Introduction
Natural killer (NK) cells represent the third largest population of lymphocytes characterized by CD3−, CD56+ cell surface phenotype [1]. NK cells have been shown to play a crucial role in clearance of malignant and virally infected cells, rejection of bone marrow transplant, maintenance of pregnancy and auto-immune disorders [2]. NK cells are important not only in innate immunity but they also play role in the regulation of adaptive immunity [3,4]. The ability of NK cells to discriminate between normal and abnormal cells is of immense importance to avoid killing of normal cells. Unlike T cells that undergo gene rearrangement to recognize MHC/peptide complexes, NK cells use inhibitory or activating receptors that do not require gene rearrangement; which has been confirmed by the normal development and cytolytic activity of NK cells in transgenic mice with disrupted RAG-1 or RAG-2 genes [5-7]. At least two class of inhibitory receptors, which interact with major histocampatibility complex (MHC) class I molecules, are expressed by NK cells in most species including human: the CD94–NKG2A C-type lectinlike receptors and the killer cell Immunoglobulin-like receptors (KIR) [8,9]. Whether a NK cell would be activated upon interaction with a potential target cell depends on the balance between the signals from the inhibitory and activating receptors [10]. Two popular hypotheses proposed regarding the activation of NK cells are ‘missing self’ and ‘induced self’ [10-12]. According to the ‘missing self’ theory, cells expressing low levels of MHC class I, which is usually observed in cancer cells or virus-infected cells, are attacked by NK cells [11]. This implies that activation of NK cells is prohibited if the target cell expresses normal levels of MHC class I. However, further studies were able to discover activating receptors on NK cells such as NKG2D, and suggested that the activation of NK cells may be determined by the lack of MHC class I as well as the expression of the ligands for NK cellactivating receptors [5,13,14]. The ‘induced self’ theory appears to be supplementary to the ‘missing self’ theory rather than an independent theory. It simply describes that cells that have undergone malignant transformation or are infected with virus express stress signals such as MICA/MICB which serve as the ligand for activating receptors expressed on NK cells for example NKG2D [15].
The inhibitory receptors in NK cells are some-what better understood than the activating receptors. While most of the activating receptors are specific for non MHC class I ligands that are expressed on both normal and abnormal (transformed or virus-infected) cells, most of the inhibitory receptors are specific for MHC class I ligand [16]. Killer-cell Immunoglobulinlike Receptor (KIR) family includes the major inhibitory receptors which recognize MHC class I molecule with locus and allele specificity [17,18].
Expression of MHC class-I by a cell and its susceptibility to NK cells:
MHC class I molecule are trimeric complexes comprised of a heavy chain that is attached non-covalently with β2-microglobulin and an 8-10 amino acids peptide [19]. Peptides play an important role in the stabilization of the trimeric complex [20]. MHC class I mediated presentation of peptides is an important cellular process by which a cell exposes samples of all the proteins synthesized by it to the immune cells. Based on the peptides presented by MHC class-I, effectors cells of adaptive immune system discriminate between normal and abnormal cells in the process of clearing abnormal cells [21]. MHC class I expression has been found to be down-regulated in many types of cancer and in viral infected cells [21]. Down-regulation of MHC class-I could be one strategy employed by malignant cells to escape T cell mediated immunesurveillance. Likewise, viruses may cause the down-regulation of MHC class-I on the infected cells to prevent clearance of the infected cells before the virus could multiply [22]. While downregulation of MHC class-I could be a strategy to protect abnormal cells from cytotoxic T cells; it can increase the susceptibility of the cells to NK cell-mediated lysis [21].
