Keywords:
Dysfunctional uterine bleeding; IHC; Estrogen and Progesterone receptors
Introduction
Dysfunctional uterine bleeding is one of the major causes of menorrhagia and it’s a diagnosis of exclusion [1]. It accounts for 33% of overall referrals to gynecologic practice [2] and is estimated to occur in 11-14% of reproductive women [3]. Over 50% of the patients who undergo hysterectomy for menorrhagia have DUB [4]. DUB has several complications such as anovulation, infertility and severe anemia.
The basic underlying mechanism of DUB is unopposed estrogenic stimulation leading to excessive endometrial proliferations and hyperplasia [5], which is a known risk factor for endometrial carcinoma. Hence, a comprehensive understanding of mechanisms of DUB is fundamental to its management.
Histological examination of endometrial aspirate is the management of choice in DUB, besides transvaginal ultrasound [6]. Histological changes in DUB are varied, with little correlation between histology and abnormal bleeding pattern [7,8].
Discovery of specific steroid receptors in endometrium, through which the ovarian hormones act, has revolutionized the medical management of DUB patients, with introduction of receptor modulating drugs [9]. The knowledge of steroid receptors in endometrium is of utmost importance, since it supports the role of hormone receptors in the etiopathogenesis of DUB [7,9] and it could start a new era in the hormonal therapy of endometrial cancer [7]. Steroid receptors can be assayed either quantitatively in tissue homogenates or qualitatively by Immunohistochemistry [10]. Immunohistochemistry helps in direct localization and analysis of receptors in tissues [11].
To date, there are very few studies on the pattern of expression of these hormonal receptors in the endometrium of DUB patients. With this aim, we undertook this study in an attempt to establish the role of these hormonal receptors in the etiopathogenesis of DUB and its implications in the management of DUB.
Tissue sample
A thorough hematologic and endocrinologic workup was done on all women aged between 20 and 50 years, who presented to obstetrics and gynecology department with menstrual irregularities. After obtaining detailed clinical and menstrual history and completel general physical examination, transvaginal ultrasound was done to assess the uterine size, endometrial width and echogenic pattern. Those with histologic evidence of uterine pathology and endocrine diseases were excluded from the study. 50 women who met the criteria for diagnosis of DUB were selected. Normal endometrium from 30 normally menstruating women between 30 and 50 years age, who underwent vaginal hysterectomy for prolapsed uterus were selected for control group.
Methods
The specimen were fixed in 10% neutral buffered formalin and subjected to routine histopathological examination. The study and control samples were classified according to the histologic appearance as early proliferative (EP), late proliferative (LP), early secretory (ES), late secretory (LS), simple endometrial hyperplasia (SEH) with/without atypia and complex hyperplasia with/without atypia.
Immunohistochemical staining
Paraffin sections were taken on poly-L-lysine coated slides and immunohistochemistry was performed using microwave heating and standard streptavidin-biotin-peroxidase complex using Super SensitiveTM IHC detection systems (BioGenex Laboratories, CA, USA). For positive tissue control, endometrial specimens with known ER and PR positivity (that were being used as positive controls for breast cancer specimens) were used and unstained areas other than nuclei in positive tissue control served as negative control. Staining of non-nuclear areas was considered false positive and such slides were considered invalid and the procedure was repeated for such cases.
Immuno-scoring
Semiquantitative assessment of ER and PR was done based on the distribution and the intensity of staining. We used a scoring system recommended by McCarty et al. Positive staining was seen as fine granular staining of nuclei of glands and stroma. A total of 100 cells were counted under oil immersion (1000X). An Immunohistochemical score was calculated by the formula Σ Pi × I, which is sum of percentage of cells for each intensity of staining [Pi=percentage of cells (0%-100%), i= intensity (0-absent, 1-weak, 2-moderate, 3- intense)] and the final score ranged from 0 to maximum of 300.
Results
The clinical profile of DUB patients is summarized (Table 1). A significant proportion (44%) of DUB patients were more than 45 years of age. The mean duration of menstrual blood flow among DUB patients was long and all had irregular cycles. 16% were moderately anemic (7-9 gm/dl) and 44% had mild anemia (9-11 gm/dl). 26% were nulliparous, remaining were biparous or multiparous.
| Profile |
Mean + SD |
| Age (years) |
38.88 ± 6.8 |
| Parity |
2 ± 1 |
| Weight (kilograms) |
56.24 ± 6.8 |
| Bleeding (days) |
8.36 ± 3.9 |
| Cycle duration (days) |
27.06 ± 7.5 |
| Hemoglobin (gm/dL) |
10.54 ± 1.6 |
Table 1: Clinical profile of DUB group.
