polymerization.J Hazard Mater 190: 359-365.
- Heginbothom M, Fitzgerald TC, Wade WG (1990) Comparison of solid media for cultivation of anaerobes.J ClinPathol 43: 253-256.
- Cover TL (2012) Perspectives on methodology for in vitro culture of Helicobacter pylori.Methods MolBiol 921: 11-15.
- Murray PR (1978) Growth of clinical isolates of anaerobic bacteria on agar media: effects of media composition, storage conditions, and reduction under anaerobic conditions. J ClinMicrobiol8: 708–714.
- Wasinger VC, Cordwell SJ, Cerpa-Poljak A, Yan JX, Gooley AA, et al, (1995) Progress with gene-product mapping of the Mollicutes: Mycoplasma genitalium. Electrophoresis 16: 1090-1094.
- Rabilloud T, Vaezzadeh AR, Potier N, Lelong C, Leize-Wagner E, et al. (2009) Power and limitations of electrophoretic separations in proteomics strategies.Mass Spectrom Rev 28: 816-843.
- Rabilloud T, Chevallet M, Luche S, Lelong C (2010) Two-dimensional gel electrophoresis in proteomics: Past, present and future.J Proteomics 73: 2064-2077.
- Rogowska-Wrzesinska A, Le Bihan MC, Thaysen-Andersen M, Roepstorff P (2013) 2D gels still have a niche in proteomics.J Proteomics 88: 4-13.
- Chirani NA, Dembahri Z, Tokarski C, Rolando C, Benmouna M (2011) Newlydesigned polyacrylamide/dextran gels for electrophoresis protein separation: synthesis and characterization. Polymer International60: 1024-1029.
- Suh KS, Mutoh M, Gerdes M, Yuspa SH (2005) CLIC4, an intracellular chloride channel protein, is a novel molecular target for cancer therapy.J InvestigDermatolSympProc 10: 105-109.
- Greaser ML, Warren CM (2012) Protein electrophoresis in agarose gels for separating high molecular weight proteins.Methods MolBiol 869: 111-118.
- Greppi GF, Roncada P (2005)La componenteproteicadel latte caprino. In: Pulina G. L’alimentazionedellacapra da latte. Avenue Media Publisher 71-99
- Wang J, Ugaz VM (2006) Using in situ rheology to characterize the microstructure in photopolymerized polyacrylamide gels for DNA electrophoresis.Electrophoresis 27: 3349-3358.
- Righetti PG, Gelfi C (1997) Electrophoresis gel media: the state of the art.J Chromatogr B Biomed SciAppl 699: 63-75.
- Anseth KS, Bowman CN, Brannon-Peppas L (1996) Mechanical properties of hydrogels and their experimental determination.Biomaterials 17: 1647-1657.
- Myung D, Waters D, Wiseman M, Duhamel PE, Noolandi J, et al. (2008) Progress in the development of interpenetrating polymer network hydrogels.PolymAdvTechnol 19: 647-657.
- Kim SJ, Yoon SG, Kim SI (2004) Synthesis and characterization of interpenetration polymer network hydrogels composed of alginate and poly(diallyldimethylammonium chloride). J ApplPolymSci91: 3705-3709.
- Wang JJ, Liu F (2013) Enhanced adsorption of heavy metal ions onto simultaneous interpenetrating polymer network hydrogels synthesized by UV irradiation. Polym Bull 70: 1415-1430.
- Hoare TR, Kohane DS (2008) Hydrogels in drug delivery: progress and challenges. Polymer 49: 1993-2007.
- Dragan ES, Lazar MM, Dinu MV, Doroftei F. Macroporous (2012) composite IPN hydrogels based on poly(acrylamide) and chitosan with tuned swelling and sorption of cationic dyes. ChemEng J204-206: 198-209
- Lee KY1, Mooney DJ (2001) Hydrogels for tissue engineering.Chem Rev 101: 1869-1879.
- Hutmacher DW1 (2001) Scaffold design and fabrication technologies for engineering tissues--state of the art and future perspectives.J BiomaterSciPolym Ed 12: 107-124.
- Bryant DM1, Mostov KE (2008) From cells to organs: building polarized tissue.Nat Rev Mol Cell Biol 9: 887-901.
