Key words
Bloodstream infection, cancer, hospital-acquired
Introduction
Infections are inevitable complications which are result of neutropenia and impaired immune response after chemotherapeutic use in cancer patients. Most of these infections are hospital-acquired in nature, because patients with cancer have prolonged and repeated contact with hospital environment and are exposed to numerous sources of infection which may involve invasive procedures. Additionally, they generally suffer from immunosuppressive chemotherapy and radiation. Aggressive antineoplastic chemotherapy is a risk factor for infections in immunocompromised patients with cancer [1-4].
Bloodstream infections in cancer patients account for approximately more than 20% of hospital-acquired infections [5-7]. Hospital-acquired bloodstream infections in cancer patients can be caused by a variety of microorganisms, but the most common pathogens are bacteria followed by fungi. The prognosis of nosocomial bloodstream infections is influenced by many factors, including the microorganism causing the sepsis, the source of infection, the absolute neutrophil count, the bone marrow status, and the presence or absence of shock.[4-6]. Bloodstream infections are often associated with high mortality rate.
Currently, gram-positive bacteria are isolated more often than gram negative bacteria in bloodstream infections in cancer patients [8]. However: in developing countries this shift is not true because of the limited sources for infection control measures [8-11].
Additionally, by prompt and appropriate empirical antibiotic therapy, mortality was shown to be decreased and over the decades it became mandatory. One of the major principles of the management of infections in patients with cancer is to recognize the variability from one time period to another. Regular local data relating causative microorganisms leading to hospitalacquired bloodstream infections are very important to control infections in these countries, such as Turkey. For this reason, local surveillance data should be maintained regularly for early and appropriate empirical therapy.
The objective of this article is to report a 3 years study of hospital- acquired bloodstream infection surveillance results in an oncology unit that is based on National Nosocomial Infections Surveillance [NNIS] system methods.
These data might serve for comparative studies with other oncology ICUs and also help to develop a more efficient surveillance system, allowing for detailed infection control measures for these high-risk patients.
Material and Methods
The study was conducted at haematology-oncology department of Cukurova University Hospital. Both hematological and solid organ malignancies were followed up by this department. Before 2006 our haematology and oncology patients were hospitalized in the same ward and after 2006 these two units were separated. Antibiotic policies have been derived by infectious diseases, haematology and oncology professionals since 2000 according to Infectious Diseases Society of America [IDSA] guidelines for febrile neutropenia [12-13].
Surveillance data of the infection control committee reviewed blood culture positive infections in these units for the years 2005-2007. An episode of bacteremia was defined as a clinically significant isolate[s] in blood cultures with compatible clinical findings of infection. An episode was considered as monomicrobial only when one organism was isolated; the recurrent isolation of the same organism from blood over a four-week period was considered as a part of the same episode. When more than one organism was isolated from a patient’s blood cultures within 48 hours, the episode was called as polymicrobial. The diagnosis of bloodstream infection was based on the Center for Disease Control [CDC] criteria and performed by infection control doctor and nurses.
Bactec [BD Becton Dickinson] and VITEC 2 [BioMerioux] systems were used for culture, identification and susceptibility testing. Standard accredited methods were used for organism isolation and identification, and sensitivity testing was performed using a comparative plate disc diffusion method [8].
Resistance to an antibiotic was defined as an intermediate or resistant according to the disc sensitivity test result. Glycopeptide resistance amongst the enterococci was confirmed using Etest methodology.
Statistical analysis was performed using the statistical package SPSS v 15.0. Comparisons were done using the Chi-square test or trend analyses. Descriptive statistics were used and data were expressed as frequency [n], percent [%], median and range.
Results
In the haematology-oncology unit, a total of 2486 patients were hospitalized in a three year period [411, 1086 and 989 in years 2005, 2006 and 2007, respectively]. There were 170 episodes of documented bloodstream infections [bacteremia/fungemia] in total of 131 patients over the three year period. The rate of bloodstream infections [episodes / total hospitalized patients in oncology and haematology unit] was 6,8%. Male:female ratio was 1,3:1. The median age in haematology and oncology unit who had bloodstream infection was 42 years [range 17-73 years] in 2005, 39 years [range 18-75 years] in 2006 and 47 years [range 20-84 years] in haematology unit and 52 years [range 26- 73 years] in oncology unit in 2007.
