Key words
|
| |
| Hydrotropy, Hydrotropes, Solubility, Solubility enhancement. |
| |
INTRODUCTION
|
| |
| The term hydrotropic agent was first introduced by Neuberg (1916) to designate anionic organic salts which, at high concentrations, considerably increase the aqueous solubility of poorly soluble solutes[1].Hydrotropy is a solubilization phenomenon whereby addition of large amount of second solute results in an increase in the aqueous solubility of another solute. However, the term has been used in the literature to designate non-micelle-forming substances, either liquids or solids, organic or inorganic, capable of solubilizing insoluble compounds. The chemical structure of the conventional Neuberg’s hydrotropic salts (proto-type, sodium benzoate) consists generally of two essential parts, an anionic group and a hydrophobic aromatic ring or ring system. The anionic group is obviously involved in bringing about high aqueous solubility, which is a prerequisite for a hydrotropic substance. The type of anion or metal ion appeared to have a minor effect on the phenomenon[2]. On the other hand, planarity of the hydrophobic part has been emphasized as an important factor in the mechanism of hydrotropic solubilization. Solute consists of alkali metal salts of various organic acids. Hydrotropic agents are ionic organic salts. Additives or salts that increase solubility in given solvent are said to “salt in” the solute and those salts that decrease solubility “salt out” the solute. Several salts with large anions or cations that are themselves very soluble in water result in “salting in” of non electrolytes called “hydrotropic salts” a phenomenon known as “hydrotropism”. Hydrotropic solutions do not show colloidal properties and involve a weak interaction between the hydrotropic agent and solute[3]. |
| |
| Solubility enhancement of various poorly soluble compounds is a challenging task for researchers and pharmaceutical scientists. Solubility is one of the important parameter to achieve desired concentration of drug in systemic circulation for pharmacological response to be shown[2]. The study on solubility yields information about the structure and intermolecular forces of drugs. Drug efficacy can be severely limited by poor aqueous solubility and some drugs also show side effects due to their poor solubility. There are many techniques which are used to enhance the aqueous solubility. The ability to increase aqueous solubility can thus be a valuable aid to increase efficiency and/or reduce side effects for certain drugs. This is true for parenterally, topically and orally administered solutions. |
| |
| General parameters affecting solubility are particle size, shape and surface area physicochemical properties of drugs, and physical forms of drugs, solvents, pH of the medium, temperature and use of surfactants[3]. The pharmacopoeia lists solubility in terms of dissolve 1g of solute. If exact solubilities are not known, the pharmacopoeia provides general terms to describe a given range. These descriptive terms are listed in (Table 1). |
| |
NEED OF SOLUBILITY
|
| |
| Therapeutic effectiveness of a drug depends upon the bioavailability and ultimately upon the solubility of drug molecules. Solubility is one of the important parameter to achieve desired concentration of drug in systemic circulation for pharmacological response to be shown[5]. Due to advanced research & development, there are varieties of new drugs & their derivatives are available. But more than 40% of lipophilic drug candidates fail to reach market due to poor bioavailability, even though these drugs might exhibit potential pharmacodynamic activities. The lipophilic drug that reaches market requires a high dose to attain proper pharmacological action. The basic aim of the further formulation & development section is to make that drug available at proper site of action within optimum dose[6]. |
| |
MECHANISM OF SOLUBILITY
|
| |
| The term ‘solubility’ is defined as maximum amount of solute that can be dissolved in a given amount of solvent. It can also be defined quantitatively as well as qualitatively. Quantitatively it is defined as the concentration of the solute in a saturated solution at a certain temperature. In qualitative terms, solubility may be defined as the spontaneous interaction of two or more substances to form a homogenous molecular dispersion. A saturated solution is one in which the solute is in equilibrium with the solvent[7-9]. |