We performed a One Sample t-test to compare the endometrial thickness of DUB patients in our study (Table 2) with a reference normal group reported from an earlier study by Chakraborty et al. [9]. There was a significant increase (p<0.001) in mean endometrial thickness (ET) in DUB group (6.70 ± 3.1mm) compared to normal group (Table 3).
| Characteristic |
Mean + SD |
| Uterine size (cm) |
6.07 ± 1.01 |
| Endometrial thickness (mm) |
6.70 ± 3.1 |
Table 2: Ultrasonographic and histologic characteristics of DUB groups.
| |
Test value=4.5 mm |
| |
95% Confidence Interval of the Difference |
| t |
df |
Sig. (2-tailed) |
Mean difference |
Lower |
Upper |
| ET (mm) |
4.82 |
49 |
p <0.001 |
2.196 |
1.2805 |
3.1115 |
Table 3: One sample t test to compare endometrial thickness of DUB group with normal or control group reported earlier in an Indian Study by Chakraborty et al. [9] wherein mean endometrial thickness was 4.5 mm.
There was a significant increase in late proliferative, early secretory and late secretory HP patterns in normal subjects and increase in simple endometrial hyperplasia and early proliferative patterns in DUB group (Table 4). The linear by linear association and Chi-square ratio was significant (χ2=3.91, p=0.05) (Table 5 and Figure 1).
| |
|
EP |
LP |
ES |
LS |
S.E.H |
AEH |
Total |
| Normal |
count |
4 |
7 |
7 |
11 |
1 |
0 |
30 |
| % Total |
5.0% |
8.8% |
8.8% |
13.8% |
1.2% |
0% |
37.5% |
| DUB |
count |
7 |
7 |
11 |
14 |
10 |
1 |
50 |
| % Total |
8.8% |
8.8% |
13.8% |
17.5% |
12.5% |
1.2% |
62.5% |
| EP: early proliferative; ES: early secretory; S.E.H: Simple endometrial hyperplasia; LP: late proliferative; LS: late secretory; A.E.H: Atypical endometrial hyperplasia |
Table 4: Chi-Square analysis of proportion of HP patterns across DUB (n=50) and Normal groups (n=30).
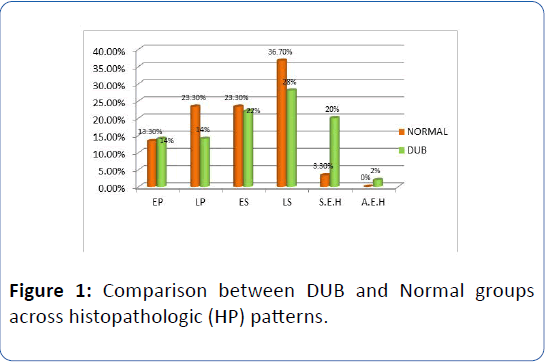
Figure 1: Comparison between DUB and Normal groups across histopathologic (HP) patterns.
| |
Value |
df |
P value (2- tailed) |
| Pearson Chi-Square |
5.793a |
5 |
0.327 |
| Likelihood Ratio |
6.966 |
5 |
0.223 |
| Linear-by-Linear Association |
3.914 |
1 |
0.048 |
| Number of valid cases |
80 |
Table 5: Comparison of changes in distribution of HP patterns across DUB and Normal groups using Chi square test.
Changes in ER/PR expression in normal and DUB groups
There was a significant increase in ER and PR receptor expression (all p<0.001) in both the uterine stroma and glands in DUB group as compared to normal on independent samples t-test (Figure 2, Tables 6 and 7).