Of the 170 episodes, 79,1% [n=31], 80,9% [n=51] and 70,5% [n=48] were monomicrobial in years 2005, 2006 and 2007, respectively.
A total of 194 microorganisms were isolated, 39 episodes of documented bloodstream infection in 30 patients in 2005, 63 episodes in 50 patients in 2006 and 68 episodes in 51 patients in 2007.
Of the 194 episodes, while the ratio of the gram-negative bacteria were slightly increasing in three years; 68,9%, 70,4% and 77%, gram-positive bacteria were decreasing 31,1%, 23,9% and 19,2%, and Candida species were causative agents in 4 episodes [5,6%] in 2006 and 3 episodes in 2007[3,8%] [p>0.05] [Table 1, figure 1].
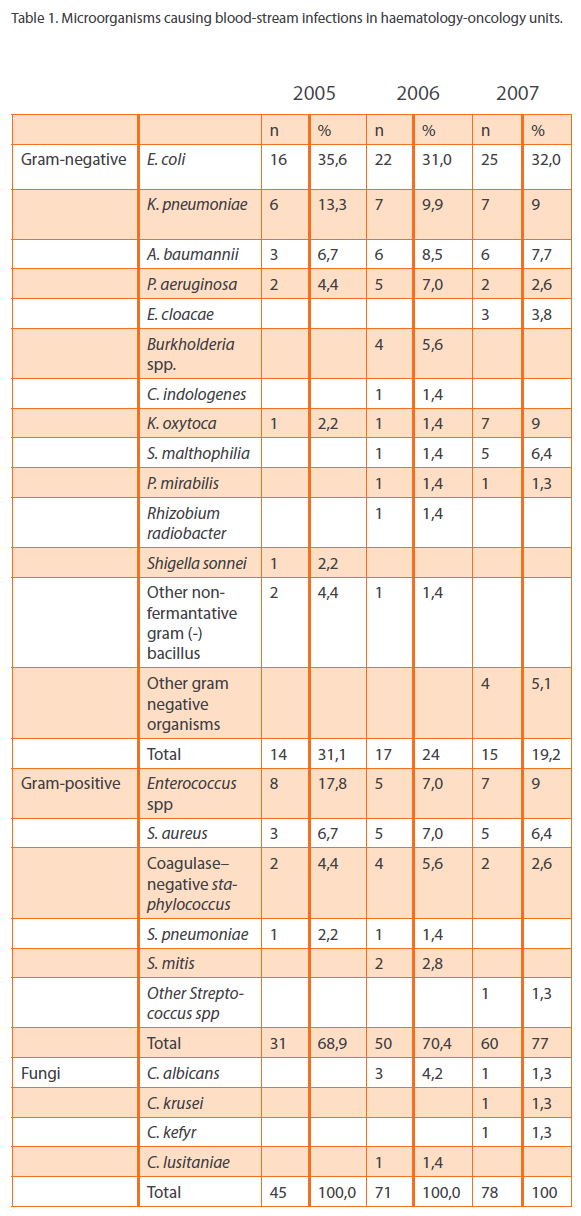
Table 1: Microorganisms causing blood-stream infections in haematology-oncology units.