| |
|
PROCESS OF SOLUBILISATION[5]
|
| |
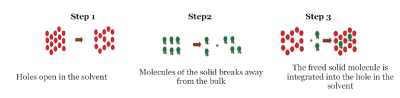
| The process of solubilisation involves the breaking of inter-ionic or intermolecular bonds in the solute, the separation of the molecules of the solvent to provide space in the solvent for the solute, interaction between the solvent and the solute molecule or ion. |
| |
 |
| |
MECHANISM OF HYDROTROPE ACTION[2]
|
| |
| A hydrotrope is a compound that solubilises hydrophobic compounds in aqueous solutions. Typically, hydrotropes consist of a hydrophilic part and a hydrophobic part (like surfactants) but the hydrophobic part is generally too small to cause spontaneous self-aggregation. Hydrotropes do not have a critical concentration above which selfaggregation 'suddenly' starts to occur. Instead, some hydrotropes aggregate in a step-wise self-aggregation process, gradually increasing aggregation size. However, many hydrotropes do not seem to selfaggregate at all, unless a solubilisate has been added. Maheshwari et al[10]; enhanced the aqueous solubility of paracetamol, a poorly water-soluble drug by use of concentrated solution of urea (a hydrotropic agent). This hydrotropic phenomenon was employed to prepare solid dispersion (SD) and syrup of paracetamol. SD was evaluated for dissolution rate and a marked increase in dissolution rate was observed with SD. IR analysis revealed that there was no complexation/interaction between paracetamol and urea. Paracetamol syrups prepared with urea showed good chemical stabilities. |
| |
SELECTION OF HYDROTROPES FOR POORLY WATER-SOLUBLE DRUGS[11]
|
| |
| It is evident from the literature survey that more is the concentration of hydrotrope; more is the aqueous solubility of poorly water-soluble drugs. Therefore, highly concentrated solutions of hydrotropic agents were used in the present investigation. Distilled water was used in making hydrotropic solutions. 2 M sodium benzoate (2 M SB), 2 M niacinamide (2 M NM), 2 M sodium salicylate (2 M SS), 4 M sodium acetate (4 M SA), 10 M urea (10 M UR) and 1.25 M sodium citrate (1.25 M SC) were employed as hydrotropic solutions. |
| |
| In order to select suitable hydrotropes (for sufficient enhancement in solubility) for various poorly watersoluble drugs, following method (an approximate solubility determination method) was used. Twenty five ml of distilled water/hydrotropic solution was taken in a 50 ml glass bottle and gross weight (including the cap) was noted. Then, few mg (by visual observation) of fine powder of drug was transferred to the bottle. The bottle was shaken vigorously (by hand). When drug got dissolved, more drugs (few mg by visual observation) were transferred to the bottle and again the bottle was shaken vigorously. Same operation was repeated till some excess drug remained undissolved (after constant vigorous shaking for 10 minutes). |
| |
| Then, again gross weight was noted. From the difference in two readings (of weight), an approximate solubility was determined and solubility enhancement ratios (solubility in hydrotropic solution/solubility in distilled water) were calculated for all selected drugs for all six hydrotropic solutions. When the determined solubility enhancement ratio was at least 5, such hydrotropic solution was selected for that drug. R K Maheshwari, Mithun Singh Rajput, Sampada Sinha[23] were developed titrimetric analysis method to solubilize the slightly water soluble topical antifungal drug, benzoic acid by 1.0 M calcium disodium edetate solution. There was more than 15 fold enhancement in aqueous solubility of benzoic acid in 1.0 M calcium disodium edentate solution as compared to the solubility in distilled water. Jayakumar C, Deepak Kumar K, Nesakumar D, Nagendra Gandhi N[15] were performed titrimetric estimation has been developed using 2 M sodium salicylate as a hydrotropic solubilizing agent for the quantitative determination of theophylline in bulk, a sparingly water?soluble keratolytic drug. There was more than a 18?fold enhancement in aqueous solubility of theophylline in 2 M sodium salicylate solution. The results of analysis obtained by the proposed method are comparable with the results of analysis obtained by the Indian Pharmacopoeial method. |
| |
METHODS TO MEASURE THE SOLUBILITY[24]
|
| |
| To determine solubility of solids in liquids following two steps are used |
| |