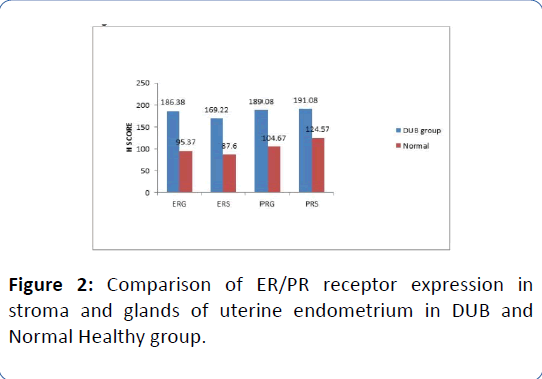
Figure 2: Comparison of ER/PR receptor expression in stroma and glands of uterine endometrium in DUB and Normal Healthy group.
| GROUP |
ERG |
ERS |
PRG |
PRS |
Normal
N=30
Mean + SD |
95.37 ± 36.3 |
87.6 ± 32.6 |
104.67 ± 35.8 |
124.57 ± 39.5 |
DUB
N=50
Mean + SD |
186.38 ± 39.1*** |
169.22 ± 37.9*** |
189.08 ± 35.5*** |
191.08 ± 32.9*** |
| ***p<0.001 using independent samples t-test |
Table 6: Comparison of mean values of ER and PR expression in endometrial stroma and glands of subjects with DUB and the Normal using Independent samples t-test.
| Receptor Status |
T |
df |
Sig. (2- tailed) |
Mean difference |
SE difference |
95% Confidence Interval of the Difference |
| Lower |
Upper |
| ERG |
10.351 |
78 |
p<0.001 |
91.013 |
8.793 |
73.508 |
108.519 |
| ERS |
9.802 |
78 |
p<0.001 |
81.620 |
8.326 |
65.043 |
98.197 |
| PRG |
10.244 |
78 |
p<0.001 |
84.413 |
8.240 |
68.009 |
100.818 |
| PRS |
8.104 |
78 |
p<0.001 |
66.513 |
8.208 |
50.173 |
82.854 |
Table 7: Independent samples t-test for ER/ PR receptor expression in endometrial stroma and glands in DUB and normal group.
ER/PR receptor expression across various HP patterns in DUB
We performed a Non-parametric median test to compare ER/PR receptor expression across various HP patterns in DUB group. Estrogen receptor expression in glands was significantly lesser in LS type more in S.E.H type on median test (χ2= 24.1, p<0.001) (Tables 8a and 8b, Figures 3-14).
| HP pattern |
Mean ± SD |
| ERG |
ERS |
PRG |
PRS |
| EP (N=7) |
196.71 ± 36.8 |
174.71 ± 44.8 |
202.14 ± 45.8 |
202.57 ± 37.6 |
| LP (N=7) |
200.14 ± 14.9 |
175.29 ± 14.6 |
191 ± 21.7 |
185.14 ± 24.4 |
| ES (N=11) |
195.82 ± 37.4 |
187.18 ± 37.1 |
196.09 ± 32.9 |
199.73 ± 38.7 |
| LS (N=14) |
152.57 ± 19.4 |
137.93 ± 28.5 |
167.07 ± 25.3 |
187.93 ± 31.5*** |
| S.E.H (N=10) |
213.80 ± 40.2*** |
189.80 ± 31.1 |
207.5 ± 36.7 |
189.7 ± 24.4 |
| A.E.H (N=1) |
113 |
123 |
131 |
115 |
| Total (N=50) |
186.38 ± 39.1 |
169.22 ± 37.9 |
189.08 ± 35.6 |
191.08 ± 32.9 |
| ***p<0.001 on Median Chi-Square test |
Table 8a: Comparison of ER/PR receptor expression in various HP patterns in patients with DUB using Median test.
| |
ERG |
ERS |
PRG |
PRS |
| Median |
187.50 |
167.00 |
187.00 |
197.00 |
| Chi-Square |
24.062a |
9.730a |
8.625b |
6.335b |
| P value |
P<0.001 |
0.083 |
0.125 |
0.275 |
Table 8b: Test statistics for the above table (Table 8a).

Figure 3: Early Proliferative Phase (ER, 400X).

Figure 4: Early Proliferative Phase (PR, 400X)

Figure 5: Early Secretory Phase (ER, 400X).
Figure 6: Early Secretory Phase (PR, 400X).
Figure 7: Late Proliferative Phase (ER, 400X).
Figure 8: Late Proliferative Phase (PR, 400X).
Figure 9: Late Secretory Phase (ER, 100X).
Figure 10: Late Secretory Phase (PR, 400X).
Figure 11: Simple Endometrial Hyperplasia (ER, 400X).