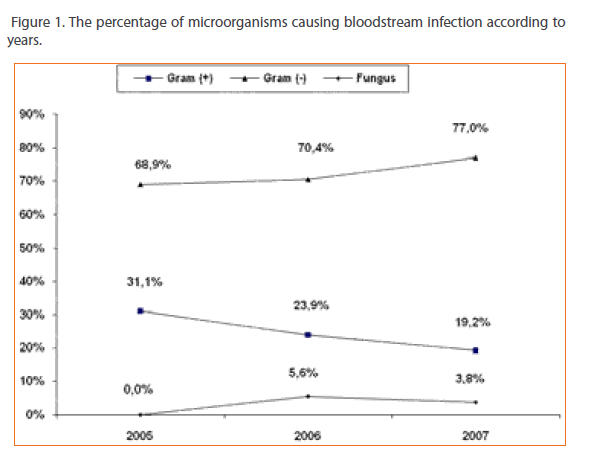
Figure 1: The percentage of microorganisms causing bloodstream infection according to years.
The majority of the cases were primary bloodstream infections. The most prevalent secondary cause of bacteremia was urinary tract infections in year 2005 [15,6%] and in year 2007 [11,6%] and pneumonia [8,5%] in 2006 [Table 2].
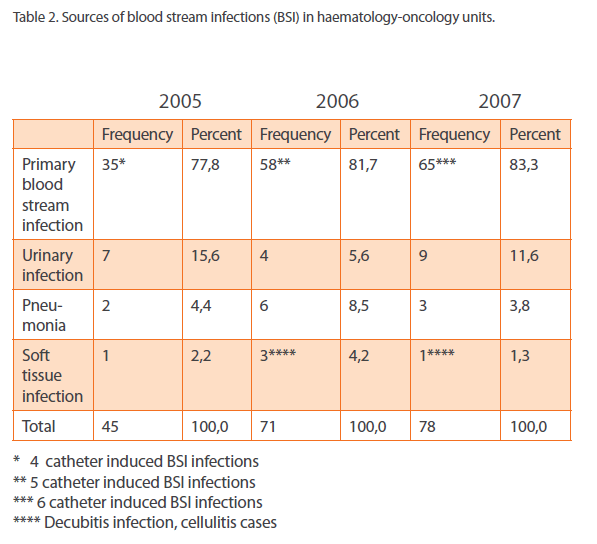
Table 2: Sources of blood stream infections (BSI) in haematology-oncology units.
years.
In our haematology and oncology unit, source of bloodstream infection was assessed and no significant difference was shown in between. But in 2005 two patients were monitorized in the same service and the source of infection was not identified. However, bloodstream infection rate [bloodstream infection episode/total number of hospitalized patients] increased from 7,3% to 13,1% in haematology unit, decreased from 5,9% to 3,8% in oncology unit between 2006 and 2007. This data is shown in Table 3.
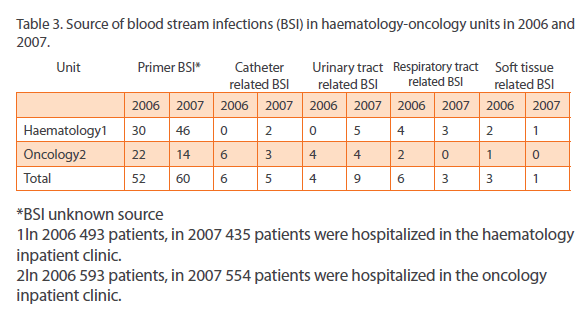
Table 3: Source of blood stream infections (BSI) in haematology-oncology units in 2006 and
High rates of resistance in both gram negative and positive pathogens were prominent. Extended spectrum beta lactamases [ESBL] percentages among E.coli and Klebsiella isolates were 69,6%, 40% and 79,2% in years 2005, 2006 and 2007 respectively. Vancomycin resistance was also high; 7/8, 3/5 and 5/7 among Enterococcus species in three years. The methicillin resistant Staphylococcus aureus [MRSA] rates were 2/3 in the year 2005, 2/5 in 2006 and 1/5 in 2007.
The most effective agents for gram negative bacteria were aminoglycosides and carbapenems. The resistance rates among the predominant pathogens Enterobactericaea are shown in Table 4.