| 1) Preparation of saturated solution: Solubility indicates the maximum amount of a substance that can be dissolved in a solvent at a given temperature. Such a solution is called saturated. Solubility is measured either in grams per 100 g of solvent (g/100 g) or number of moles per 1 L of the solution. |
| |
| 2) Analysis of saturated solution : |
| |
| Once the saturated solution is prepared its analysis is carried out to check the solubility. It depends upon the nature of the solute and accuracy of the method employed. Following methods are used for analysis. |
| |
| a) Evaporation method |
| |
| b) Volumetric method |
| |
| c) Gravimetric method |
| |
| d) Instrumental method |
| |
DETERMINATION OF INTERFERENCE OF HYDROTROPIC AGENTS IN THE SPECTROPHOTOMETRIC ESTIMATION OF DRUGS [11]
|
| |
| A UV-Visible recording spectrophotometer with 1 cm matched silica cells was employed for spectrophotometric determinations. For determination of interference of hydrotropic agents in the spectrophotometric estimation of the standard solutions of drugs were determined in distilled water alone and in the presence of the maximum concentration of the hydrotropic agent employed for spectrophotometric analysis. The absorbances were recorded against respective reagent blanks at appropriate wavelengths.Titrimetric analysis method employed for determining equilibrium solubility at room temperature. Enhancement ratios in solubilities were determined by following formula - Enhancement ratio = Solubility in hydrotropic solution÷ Solubility in distilled water. |
| |
| Smita Sharma, Mukesh C. Sharma[25] were investigate that hydrotropic solution of 8M urea has been employed as solubilizing agent to solubilization poorly water soluble drug Pseudoephedrine Sulphate, Desloratidine, from fine powder of its tablet dosage form for spectrophotometric determination in ultraviolet region. Pseudoephedrine Sulphate, Desloratidine shows maximum absorbance at resulting solutions were measured at 274.4 nm and 289.1nm. R. K. Maheshwari, s. R. Bishnoi, d. Kumar, murali krishna[26] , in the present investigation, hydrotropic solution of ibuprofen sodium (0.5M) was employed as solubilizing agent to solubilize the poorly water-soluble drug, ornidazole from fine powder of its tablets for spectrophotometric determination. Ornidazole shows its maximum absorbance at 320 nm and Beer’s law was obeyed in concentration range of 5-25 mcg/ml. |
| |
ADVANTAGES OF HYDROTROPIC SOLUBILIZATION TECHNIQUE[2]
|
| |
| 1. Hydrotropy is suggested to be superior to other solubilization method, such as miscibility, micellar solubilization, co solvency and salting in, because the solvent character is independent of pH, has high selectivity and does not require emulsification |
| |
| 2. It only requires mixing the drug with the hydrotrope in water. |
| |
| 3. It does not require chemical modification of hydrophobic drugs, use of organic solvents, or preparation of emulsion system. |
| |
|
MIXED HYDROTROPY
|
| |
| Mixed hydrotropic solubilization technique is the phenomenon to increase the solubility of poorly water-soluble drugs in the blends of hydrotropic agents, which may give miraculous synergistic enhancement effect on solubility of poorly water soluble drugs, utilization of it in the formulation of dosage forms of water insoluble drugs and to reduce concentration of individual hydrotropic agent to minimize the side effects (in place of using a large concentration of one hydrotrope a blend of, say, 5 hydrotropes can be employed in 1/5th concentrations reducing their individual toxicities[27]. Veena Nair, Mithun S Rajput[28] were developed a novel, safe and sensitive method of spectrophotometric estimation in the ultraviolet region using a mixed hydrotropic solution, containing a blend of 30% w/v urea, 13.6% w/v sodium acetate and 11.8% w/v sodium citrate for the quantitative determination of ketoprofen, a poorly water soluble drug, in tablet dosage form. Beer's law was obeyed in the concentration range of 4–20 μg/ml. There was more than 570-fold enhancement in aqueous solubility of ketoprofen in mixed hydrotropic solution as compared with the solubility in distilled water precluding the use of organic solvents. Nilesh Jain, Ruchi Jain, Navneet Thakur, Brahm Prakash Gupta, Jitendra Banweer and Surendra Jain[13] were developed Spectrophotometric method using 2 M sodium acetate and 8 M Urea solution as hydrotropic solubilizing agent for the quantitative