Figure 12: Simple Endometrial Hyperplasia (PR, 400X).
Figure 13: Atypical Endometrial Hyperplasia (ER, 400X).
Figure 14: Atypical Endometrial Hyperplasia (PR, 400X).
The Figures 3 and 4 illustrate trend in the concentration of both ER and PR in glands and stroma of DUB patients across the different phases of menstrual cycle. There was an increasing trend in ER glands in proliferative phase, and decreasing trend in secretory phase. In late secretory phase, the mean ER and PR scores were significantly lower compared to other phases. The ER stroma was lower than ER glands in all phases PR showed decreasing trend from early to late proliferative phase, thereafter increasing in early secretory phase before falling again in late secretory phase, wherein PR in stroma was higher than that in glands (mean=167.07 ± 25.3). In atypical endometrial hyperplasia, ER and PR were significantly lower than in all other HP patterns (Figures 5-16).
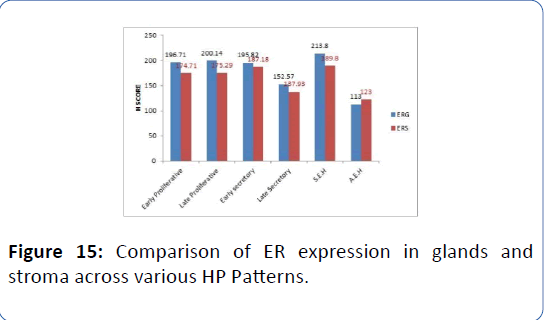
Figure 15: Comparison of ER expression in glands and stroma across various HP Patterns.
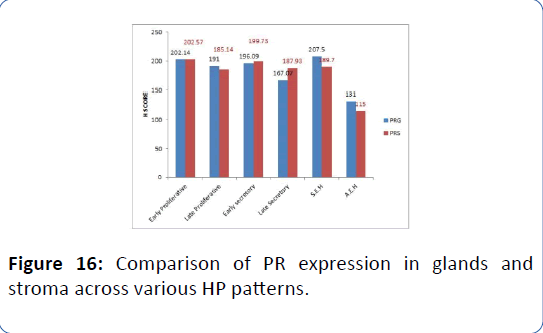
Figure 16: Comparison of PR expression in glands and stroma across various HP patterns.
Discussions
The response of the tissues to steroid hormones depends upon the availability of corresponding receptors in the target organs. These receptors can be analyzed by different methods. Tissue homogenization and biochemical methods are time consuming; complicated and less reliable [12]. Immunohistochemistry allows tissue localization of these receptors in endometrial glands and stroma. Very few studies have been done on immunohistochemical assessment of hormonal receptors in the endometrium of patients with dysfunctional uterine bleeding.
In our study, we investigated the hormonal milieu in the endometrium of patients with dysfunctional uterine bleeding by Immunohistochemistry, by the use of specific monoclonal antibodies against estrogen and progesterone receptors.
In concordance with the results of Levy et al. [13], Chakraborty et al. [9] and Gleeson et al. [4], there was an increasing trend in ER and PR in proliferative phase and decreasing trend in secretory phase in DUB group. This was consistent with the findings in normal endometrium by previous investigators [7,14,15]. Our findings confirm the cyclical variation of the steroid receptors. Also we suggest that the estrogen hormone induces the synthesis of both the receptors during the proliferative phase, and the progesterone hormone suppresses the synthesis of these receptors in secretory phase.
Histologically we observed predominance of early proliferative pattern, endometrial hyperplasia and increased endometrial thickness in DUB patients compared to normal as in earlier studies [16,17]. Since the concentrations of both ER and PR were increased in endometrial glands and stroma of DUB patients, our findings support the role of ovarian steroid hormones in pathogenesis of DUB through increased local concentration of these receptors in endometrium [18], with subsequent unopposed estrogen effect leading to excess endometrial proliferation and hyperplasia [6]. This, in addition to uneven breakdown of endometrium may be responsible for increased endometrial thickness in DUB patients.
We demonstrated higher variation in ER of glands than other receptors in simple endometrial hyperplasia (χ2=24.1, p<0.001), which was consistent with findings of Thornton et al. [19]. This might be due to increased proliferation of glands and increased gland to stroma ratio in endometrial hyperplasia.
