Keywords
Neonate; Hypoxia; Ischemia; Labour; Contractions
Introduction
The terms Perinatal Asphyxia, Neonatal Asphyxia, Birth Asphyxia, Neonatal Encephalopathy, Hypoxic-ischemic Encephalopathy, all relate to the clinical observation [1] that fetuses that have apparently progressed normally may develop profound hypoxic injury during and following the birth process. This causes death or disability in 2-3 per 1000 cases and accounts for about 20% of all cases of cerebral palsy.
Many mechanisms [2-4] have been proposed, with corresponding variation of treatments, but the only protocol showing promise is the empirical one of cooling of the infant [5]. The mechanism initiating such damage cascades has not yet been determined. The possible involvement of intrauterine pressure during contractions does not appear to have previously been considered. That is the subject of this article.
Methodology
The hydro-mechanical neonatal encephalopathy (HNE) hypothesis
This hypothesis concerns the movement of Extra Cellular Fluid (referred to hereafter as Tissue Fluid) occurring during the birthing process. It is postulated that HNE comes in two phases, an initial mechanical trauma which happens during delivery, and a second Hypoxic-Ischemic injury phase that results from vascular derangement during delivery.
The initial mechanical phase requires access to uterine contraction pressure and so cannot occur before or after delivery. It can only happen during delivery. The second (ischemic) phase is temporary, awaiting the dispersal of profound edema set up in the first phase. The following indicates a typical predicted sequence.
Phase I: Mechanical Trauma
Initial pressurizing (Early labor, cervix still closed)
Figure 1 is a re-plot of part of the example given in reference [6]. This is the intrauterine pressure during labor registered via a catheter inserted into the amniotic fluid. Between contractions the intrauterine pressure was slightly less than 20 mmHg, but during contractions it reached 100 mmHg.
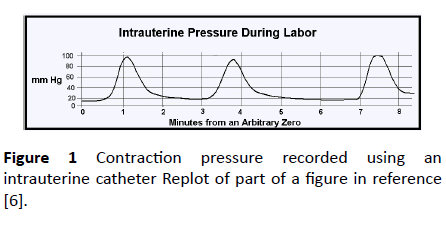
Figure 1: Contraction pressure recorded using an intrauterine catheter Replot of part of a figure in reference [6].
Pre-delivery, the fetus and placenta are totally enveloped in the uterus (Figure 2a). If the uterine muscle contracts, all the tissue contained in the uterus will be subjected to the same additional raised pressure. For example, venous pressure in a fetus is typically 15 mmHg [7]. Suppose a Braxton Hicks contraction [8] exerts a pressure of 20mmHg. Then the fetal absolute venous pressure (measured from outside) will be (15+20) or 35mmHg. But the pressure in the tissue fluid surrounding all the local vessels would also have been raised by 20 mmHg, so the pressure distending a capillary would not have changed. Similarly, in the case shown in Figure 1, the absolute pressure measured via an intrauterine catheter rose to 100 mmHg, but so also would the pressure in the surrounding tissue fluid. The vascular distending pressure would have hardly changed. Some maternal blood may have been displaced from the maternal side of the placenta, but fetal vessels would not be damaged at this stage.
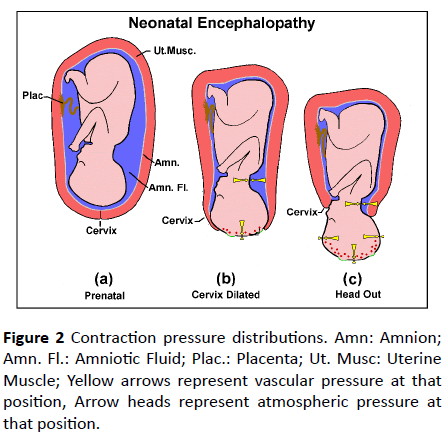
Figure 2: Contraction pressure distributions. Amn: Amnion; Amn. Fl.: Amniotic Fluid; Plac.: Placenta; Ut. Musc: Uterine Muscle; Yellow arrows represent vascular pressure at that position, Arrow heads represent atmospheric pressure at that position.
Pressures during delivery
Now the situation during parturition when the cervix has started to dilate will be considered during contractions (Figures 2b and 2c). The scalp has lost the support of the uterus, it is exposed to air pressure only. Tissue fluid pressure under the newly exposed scalp surface will be virtually zero (ambient air pressure). However, the capillaries passing through this region are still connected to vessels in the fetus and so their lumens are at uterine contraction pressure. Capillary walls will be subjected to the full uterine contraction pressure. This may rupture some cerebral vessels, depriving local neurons of circulation, forming a diffuse injury. These ruptures will be obvious at autopsy and do not form part of HNE.
Flooding
It is normal for fluid to move through the walls of capillaries. The mean pressure in the lumen of typical capillaries is similar to that in the surrounding interstitium, slightly higher at the arteriolar end, slightly lower at the venule end. This facilitates a local fluid flow bringing oxygen to cells in the interstitium and carrying carbon dioxide away. Normally this pressure difference is only a few mm Hg, but, as previously indicated, during contractions pressure in cerebral capillaries may be 100 mm Hg while they are surrounded by tissue fluid at 20 mmHg pressure, a transmural pressure of 80 mm Hg. Such a high transmural capillary pressure, together with the increased permeability of the stretched walls, will rapidly force excessive volumes of plasma out into the surrounding interstitium.