- Zhou M, Smith AM, Das AK, Hodson NW, Collins RF, et al. (2009) Self-assembled peptide-based hydrogels as scaffolds for anchorage-dependent cells.Biomaterials 30: 2523-2530.
- Liu H J, Hu YH, H. M. Wang, J. Y. Wang, D. L. Kong, et al.( 2011)Soft Matter7: 5430–5436
- Yang C, Li D, Liu Z, Hong G, Zhang J, et al. (2012) Responsive small molecular hydrogels based on adamantane-peptides for cell culture.J PhysChem B 116: 633-638.
- Wang H, Yang Z (2012) Short-peptide-based molecular hydrogels: novel gelation strategies and applications for tissue engineering and drug delivery.Nanoscale 4: 5259-5267.
- Kaivosoja E, Barreto G, Levón K, Virtanen S, Ainola M, et al. (2012) Chemical and physical properties of regenerative medicine materials controlling stem cell fate.Ann Med 44: 635-650.
- Khademhosseini A, Langer R (2007) Microengineered hydrogels for tissue engineering.Biomaterials 28: 5087-5092.
- Mironov V, Boland T, Trusk T, Forgacs G, Markwald RR (2003) Organ printing: computer-aided jet-based 3D tissue engineering.Trends Biotechnol 21: 157-161.
- Tanzi MC, Bianchi A, Farè S, Mantero S, Raimondi MT, et al. (2013) GruppoNazionale di Bioingegneria n°32: ApproccioIntegrato per la MedicinaRigenerativa. PàtronEditore.
- Weiss P (1934) In Vitro Experiments on the Factors Determining the Course of the Outgrowing Nerve Fiber. Journal of Experimental Zoology 69: 393–448.
- Clark P, Connolly P, Curtis AS, Dow JA, Wilkinson CD (1991) Cell guidance by ultrafine topography in vitro.J Cell Sci99 : 73-77.
- Saito AC, Matsui TS, Ohishi T, Sato M, Deguchi S (2014) Contact guidance of smooth muscle cells is associated with tension-mediated adhesion maturation.Exp Cell Res 327: 1-11.
- Elisseeff J, Anseth K, Sims D, McIntosh W, Randolph M, et al. (1999) Transdermal photopolymerization for minimally invasive implantation.ProcNatlAcadSci U S A 96: 3104-3107.
- Elisseeff J, McIntosh W, Anseth K, Riley S, Ragan P, et al. (2000) Photoencapsulation of chondrocytes in poly(ethylene oxide)-based semi-interpenetrating networks.J Biomed Mater Res 51: 164-171.
- Massia SP, Stark J, Letbetter DS (2000) Surface-immobilized dextran limits cell adhesion and spreading.Biomaterials 21: 2253-2261.
- Holland NB, Qiu Y, Ruegsegger M, Marchant RE (1998) Biomimetic engineering of non-adhesive glycocalyx-like surfaces using oligosaccharide surfactant polymers.Nature 392: 799-801.
- Liu Y, Chan-Park MB (2010) A biomimetic hydrogel based on methacrylated dextran-graft-lysine and gelatin for 3D smooth muscle cell culture.Biomaterials 31: 1158-1170.
- Ward AG, Courts A (1997) The Science and Technology of Gelatin. Academic Press 66: 373-374
- Type A & B Process Definition. Vyse Gelatin Company. https://www.vyse.com/ (2009). Accessed Feb 2015.
- Das S, Pati F, Choi YJ, Rijal G, Shim JH, et al. (2015) Bioprintable, cell-laden silk fibroin-gelatin hydrogel supporting multilineage differentiation of stem cells for fabrication of three-dimensional tissue constructs.ActaBiomater 11: 233-246.
- Ignatova M1, Manolova N, Rashkov I (2013) Electrospun antibacterial chitosan-based fibers.MacromolBiosci 13: 860-872.
- Jiang T, Deng M, James R, Nair LS, Laurencin CT (2014) Micro- and nanofabrication of chitosan structures for regenerative engineering.ActaBiomater 10: 1632-1645.