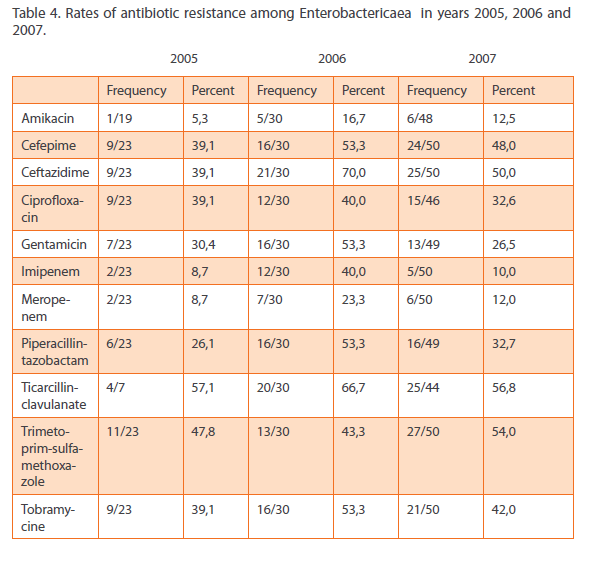
Table 4: SourceRates of antibiotic resistance among Enterobactericaea in years 2005, 2006 and 2007.
Discussion
Malignant hematologic diseases require intensive treatment regimens. Neutropenia following chemotherapy in this patient population is common, often profound and prolonged, with an increased risk of infection [14]. Neutropenia is recognized as an independent risk factor for central line related blood stream infection [15].
Our study revealed that Gram negative bacteria were predominant. This has been an observation among similar studies done in patients in the developing countries [9-11]. The reasons for this could be the relatively lower use of indwelling catheters and other portal devices. Another reason can be the lack of the application of prophylactic antibiotic regimens in neutropenic patients. Our institute does not use empirical antibiotics for prevention of bacterial infections among cancer patients.
Despite the 4-fluoroquinolones are not part of a prophylactic regimen on either unit, fluoroquinolone resistance was a significant problem among strains of Enterobacteriacea isolated from blood. Resistance was, however, seen in nearly 37% isolates of E.coli, K. pneumoniae and other coliforms and in 22% of P. aeruginosa isolates of. MRSA bacteremia is not endemic in either unit and therefore active MRSA infection control measures are worthwhile. It is interesting to note that documented fungemia is low in last three years. While it is recognized that blood cultures have a low sensitivity for detection of fungemia, in studies using rRNA PCR and sequencing methodology showed no evidence of fungemia [16].
Over the last decades there has been a shift from gram negative causative agents to gram positive pathogens especially coagulase negative staphylococci [CoNS], S. aureus and viridans streptococci. Therefore, in some European countries gram negative bacilli reemerged as etiological agents in cancer patients where as gram positive predominance continued in USA. These data, including changing epidemiology and resistance patterns of microorganisms, presents the necessity of continuous surveillance of blood stream infections in haematology-oncology units [17-19].
The occurrence of methicillin-resistant S. aureus [MRSA] was high [5/13] in our study. Because of high rates of methicillinresistance, utilization of empirical vancomycin at our institute must be throughly scrutinized. The indiscriminate use of vancomycin has promoted resistance and this is evident by the high occurrence of vancomycin-resistant Enterococcus isolates [15/20 of isolates].
The high occurrence of non-lactose fermenters [10-15%] especially Pseudomonas aeruginosa and Acinetobacter baumannii was of concern. Both of these bacteria are associated with a high degree of resistance to antibiotics. Blood stream infections with P. aeruginosa have been associated with increased mortality in some studies. Acinetobacter spp. have emerged as prominent multidrug-resistant bacteria in several intensive care units all over the world, and their occurrence in the setting of malignancy could be disastrous [20-21].
Intravascular devices are considered the main sources of primary BSI [22]. Our data showed that only 10.1% of BSI’s were considered CVC-related. CVC-related infection is one of the major complications in haematology and oncology patients, and usually requires removal of the infected CVC to cure the infection [23]. Intravascular catheters are often used for chemotherapy and fluid replacement, which frequently result in catheter-induced infections. A determination of the disinfection method to be employed is often possible when the potential risks of infection associated with the use of certain patient care materials in the hospital environment are considered [24].