In 1980’s several studies suggested that there exists an inverse relation between the levels of MHC class I expression on target cell and its susceptibility to NK cell mediated lysis [23,24]. These studies used diverse MHC class-I modulating treatments such as incubation in cytokines, manipulations of growth environments both in vitro and in vivo, and mutagenesis. The existence of inverse relation between relation between the levels of MHC class I expression on target cell and its susceptibility to NK cellmediated lysis provided some of the first evidence implicating MHC class I molecules as potential ligands for NK cell receptors [23,24]. A study published by Strokus et al. in 1989 elegantly showed that MHC class I expression on target cell has an inverse relation with its sensitivity to NK cells [20,25]. In this study, the authors showed that C1R, a human B cell line null for HLA-A and B and sensitive to NK mediated killing, became resistant to NK cells upon transfection with HLA-A3 or -B7 or -B27 or -Bw58 genomic clones. However, transfection with HLA-A2 clone failed to protect C1R cells from NK cells. From a subsequent study the same authors mapped the protective phenotype of class I molecules to the outer α-1/α-2 domains of the HLA class-I molecule and they also identified a non-permissive residue (His74) in the non-protective HLA-A2.1 molecule [20,21]. Also, the authors found that treatment with exogenous peptides reduced the protection, from NK cells, of C1R expressing transfected HLA class-I molecules. Based on these early works, two models were proposed to explain the role of MHC class-I in protection of target cells from NK cells: According to the first model NK cells recognize the ‘self’ endogenous peptide bound to MHC class-I on the surface of target cells which Results in the triggering of negative signal ultimately aborting the cytolytic programming of NK cells [11,20]. The alternate model also known as ‘masking hypothesis’ postulates that “NK cells are triggered by a target structure bound to MHC class–I and released by peptide binding” [21,26]. If the second model was true it would mean that empty MHC class-I would better interact with NK target structure and hence would be more protective.
Killer-cell Immunoglobulin-like Receptor (KIR)
Among many types of inhibitory receptors expressed by NK cells, receptors belonging to the KIR family are considered to play key role in the development and function of human NK cells [2]. KIRs have evolved to generate a highly polymorphic system resulting in diversity comparable to that of MHC class I molecule. In human, the HLA (human leukocyte antigen) -A, -B and -C are polymorphic MHC class-I whereas the HLA-E is relatively conserved, oligomorphic2. Consistent with the co-evolution of KIRs and their ligand (MHC class I), KIRs bind to highly diversified HLA-A, HLA-B and HLA-C whereas the CD94–NKG2A binds to the relatively conserved HLA-E molecule (Figure 1). KIRs may contain two (KIR2D) or three (KIR3D) extracellular immunoglobulin domains. Also, they may exhibit a short (S) or long (L) intracytoplasmic tail which transduces stimulatory or inhibitory signals, respectively [27-30]. Fourteen KIRs have been identified in human which serve either inhibitory function (KIR3DL1-3, KIR2DL1-3, KIR2DL5) or activating (KIR3DS1, KIR2DS1-5) or both (KIR2DL4). The inhibitory KIRs, with long cytoplasmic tail, upon interaction with MHC class-I molecule recruit tyrosine phosphatase SHP-1 to the immunoreceptor tyrosine-based inhibition motifs (ITIM) in their cytoplasmic tail resulting in the complete and proximal blockage of NK activation [31]. However, KIRs with short cytoplasmic tails lack ITIM and instead possess a positively charged residue in their transmembrane domain that interacts with an adapter containing activation motifs ultimately triggering activation signal upon engagement of short-tailed KIRs with their ligands [31].
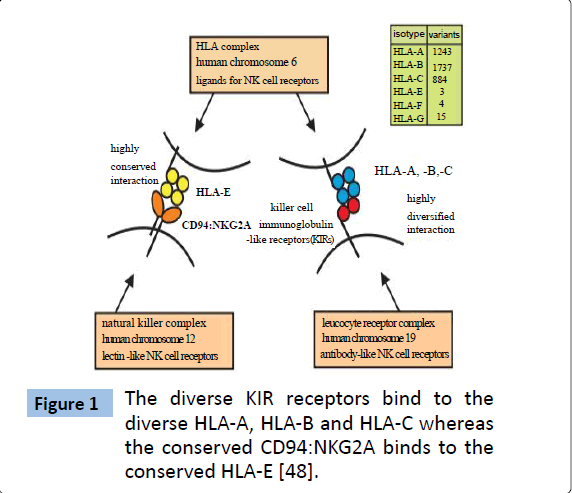
Figure 1: The diverse KIR receptors bind to the diverse HLA-A, HLA-B and HLA-C whereas the conserved CD94:NKG2A binds to the conserved HLA-E [48].