- Chen T, Embree HD, Brown EM, Taylor MM, Payne GF (2003) Enzyme-catalyzed gel formation of gelatin and chitosan: potential for in situ applications.Biomaterials 24: 2831-2841.
- Tezel A1, Fredrickson GH (2008) The science of hyaluronic acid dermal fillers.J Cosmet Laser Ther 10: 35-42.
- Lam J, Truong NF, Segura T (2014) Design of cell-matrix interactions in hyaluronic acid hydrogel scaffolds.ActaBiomater 10: 1571-1580.
- West DC, Kumar S (1989) Hyaluronan and angiogenesis.Ciba Found Symp 143: 187-201.
- Tempel C, Gilead A, Neeman M (2000) Hyaluronic acid as an anti-angiogenic shield in the preovulatory rat follicle.BiolReprod 63: 134-140.
- Slevin M, West D, Kumar P, Rooney P, Kumar S (2004) Hyaluronan, angiogenesis and malignant disease.Int J Cancer 109: 793-794.
- Kisiel M, Martino MM, Ventura M, Hubbell JA, Hilborn J, et al. (2013) Improving the osteogenic potential of BMP-2 with hyaluronic acid hydrogel modified with integrin-specific fibronectin fragment.Biomaterials 34: 704-712.
- Shu XZ, Liu Y, Palumbo F, Prestwich GD (2003) Disulfide-crosslinkedhyaluronan-gelatin hydrogel films: a covalent mimic of the extracellular matrix for in vitro cell growth.Biomaterials 24: 3825-3834.
- Munarin F, Tanzi MC, Petrini P (2012) Advances in biomedical applications of pectin gels.Int J BiolMacromol 51: 681-689.
- McKenna BA, Nicholson TM, Wehr JB, Menzies NW (2010) Effects of Ca, Cu, Al and La on pectin gel strength: implications for plant cell walls.Carbohydr Res 345: 1174-1179.
- Munarin F, Guerreiro SG, Grellier MA, Tanzi MC, Barbosa MA, et al. (2011) Pectin-based injectable biomaterials for bone tissue engineering.Biomacromolecules 12: 568-577.
- Jang J, Seol YJ, Kim HJ, Kundu J, Kim SW, et al. (2014) Effects of alginate hydrogel cross-linking density on mechanical and biological behaviors for tissue engineering.J MechBehav Biomed Mater 37: 69-77.
- Guillaume O, Daly A, Lennon K, Gansau J, Buckley SF, et al. (2014) Shape-memory porous alginate scaffolds for regeneration of the annulus fibrosus: effect of TGF-β3 supplementation and oxygen culture conditions.ActaBiomater 10: 1985-1995.
- Shteyer E, Ben Ya'acov A, Zolotaryova L, Sinai A, Lichtenstein Y et al. (2014) Reduced liver cell death using an alginate scaffold bandage: A novel approach for liver reconstruction after extended partial hepatectomy. ActaBiomater. 10: 3209-3216.
- Ziv K, Nuhn H, Ben-HaimY, Sasportas LS, Kempen PJ, et al. (2014) A tunable silk-alginate hydrogel scaffold for stem cell culture and transplantation.Biomaterials 35: 3736-3743.
- Burdick JA, Murphy WL (2012) Moving from static to dynamic complexity in hydrogel design.Nat Commun 3: 1269.
- Douezan S, Dumond J, Brochard-Wyart F (2012) Wetting trasitions of cellular aggregates induced by substrate rigidity. Soft Matter 8: 4578-4583.
- Jiang LY, Luo Y (2013) Guided assembly of endothelial cells on hydrogel matrices patterned with microgrooves: a basic model for micro vessel engineering. Soft Matter. 9: 1113-1121.
- Tse JR, Engler AJ (2010) Preparation of hydrogel substrates with tunable mechanical properties.CurrProtoc Cell Biol Chapter 10: Unit 10.
- Zhu J (2010) Bioactive modification of poly(ethylene glycol) hydrogels for tissue engineering.Biomaterials 31: 4639-4656.
- Carmeliet P (2000) Mechanisms of angiogenesis and arteriogenesis.Nat Med 6: 389-395.