During the last two decades, the incidence of CVC-related Gramnegative bacteremia and septicemia caused by Stenotrophomonas maltophilia have increased [25-26]. S. maltophilia, as well as other non-fermentative Gram-negative bacilli, may contaminate the infusate and enter the catheter. In our patient population in 2007 S. maltophilia was the fifth most prevalent Gram-negative pathogen responsible from bloodstream infection.
Considerably higher rates of resistance in both gram negative and positive pathogens were striking in our study. This may be because of the antibiotic policies, severe underlying diseases, chemotherapeutics used, prolonged hospital stay and ineffective infection control measures. Strict infection control policies for this very susceptible patient group seem to be essential as with the other parts of the hospital.
Conclusion
As a conclusion, gram negative bacteria especially the Enterobacteriaecea are the major cause of bacteremia in the haematology and oncology patients in our institute. Regarding the high rates of resistance among gram negative and positive microorganisms, combination therapy of aminoglycosides with cephalosporins and piperacillin-tazobactam, and monotherapy with carbapenems seems to be appropriate as recommended by IDSA febrile neutropenia guidelines. Linezolid therapy should also be kept in mind for resistant gram positive infections. This study also demonstrates the importance of surveillance of infection in haematology and oncology patients to detect trends in infection and the emergence of multi-resistant organisms, e.g. vancomycin resistant enterococci [VRE].
In addition, the present study describes the predominance of primary BSI, most of them from unknown sources; the importance of the urinary and respiratory tract as the main source for secondary BSI. The emerging trends in antibiotic resistance and their implications for empirical therapy indicate that institutions caring for cancer patients should have active ongoing microbiological surveillance studies with the objective of monitoring infections due to antibiotic-resistant pathogens, in order to improve their current antimicrobial regimens.
283
References
- Viscoli C. The evolution of the empirical management of fever and neutropenia in cancer patients. J AntimicrobChemother 1998; 41[Suppl. D]: 65-80.
- Oppenheim BA. The changing pattern of infection in neutropenic patients. J AntimicrobChemother 1998; 41 [Suppl. D]: 7-11.
- Klastersky J. Science and pragmatism in the treatment and prevention of neutropenicinfection. J AntimicrobChemother 1998; 41[Suppl. D]: 13-24.
- EORTC International Antimicrobial Therapy Project Group. Three antibiotic regimes in the treatment of infection in febrile granulocytopenic patients with cancer.J Infect Dis 1978; 137: 14-29.
- EORTC International Antimicrobial Therapy Cooperative Group. Efficacy and toxicity of single daily doses of amikacin and ceftriaxone versus multiple daily doses of amikacin and ceftazidime for infection in patients with cancer and granulocytopenia.Ann Intern Med 1993; 119: 584-593.
- Cometta A, Zinner S, de Bock R, Calandra T, Gaya H, Klastersky J, et al. Piperacillintazobactam plus amikacin versus ceftazidime plus amikacin as empiric therapy for fever in granulocytopenic patients with cancer. Antimicrob Agents Chemother1995; 39: 445-452.
- Cometta A, Calandra T, Gaya H, SHZinner, R de Bock, A Del Favero et al. Monotherapywith meropenem versus combination therapy with ceftazidime plus amikacinas empiric therapy for fever in granulocytopenic patients with cancer. AntimicrobAgents Chemother 1996; 43: 1108-1115.
- Working Party Report of the British Society for Antimicrobial Chemotherapy. A guide to sensitivity testing.J AntimicrobChemother. 1991; 27[Suppl. D]: 1-50.
- Chen CY, Tang JL, Hsueh PR, Yao M, Huang SY, Chen YC, et al. Trends and antimicrobial resistance of pathogens causing bloodstream infections among febrile neutropenic adults with hematological malignancy. J Formos Med Assoc2004;103:526-32.