Phase II: Hypoxic-Ischemia and Treatment
Capillary constriction
Constriction of capillaries will occur, not by the action of precapillary sphincters but by hydrostatic pressure in the tissue fluid. Figure 3 illustrates this process. Red signifies blood vessels and blue the interstitium surrounding them. Consider them to be capillaries fed from the left and drained to the right (Diagramatic, not to any scale).
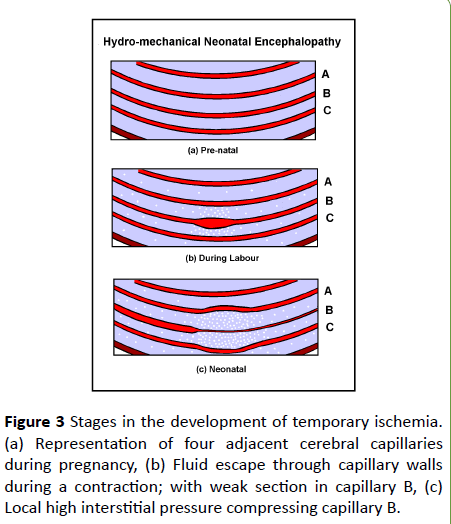
Figure 3: Stages in the development of temporary ischemia. (a) Representation of four adjacent cerebral capillaries during pregnancy, (b) Fluid escape through capillary walls during a contraction; with weak section in capillary B, (c) Local high interstitial pressure compressing capillary B.
Figure 3a represents four adjacent capillaries in late pregnancy. Capillary B is considered to have weaker and more porous walls compared to capillaries A and C. This need not have any effect at normal fetal blood pressures and flows.
Figure 3b represents the situation during a contraction in early labor. The high lumen pressure is causing all of them to leak fluid (represented by white spots) especially capillary B. Capillary B leaks faster both because its nature is more permeable and because its walls are stretched. Between contractions, fluid will be driven back into the capillaries by the much smaller edemous pressure, so fluid will accumulate in the local interstitium, building up local edemous pressure.
Figure 3c represents the situation after delivery. The accumulated fluid around capillary B has caused the local interstitium to swell and deflect capillaries A and C. Capillary B is surrounded by pressurised interstitium. This locally elevated interstitial pressure will constrict vessels by compressing them. Downstream of such constrictions (to the right in the figure) blood flow will be reduced, and upstream, flow will be redirected to unobstructed paths impairing delivery of oxygen and removal of carbon dioxide.
Thus, during contractions plasma forced through vessel walls into their surrounding interstitium will form highpressure cuffs around vessels. Between uterine contractions, and following delivery, this additional intrauterine pressure component will no longer be there. The cuffs will then press on the capillaries, narrowing them, and so restricting flow through them, causing local Ischemia.
Ischemia
Quite small compressions will make significant increases in flow resistance. The relationship between driving pressure and flow in a rigid tube can be described by a form of the Poiseuille Equation below [9]:
Q = K × D4 × P
Where Q is the flow rate, K is a constant describing the tube, and P is the pressure driving the flow.
Capillaries are not rigid nor are they necessarily circular, but the fundamental approximation that for a given driving pressure flow through a tube varies as the fourth power of its radius, 104, is valid. This means that quite small reductions in radius make a big difference in fluid flow resistance. If an interstitional pressure reduces capillary radius by 10%, flow resistance will increase by roughly 50%. If the diameter is halved, flow will will be reduced to 6% of original. The magnitude of such flow reductions will vary from vessel to vessel producing a diffuse pattern of injury, whose local magnitude will depend both on the strength of the contractions and the maturity of the vasculature. Neighbouring vessels may be unaffected.
This Ischemia and consequential tissue hypoxia is only temporary, normoxia will return when the excess interstitial fluid disperses and its pressure on capillaries is removed [6]
Therapeutic hypothermia
The protective action of hypothermia will occur during the second phase of HNE. It involves keeping neurons alive, though not necessarily functional, in the temporary ischemic conditions arising following delivery. Kerenyi et al. [10] quote Sahuquillo and Vilata [11] that hypothermia reduces metabolic rate by 7-9% per 1oC core temperature reduction, with parallel deceases in oxygen consumption and carbon dioxide production. Empirically, cooling works, but the optimum cooling temperature range and duration have yet to be determined. The following is based on the Hydro-Mechanical concept of insult arising during delivery.
Temperature cooling range: Kerenyi et al. [10] studied systemic effects of the temperature when piglets were cooled during therapeutic hypothermia. They found that there was no difference in the relative risk of any neurodevelopmental outcome among piglets who received hypothermia with target core temperature > 34°C as compared to the control group. Therefore, the optimal temperature for neural rescue was likely to lie at some point below 34°C. They also found more fatalities wth cooling to 30°C compared to normothermia or cooling to 35°C. There was considerable risk of cardiac irregularities, more cardiac arrhythmias.