Peptide bound to MHC class-I can regulate recognition by KIR
It is a well-known fact that T cell receptors (TCR) are highly specific for peptide-MHC complex [32,33]. Interestingly, three dimensional structures of MHC-C1–KIR2DL2 and MHC-C2–KIR2DL1 complexes show that the interaction of KIRs with MHC class I is similar to the interaction of TCR with MHC class I in terms of structure: TCR and KIRs interacts with the same face of MHC class-I molecule with the top helices and exposed regions of the MHC bound peptides [33,34]. Analysis of crystal structures depict that TCR covers most part of this face while KIRs mainly cover the ‘right-hand’ side of the face which includes the carboxyl (C)-terminal region of α1-helix, residues 7 and 8 of the bound peptide and amino (N)- terminal region of α2-helix [33]. Several studies published in mid 1990s demonstrated that KIRs are sensitive to the sequence of peptides bound by MHC class-I. Malnati et al. in 1995, for the first time, demonstrated that binding of KIR to MHC class I molecule is specific to the peptide bound to the MHC class-I bound peptide [26]. In this study they showed that mutation of single amino acids in the peptide binding site of HLA-B*2705 resulted into variation in protection from HLA-B*2705-specifi NK clones. Moreover, they found that in a transporter-associated protein (TAP)-deficient cell line, only one of several self-peptides loaded onto HLA-B*2705 protected the cells from NK cells. This requirement of the specific peptide for protection of target cells from NK-mediated lysis ruled out the previously proposed ‘masking hypothesis’. This specificity was originally shown for KIR3DL1 and HLA-B*2705, subsequent studies showed that KIR2DL1, KIR2DL2, KIR2DL3, and KIR3DL2 also have specificity for the sequence of the peptide bound by MHC class-I [26,35-39]. In fact, peptide selectivity has been shown for all inhibitory KIRs tested to date [38]. Most of the studies showed that certain amino acid residues at position 7 and 8 of the bound peptide failed to provide protection from NK cell lysis. These findings comply with the finding from the crystal structures of KIR bound to MHC-peptide complexes.
Peptide Antagonism and NK Cell Activation
By 2010 many studies showed that the recognition of MHC class-I by KIR and the resulting inhibition of NK mediated-lysis are sensitive to the sequence of peptide bound to MHC class I [40]. Fadda et al. in 2010 showed that the inhibition of NK by peptide- MHC complexes on the target cells can be relieved by specific peptide sequence variants that function as antagonist [40,41]. In this study the authors used T2 cells which lack TAP protein to screen a series of peptide variants that confer binding of KIR2DL2 and KIR2DL3 to HLA class I. The T2 cell line expresses HLA-Cw*0102 in the absence of exogenous peptides. MHC class I expressed on the surface of T2 cell is bound weekly to self-peptide which likely dissociates rapidly and hence does not inhibit NK cells. The peptides used in this study were the variants of the nonamer peptide VAPWNSLSL which is the most abundant identified peptide eluted from a HLA-Cw*0102 expressing 721.221 cells and is derived from the TIMP1 protein [42]. The peptide derivatives retained the anchor residues for MHC class-I binding but varied in the amino acid residues at position 7 and 8, the two positions previously shown to affect KIR binding. Among many peptides variants used VAPWNSFAL was found to bind strongly to the HLA-Cw*0102 whereas VAPWNSWSL, VAPWNSRAL and VAPWNSYSL were found to bind weakly to the HLA-Cw*0102. Whereas the strong binding peptide induce strong binding of both KIR2DL2 and KIR2DL3 to HLA-Cw*0102 and strongly inhibited NK cell activation, the weak binding peptides did not induce binding of KIRs to HLA-Cw*0102 and failed to inhibit the activation of NK cells. Surprisingly, combination of peptides that conferred weak and strong recognition of HLA-Cw*0102 by KIRs, was less potent compared to the strong binding peptide alone in inhibiting NK cells. This suggested that peptides that bind to MHC class-I molecule with low-affinity can act as an antagonist to the peptides that bind MHC class-I with high affinity. The authors also showed that peptide antagonism was independent of KIR genotype, and presence or absence of activating KIR (KIR2DS2, short-tailed) does not affect peptide antagonism. This study elegantly showed that the inhibition of activation signals in NK cells can itself become the target of antagonistic peptide leading to the activation of NK cells [41].