- Velasco E, Byington R, Martins CS, Schirmer M, Dias LC, Gon_alvesVM. Bloodstream infection surveillance in a cancer centre: A prospective look at clinical microbiology aspects. ClinMicrobiol Infect 2004;10:542-9.
- Paul M, Gafter-Gvili A, Leibovici L, Bishara J, Levy I, Yaniv I, et al. The epidemiology of bacteremia with febrile neutropenia: Experience from a single center, 1988- 2004. Isr Med Assoc J 2007;9:424-9.
- Hughes WT, Armstrong D, Bodey GP, et al. 1997 guidelines for the use of antimicrobial agents in neutropenic patients with unexplained fever.Infectious Diseases Society of America.Clin Infect Dis 1997;25:551–73.
- Hughes WT, Armstrong D, Bodey GP, Bow EJ, Brown AE, Calandra T, et al. 2002 guidelines for the use of antimicrobial agents in neutropenic patients with cancer. Infectious Diseases Society of America.Clin Infect Dis 2002;34:730-751.
- Pizzo PA. Fever in immunocompromised patients.N Engl J Med 1999; 341: 893- 900.
- Wurzel CL, Halom K, Feldman JG, Rubin LG. Infection rates of Broviac-Hickman catheters and implantable venous devices. Am J Dis Child 1988; 142:536-540.
- Jugo J, Kennedyy R, Crowe MJ, G. Lamrocky, McClurgRB, Rooney PJ, et al. Trends in bacteraemia on the haematology and oncology units of a UK tertiary referral hospital. Journal of Hospital Infection 2002; 50: 48-55.
- Sigurdardottir K, Digranes A, Harthug S, Nesthus I, TangenJM, Dybdahl B et al. A multi-center prospective study of febrile neutropenia in Norway: Microbiologycalfindings and antimicrobial susceptibility. Scan J Inf Dis 2005; 37: 455-464.
- Baskaran ND, GanGG, Adeeba K, Sam IC. Bacteremia in patients with febrile neutropenia after chemotherapy at a university medical center in Malaysia.Int J InfDis 2007; 11 [6]: 513-17.
- Akova M. Emerging problem pathogens: A review of resistance patterns over time. Int J Inf Dis 2006; 10: 3-8.
- Cherif H, Kronvall G, Bj_rkholm M, Kalin M. Bacteraemia in hospitalised patients with malignant blood disorders: A retrospective study of causative agents and their resistance profiles during a 14-year period without antibacterial prophylaxis. Hematol J 2003;4:420-6.
- Spanik S, Kukuckova E, Pichna P, Grausova S,Krupova I, Rusnakova V, et al. Analysis of 553 episodes of monomicrobialbacteraemia in cancer patients: Any association between risk factors and outcome to particular pathogen? Support Care Cancer 1997;5:330-3.
- Sherertz RJ. Practical healthcare epidemiology: surveillance for infections associated with vascular catheters. Infect ContrHospEpidemiol 1996;17:746-52.
- Hanna H, Afif C, Alakech B, Boktour M, Tarrand J,Hachem R et al. Central venous catheter related bacteremia due to gram-negative bacilli: significance of catheter removal in preventing relapse. Infect Control HospEpidemiol 2004; 25: 646–649.
- Goren D, Fen T. Nosocomial infections in haematology and oncology clinics: preventive measures: Review. TurkiyeKlinikleri J Med Sci 2005; 25:706-723.
- Lai CH, Chi CY, Chen HP, Chen TL, Lai CJ, Fung CP, et al. Clinical characteristics and prognostic factors of patients with Stenotrophomonasmaltophilia bacteremia. J MicrobiolImmunol Infect 2004; 37: 350–358.
- Lai CH, Wong WW, Chin C, Lin HH, Chen WF, Yu KW, et al. Central venous catheter- related Stenotrophomonasmaltophiliabacteraemia and associated relapsing bacteraemia in haematology and oncology patients. Clinical Microbiology and Infection 2004; 12 [10]: 986-991.