- Silva AK, Richard C, Bessodes M, Scherman D, Merten OW (2009) Growth factor delivery approaches in hydrogels.Biomacromolecules 10: 9-18.
- Hubbell JA (2003) Materials as morphogenetic guides in tissue engineering.CurrOpinBiotechnol 14: 551-558.
- Schmoekel HG, Weber FE, Schense JC, Grätz KW, Schawalder P, et al. (2005) Bone repair with a form of BMP-2 engineered for incorporation into fibrin cell ingrowth matrices.BiotechnolBioeng 89: 253-262.
- Martino MM, Tortelli F, Mochizuki M, Traub S, Ben-David D, et al. (2011) Engineering the growth factor microenvironment with fibronectin do mains to promote wound and bone tissue healing.SciTransl Med 3: 100ra89.
- Kim S, Bedigrew K, Guda T, Maloney WJ, Park S, et al. (2014) Novel osteoinductive photo-cross-linkable chitosan-lactide-fibrinogen hydrogels enhance bone regeneration in critical size segmental bone defects.ActaBiomater 10: 5021-5033.
- Bae MS, Ohe JY, Lee JB, Heo DN, Byun W, et al. (2014) Photo-cured hyaluronic acid-based hydrogels containing growth and differentiation factor 5 (GDF-5) for bone tissue regeneration.Bone 59: 189-198.
- Ueno Y, Kitamura C, Terashita M, Nishihara T (2006) Re-oxygenation improves hypoxia-induced pulp cell arrest.J Dent Res 85: 824-828.
- Kawagishi E, Nakakura-Ohshima K, Nomura S, Ohshima H (2006) Pulpal responses to cavity preparation in aged rat molars.Cell Tissue Res 326: 111-122.
- Nakashima M, Iohara K, Zheng L (2006) Gene therapy for dentin regeneration with bone morphogenetic proteins.Curr Gene Ther 6: 551-560.
- Madan AK, Kramer B (2005) Immunolocalization of fibroblast growth factor-2 (FGF-2) in the developing root and supporting structures of the murine tooth.J MolHistol 36: 171-178.
- Annabi B, Naud E, Lee YT, Eliopoulos N, Galipeau J (2004) Vascular progenitors derived from murine bone marrow stromal cells are regulated by fibroblast growth factor and are avidly recruited by vascularizing tumors.J Cell Biochem 91: 1146-1158.
- Yamamoto M, Ikada Y, Tabata Y (2001) Controlled release of growth factors based on biodegradation of gelatin hydrogel.J BiomaterSciPolym Ed 12: 77-88.
- Nakayama J, Fujioka H, Nagura I, Kokubu T, Makino T, et al. (2009) The effect of fibroblast growth factor-2 on autologous osteochondral transplantation.IntOrthop 33: 275-280.
- Nakahara T, Nakamura T, Kobayashi E, Inoue M, Shigeno K(2003) Novel approach to regeneration of periodontal tissues based on in-situ tissue engineering: effects of controlled release of basic fibroblast growth factor from a sandwich membrane. Tissue Eng. 9: 153-162.
- Yasuda Y, Koyama H, Tabata Y, Fujihara Y, Oba M, et al. (2008) Controlled delivery of bFGF remodeled vascular network in muscle flap and increased perfusion capacity via minor pedicle.J Surg Res 147: 132-137.
- Laçin NT (2014) Development of biodegradable antibacterial cellulose based hydrogel membranes for wound healing.Int J BiolMacromol 67: 22-27.
- Lee YH, Chang JJ, Yanga MC, Chienc CT, Lai WF (2012) Acceleration of wound healing in diabetic rats by layered hydrogel dressing. Carbohydrate Polymers. 88: 809-819.
- Tao Wang, Xiao-Kang Zhu, Xu-Ting Xue, Da-Yang Wu (2012) Hydrogel sheets of chitosan, honey and gelatin as burn wound dressing. Carbohydrate Polymers. 88: 75–83.
- Kanokpanont S, Damrongsakkul S, Ratanavaraporn J, Aramwit P (2012) An innovative bi-layered wound dressing made of silk and gelatin for accelerated wound healing.Int J Pharm 436: 141-153.