High concentration of both endometrial estrogen (p<0.05) and progesterone (p<0.05) receptors in late secretory phase in DUB patients compared to normal group in our study, suggests increased synthesis of these receptors induced by high local estrogen concentration during this phase.
The most interesting finding in our study was demonstration of significant increase (all with p<0.001) in both ER and PR in glands and stroma of endometrium in women with DUB compared to normal group. Since serum estrogen and progesterone were normal in our patients, we suggest the role of increased local concentration of these receptors in the etiopathogenesis of DUB.
Earlier studies [9,20] have demonstrated down regulation of both ER and PR in atypical endometrial hyperplasia. We report one such case. These findings imply that with the development of atypical hyperplasia, there is down regulation of the hormonal receptors.
Our findings were in contrast to Gleeson et al. [4] and Critchley et al. [20] who found no significant difference in estrogen and progesterone receptors between dysfunctional uterine bleeding and normal endometrium. This could be due to difference in the method of selection of patients for the study. Both Critchley et al. and Gleeson et al excluded patients with histologic evidence of simple endometrial hyperplasia and endometrial maturation and menstrual dating discordance.
Although we observed peak concentration of ER and PR in endometrial hyperplasia, but not as exaggerated as noted by Chakraborty by et al. [9].
To the best of our knowledge, there are no reports available on correlation between the ER/PR and the endometrial thickness in DUB. Ohno et al. studied relationship of endometrial ER and PR with sonographic appearance of endometrium in normal and pregnant women. They found no significant relationship between the endometrial thickness and ER, PR receptors. In contrast, we demonstrated a significant negative correlation (p=0.03) between the endometrial thickness and progesterone receptors in glands.
There is various treatment modalities available for DUB, depending on reproductive status of women and individual’s choice. The current most effective treatment strategy for DUB is hormonal therapy. Surgical interventions are reserved for intractable cases. Majority of patients with simple endometrial hyperplasia undergo endometrial ablation or hysterectomy, since they are less amenable to medical management.
A recent development in the management of dysfunctional uterine bleeding is the identification of progesterone antagonists, such as Mifepristone, and selective Progesterone receptor modulators, such as Mesoprogestin J1042 [21,22]. Some Progesterone antagonists, such as ZK 137 316 and ZK 230 211 have been studied in experimental models with promising results in inhibition of endometrial proliferation and induction of amenorrhea [23].
Conclusions
Immunohistochemical analysis of estrogen and progesterone receptors in endometrium is a very useful adjuvant investigation in the management of DUB patients. We support the role of these receptors in the etiopathogenesis of DUB and consequently the development of simple endometrial hyperplasia, which is a pre-cancerous lesion. Our correlation of ER/PR with sonographic endometrial thickness in DUB implies that the later may help in predicting the behavior of these receptors in endometrium. There are limited reports available on the study of both glandular and stromal hormonal receptors in simple endometrial hyperplasia. We conclude that glandular estrogen receptor is a better predictor of disease behavior than other receptors in these patients. There is a subset of DUB patients with increased concentration of ER and PR, who might benefit from the receptor-targeted drugs, such as progesterone antagonists and receptor modulators. This obviates the need for invasive surgeries. We advocate the use of these drugs in the treatment of patients with dysfunctional uterine bleeding and endometrial hyperplasia. However this needs to be validated by long term follow-up studies.
Acknowledgements
Dr. Prakash CJ, Ex-Professor and Head, Department of Pathology, Vydehi Institute of Medical sciences and Research Center, Bangalore.
Dr. Santhosh KV, Ex-Professor, Department of Pathology, Vydehi Institute of Medical sciences and Research Center, Bangalore.
Technical Staff, Department of Pathology, Vydehi Institute of Medical sciences and Research Center, Bangalore.
23623
References
- Speroff L, Fritz MA (2005) Dysfunctional uterine bleeding: Clinical gynaecologic endocrinology and infertility. 7th edn, Lippincott, USA.
- Abbott JA, Garry R (2002) The surgical management of Menorrhagia. Hum Reprod Update 8: 68-78.
- Barr F, Brabin L, Agbaje S, Buseri F, Ikimalo J, et al. (1998) Reducing iron deficiency anaemia due to heavy menstrual blood loss in Nigerian rural residents. Public Health Nutr 1: 249-257.