However, cooling to 33-34°C showed no physiological or clinical differences. This hypothetical hydro-mechanical model suggests that the requirement is to balance the benefits of slowing neurological metabolism against the hazards of slowing that of the heart sufficiently to affect its pacemaker.
Timing: After delivery the extravased fluid will start to drain away, and the vulnerable vessels will start to reopen. This is a complex phase because restoration of normal circulation is taking place while injury cascades have been triggered. This drainage time will vary from vessel to vessel because the vessels required to carry away the excess fluid are the same ones in which flow is restricted.
It would seem important to maintain cooling until more normal pressures have returned to the vulnerable regions. The 72-hour period set in the TOBY study [5] seems to have been adequate on an empirical basis.
Summarizing: If this Hydro-mechanical Neonatal Encephalopathy hypothesis is valid-
1. Phase 1 HNE in an individual infant can only occur during labour. It requires the excessive vascular pressures generated during uterine contractions. It cannot recur postnatally.
2. Local hypoxic stress in the cerebrum may begin in late stage one labour as the cervix starts to dilate.
3. Fluid is expelled through the walls of venules and capillaries by their excessive lumen pressure during contractions, raising local tissue fluid pressure.
4. Between contractions and in the neonatal period, this locally raised tissue fluid pressure may temporarily constrict venules, capillaries or even arterioles.
5. This temporary reduction of circulation will last until the excess fluid in the local interstitium surrounding vessels has dispersed, allowing the vessels to reopen.
6. Hypothermia works by enabling neurons to survive this temporary reduction of blood flow (ischemia) by reducing their metabolic activity.
7. Optimum cooling (33-34°C) lies in the small range of temperature over which neural metabolic activity is reduced but cardiac pacemaker activity is not affected.
Discussion
The therapeutic benefit of hypothermia on this form of Hypoxic-Ischemic Encephalopathy lies in reducing neuronal activity and metabolic demands to keep neurons alive until local interstitial pressures have subsided, and normal blood flow is restored. It should be started as early as possible to conserve cell reserves.
Permanence
Analysis of neurological outcomes of various studies at 18 months of age [12] showed that treatment with hypothermia was consistently associated with an increased rate of normal survival. Among infants who survived to 18 months of age, those treated with hypothermia had significantly lower rates of severe disability, cerebral palsy, severe neuromotor delay, severe neurodevelopmental delay and blindness. This is consistent with the HME concept that conditions for the primary injury (vessel lumen at uterine contraction pressure, surrounded by tissue fluid at zero pressure) only exist relatively briefly. It starts late in the First Stage of labor and lasts while the head emerges but only until the body is free of uterine pressure.
Further damage may follow as a result of the hypoxia and ischemic injury, but the accumulation of expressed fluid itself cannot occur without access to the excessive intrauterine pressure.
Indicated treatment
Once recognised the infant should be cooled as soon as possible to conserve nutrients and slow down metabolism to match the meagre oxygen supply. Continue until the local tissue fluid accumulations have dispersed and normal capillary circulation has been restored.
Conclusions
1. There exists a form of Neonatal Encephalopathy that is entirely mechanical in origin. It results in temporary cerebral ischemia.
2. Hypothermia acts by reducing neural metabolic rate, to favour neural survival during this ischemic period.
3. Hydro-Mechanical Encephalopathy can only arise during delivery.
4. Hydro-Mechanical Encephalopathy is unrelated to previous events in that pregnancy.
21845
References
- Lally P, Pauliah S, Montaldo P, Chaban B, Oliveira V, et al. (2015) Magnetic resonance biomarkers in neonatal encephalopathy (MARBLE): A prospective multicountry study. BMJ Open 5: e008912.
- Azzopardi D, Strohm B, Edwards D, Dyet L, Haliday HL, et al. (2009) Moderate hypothermia to treat perinatal asphyxial encepphalopathy. N Eng J Med 361: 1349-1358.
- Ganong WF (1987) Dynamics of blood and lymph flow. In: Ganong WF, editor. Review of medical physiology (13th edn) Connecticut: Prentice-Hall International Inc. pp: 477-492.
- Kerenyi A, Kelen D, Faulkner SD, Bainbridge A, Chandrasekare M, et al. (2012) Systemic effects of whole-body cooling to 35°C, 33.5°C and 35°C in a piglet model of perintal asphyxia:implications for therapeutic hypothermia. Pediatric Research 71: 573-582.
- Sahuquillo J, Vilalta A (2007) Cooling the injured brain: How does moderate hypothermia influence the pathophysiology of traumatic brain injury. Curr Pharm Des 13: 2310-2322.
- Edwards D, Brocklhurst P, Gunn AJ, Halliday H, Juszcak E, et al. (2010) Neurological outcomes at 18 months of age after moderate hypothermia for perinatal hypoxic ischaemic encephalopathy: Synthesis and meta-analysis of trial data. BMJ 340: 363.