- Gurny R, Kaufmann B, Moeller M(2012) International Patent WO 2012013670 A1. Process for the esterification of hyaluronic acid with hydrophobic organic compounds
- Bertrand N, Leroux JC (2012) The journey of a drug-carrier in the body: an anatomo-physiological perspective.J Control Release 161: 152-163.
- Ashley GW, Henise J, Reid R, Santi DV (2013) Hydrogel drug delivery system with predictable and tunable drug release and degradation rates.ProcNatlAcadSci U S A 110: 2318-2323.
- Marin MA, Mallepally RR, McHugh MA(2014) Silk fibroin aerogels for drug delivery applications. J Supercrit Fluids 91: 84-89.
- Patra C, Talukdar S, Novoyatleva T, Velagala SR, Mühlfeld C, et al. (2012) Silk protein fibroin from Antheraeamylitta for cardiac tissue engineering.Biomaterials 33: 2673-2680.
- Park CH, Jeong L, Cho D, Kwon OH, Park WH (2013) Effect of methylcellulose on the formation and drug release behavior of silk fibroin hydrogel.CarbohydrPolym 98: 1179-1185.
- Mandal BB, Kundu SC (2008) A novel method for dissolution and stabilization of non-mulberry silk gland protein fibroin using anionic surfactant sodium dodecyl sulfate.BiotechnolBioeng 99: 1482-1489.
- Yesilirmak N, AltÄnors DD (2013) A silicone hydrogel contact lens after 7 years of continuous wear.Cont Lens Anterior Eye 36: 204-206.
- Phan CM, Subbaraman L, Jones L (2014) Contact lenses for antifungal ocular drug delivery: a review.Expert Opin Drug Deliv 11: 537-546.
- Wilson CG (2004) Topical drug delivery in the eye.Exp Eye Res 78: 737-743.
- Cohen RA, Gebhardt BM, Insler MS(1996) Comparison of contact lens delivery and topical instillation of 4% lidocaine in the normal cornea and limbal conjunctiva. Invest Ophthal Vis Sci. 37: 332.
- Eugen B, Liliana V, Thomas GN, John T (2006) Polymeric materials for ophthalmic drug delivery: trends and perspectives. J Mater Chem16: 3439-3443.
- Lin HR, Sung KC (2000) Carbopol/pluronic phase change solutions for ophthalmic drug delivery.J Control Release 69: 379-388.
- Jun Chen, Xiaojian Li, LiqianGao, Yi Hu, Wen Zhong, et al. (2014)Nano LIFE.4: 1441004
- Lowe CP1, Dreischor MW (2005) The size of a polymer in a symmetric solvent.J ChemPhys 122: 84905.
- Stevens MM1, George JH (2005) Exploring and engineering the cell surface interface. Science 310: 1135-1138.
- A. Sionkowska (2011) Current research on the blends of natural and synthetic polymers as new biomaterials: review. ProgPolymSci 36: 1254-1276
- A. Sivashanmugam (2015)An overview of injectable polymeric hydrogels for tissue engineering, Eur.Polym. J.
- Hehl EM1, Beck R, Luthard K, Guthoff R, Drewelow B (1999) Improved penetration of aminoglycosides and fluorozuinolones into the aqueous humour of patients by means of Acuvue contact lenses. Eur J ClinPharmacol 55: 317-323.
- Dmochowski RR, Appell RA (2000) Injectable agents in the treatment of stress urinary incontinence in women: where are we now? Urology 56: 32-40.
- Lightner DJ1 (2002) Review of the available urethral bulking agents. CurrOpinUrol 12: 333-338.
- Bent AE, Tutrone RT, McLennan MT, Lloyd LK, Kennelly MJ, et al. (2001) Treatment of intrinsic sphincter deficiency using autologous ear chondrocytes as a bulking agent. NeurourolUrodyn 20: 157-165.
- Yang X, Sun Y, Kootala S, Hilborn J, Heerschap A, et al. (2014) Injectable hyaluronic acid hydrogel for 19F magnetic resonance imaging.CarbohydrPolym 110: 95-99.
- Choudhury NA, Sampath S, Shukla AK (2008) Hydrogel-polymer electrolytes for electrochemical capacitors: an overview. Energy Environ Sci 2: 55-67.