- Gleeson N, Jordan M, Sheppard B, Bonnar J (1993) Clinical variation in endometrial estrogen and progesterone receptors in women with normal menstruation and dysfunctional uterine bleeding. Eur J Obstet Gynecol Reprod Biol 48: 207-214.
- Livingstone M, Fraser IS (2002) Mechanisms of abnormal uterine bleeding. Hum Reprod Update 8: 60-67.
- Hunter DC, McClure N (2001) Abnormal uterine bleeding: An evaluation endometrial biopsy, vaginal ultrasound and outpatient hysterectomy. Ulster Med J 70: 25-30.
- Mylonas I, Makovitzky J, Friese K, Jeschke U (2009) Immunohistochemical labeling of steroid receptors in normal and malignant human endometrium. Acta Histochem 111: 349-359.
- Moore JG, Singh BP, Holzman RS (1954) Functional uterine bleeding-etiologic factors and therapy. California Medicine 81: 316-320.
- Chakraborty S, Khurana N, Sharma JB, Chaturvedi KU (2005) Endometrial hormone receptors in women with dysfunctional uterine bleeding. Arch Gynecol Obstet 272: 17-22.
- Lessey BA, Killam AP, Metzger DA, Haney AF, Greene GL, et al. (1988) Immunohistochemical analysis of human uterine estrogen and progesterone receptors throughout the menstrual cycle. J Clin Endocrinol Metabl 67: 334-340.
- Press MF, Udove JA, Greene GL (1988) Progesterone receptor distribution in the human endometrium. Am J Pathol 131: 112-124
- Bergeron A, Toft FDO, Schneider W, Shyamala G (1988) Immunohistochemical study of progesterone receptors in the human endometrium during the menstrual cycle. Lab Invest 59: 862-869.
- Bergqvist, Ferno M, Skoog L (1997) Qualitative enzyme immunoassay and semiquantitative immunohistochemistry of oestrogen and progesterone receptors in endometriotic tissue and endometrium. J Clin Pathol 50: 496-500.
- Mylonas I, Jeschke U, Shabani N, Kuhn C, Balle A, et al. (2004) Immunohistochemical analysis of estrogen receptor alpha, estrogen receptor beta and progesterone receptor in normal human endometrium. Acta Histochemica 106: 245-252.
- Noe M, Kunz G, Herbertz M, Mall G, Leyendecker G (1999) The cyclic pattern of the immunohistochemical expression of oestrogen and progesterone receptors in human myometrial and endometrial layers: Characterization of the endometrialsubendometrial unit. Hum Reprod 14: 190-197.
- Israel R, Mishell DR Jr, Labudovich M (1970) Mechanisms of normal and dysfunctional uterine bleeding. Clin Obstet Gynecol 13: 386-392.
- Takreem A, Danish N, Razaq S (2009) Incidence of endometrial hyperplasia in 100 cases presenting with polymenorrhagia/menorrhagia in perimenopausal women. J Ayub Med Coll Abbottabad 21: 60-63.
- Fraser IS, Hickey M, Song J (1996) A comparison of mechanisms underlying disturbance of bleeding caused by spontaneous dysfunctional uterine bleeding or hormonal contraception. Hum Reprod Update 11: 165-178.
- Thornton JG, Wells M (1987) Oestrogen receptor in glands and stroma of normal and neoplastic human endometrium: A combined biochemical, immunohistochemical, and morphometric study. J Clin Pathol 40: 1437-1442.
- Critchley HO, Abberton KM, Taylor NH, Healy DL, Rogers PA (1994) Endometrial sex steroid receptor expression in women with menorrhagia. Br J Obstet Gynaecol 101: 428-434.
- Buffet NC, Meduri G, Bouchard P, Spitz IM (2005) Selective progesterone receptor modulators and progesterone antagonists: mechanisms of action and clinical applications. Hum Reprod Update 11: 293-307.
- Chwalisz K, Brenner RM, Fuhrmann UU, Hess-Stumpp H, Elger W (2000) Antiproliferative effects of progesterone antagonists and progesterone receptor modulators on the endometrium. Steroids 65: 741-751.
- Slayden OD, Chwalisz, Brenner RM (2001) Reversible suppression of menstruation with progesterone antagonists in rhesus macaques. Hum Reprod 16: 1562-1574.






