determination of poorly water?soluble hydrochlorothiazide in tablet dosage form. There were more than 55 and 70 fold enhancements in the solubility of hydrochlorothiazide increases in 2 M sodium acetate and 8 M Urea solution as compared to solubilities in distilled water. Hydrochlorothiazide shows maximum absorbance at 272 nm. Sodium acetate and urea did not show any absorbance above 240 nm, and thus no interference in the estimation was seen. Hydrochlorothiazide is obeyed Beer’s law in the concentration range of 10 t0 50μg/ml (r2= 0.999) in sodium acetate and 5 to 25 μg/ml (r2= 0.999) in urea with mean recovery 98.74 and 99.99% in sodium acetate and urea respectively. |
| |
ADVANTAGES OF MIXED HYDROTROPIC SOLUBILIZATION[2]
|
| |
| 1. It may reduce the large total concentration of hydrotropic agents necessary to produce modest increase in solubility by employing combination of agents in lower concentration. |
| |
| 2. It is new, simple, cost-effective, safe, accurate, precise and environmental friendly method for the analysis (titrimetric and spectrophotometric) of poorly water-soluble drugs titrimetric and spectrophotometric precluding the use of organic solvents. |
| |
| 3. It precludes the use of organic solvents and thus avoids the problem of residual toxicity, error due to volatility, pollution, cost etc. |
| |
| Vikas Pareek, Santosh Tambe, Santosh Bhalerao, Rupali Shinde, Lalit Gupta[12] were employed Conventional Spectrophotometric Estimation (Method I) and Area Under Curve Method (Method II) for quantitation of Cefprozil by using five different hydrotropic agents. These include Potassium acetate (6.0M), Potassium citrate (1.5M), Sodium acetate (4.0M), Sodium citrate (1.25M) and Urea (10.0M). All these agents do not show absorbance above 245 nm and hence do not interfere with absorbance of Cefprozil (λmax? 280 nm). |
| |
NOVEL PHARMACEUTICAL APPLICATIONS OF HYDROTROPIC SOLUBILIZATION IN VARIOUS FIELDS OF PHARMACY[02]
|
| |
| 1. Quantitative estimations of poorly watersoluble drugs by UV-Visible spectrophotometric analysis precluding the use of organic solvents. |
| |
| 2. Quantitative estimations of poorly watersoluble drugs by titrimetric analysis.such as ibuprofen, flurbiprofen and naproxen using sodium benzoate[29] . |
| |
| 3. Preparation of hydrotropic solid dispersions of poorly water-soluble drugs precluding the use of organic solvents. Such as felodipine[30] using poly-ethylene glycol 6000 and poly-vinyl alcohol. |
| |
| 4. Preparation of dry syrups (for reconstitution) of poorly water-soluble drugs. |
| |
| 5. Preparation of topical solutions of poorly water-soluble drugs, precluding the use of organic solvents. Such as tinidazole, metronidazole and salicylic acid using sodium benzoate and sodium citrate. |
| |
| 6. Preparation of injection of poorly water soluble drugs. |
| |
| 7. The use of hydrotropic solubilizers as permeation enhancers. |
| |
| 8. The use of hydrotropy to give fast release of poorly water-soluble drugs from the suppositories. |
| |
| 9. Application of mixed- hydrotropy to develop injection dosage forms of poorly water-soluble drugs. |
| |
| 10.Application of hydrotropic solubilization in nanotechnology (by controlled precipitation). |
| |
| 11. Application of hydrotropic solubilization in extraction of active constituents from crude drugs (in pharmacognosy field). |
| |
| 12.Hydrotropic solutions can also be tried to develop the dissolution fluids to carry out the dissolution studies of dosage forms of poorly water-soluble drugs |
| |
CONCLUSION
|
| |
| By this Study we can conclude that, Solubility is the most important physical characteristic of a drug for its oral bioavailability, formulation, development of different dosage form of different drugs and for quantitative analysis. Solubility can be enhanced by many techniques among them hydrotropy is of very much importance. Hydrotropy is defined as a solubilisation process whereby addition of a large amount of second solute results in an increase in the aqueous solubility of another solute and the chemicals which are used in hydrotropy are called hydrotropes. For example sodium benzoate, urea, sodium salicylate and ibuprofen sodium etc. In present scenario this method is getting lot of values and may be proved the best method in future. |
| |
Conflict of Interest
|
| |
| NIL |
| |
Source of Support
|
| |
| NONE |
| |
Tables at a glance
|
 |
 |
| Table 1 |
Table 2 |
|
| |