The study by Fadda et al. not only showed that peptide antagonism can be an alternate mechanism of NK cell activation but also showed that peptide antagonism is more effective in NK cell activation compared to MHC class-I down-regulation. The authors concluded this based on the observation that KIRpositive NK cells are more responsive to the changes in peptide than to changes in MHC class I expression. This implies that even if cancer cells and virus infected cells do not substantially downregulate MHC class-I, they might substantially change the peptide repertoire [26,40]. Therefore, peptide selectivity bestows NK cells with an extra sensitive mechanism for clearing cancerous, and virus infected cells.
Requirement of MHC class-I Bound Peptide for KIR Recognition
Whether bound peptide is always necessary for KIR recognitionhas been a matter of controversy. In 1995, Malnati et al. showed that recognition of HLA-B2705 by human NK cells was not only peptide dependent but also peptide specific [26]. In the same year Mandelboim et al. published their work showing that presence of an ‘empty’ MHC class-I on the surface of RMA-S cell is sufficient to protect the cell from NK-mediated lysis [43]. The authors showed that the protection of the RMA-S cells from the NK cells was because of the interaction of KIR with the HLA-C (HLA-Cw6 and HLA-Cw7). In 1997, Zappacosta et al. using the same cell line (RMA-S) showed that in the absence of exogenous peptide the HLA-Cw*0304 transfectants were killed to almost the same extent by Nk cells as the untransfecetd cells [19]. Also, they found that addition of exogenous peptide protected the HLA-Cw*0304 transfected but not the untransfected cells from Nk cells. Based on these studies the authors concluded that presence of MHC class-I bound peptide is crucial for KIR mediated inhibition of NK cells. Fadda et al. in 2010 found that T2 cells, TAP-deficient human cell line, expressing HLA-Cw*0102 were not protected by NK cells [40]. This study not only showed that peptide is required for KIR recognition but also showed that the peptide should bind strongly to MHC class-I in order to induce KIR binding to the MHCpeptide complex.
While most of the studies using different variants of HLA in mouse (RMA-S) and human (T2) cell lines have shown that MHC class-I bound peptide is necessary for KIR recognition, the study by Mandelboim et al. shows that peptide is not necessary for KIR recognition as ‘empty’ MHC class-I on the surface of cell is sufficient for the protection of the cell from NK mediated lysis. Given the fact that different HLA istoypes are recognized by different KIRs, it might be possible that MHC-bound peptide is crucial for the recognition of most of the HLA ligands but not all.
The requirement of MHC class-I bound peptide for most KIRs to recognize their ligand could serve an important purpose of killing cancerous or viral infected cells that may express decoy MHC class-I. For example, murine cytomegalovirus virus (MCMV) has been shown to down-regulate MHC class-I expression on the infected cells to protect the infected cell from cytotoxic T-cells [44]. However, since down-regulation of MHC class-I increases the susceptibility of the infected cell to NK cells, therefore to evade NK-mediated lysis MCMV expresses decoy MHC class-I molecule that do not bind peptide [44,45]. Interaction of Ly49, the murine equivalent of KIR, with MHC class-I is mostly independent of the bound peptide [46,47]. Decoy MHC class-I (without bound peptide) on the surface of MCMV-infected cells could evade NK-mediate lysis probably because of the peptide independent interaction of Ly49 with MHC class-I. To date no cancer type or no human virus has been shown to express decoy MHC class-I. However, the peptide-dependence of KIRs may mean that our NK cells could be able to attack if a cell expresses decoy MHC class-I.
Conclusion
Natural killer cells are the major effector cells in the innate immunity. Activation of NK cells depends on the balance between the inhibitory and activating signals from its surface bound receptors. KIR represents a family of NK cell receptors that are the key transducers of inhibitory signals. KIRs are thought to be evolving rapidly with diversity comparable to that of MHC class. KIRs are sensitive to the amino acid sequence, especially at position 7 and 8, of peptide bound to the MHC class-I. Peptides that bind to MHC class-I with strong affinity highly promote the KIR interaction with MHC-peptide complex resulting into higher protection of the target cell from NK-mediated lysis. However, peptides that binds weakly to MHC class-I do not protect target cell; rather they play antagonistic role and reduce the protection caused by strong binding peptide. Thus, peptide bound to MHC class-I regulate the KIR recognition which can confer NK cells with an additional mechanism for sensing cancerous or virus infected cells through ‘peptide antagonism’. However, so far peptide antagonism has been tested only in one receptor:ligand system, in vitro. Most of KIRs tested to date require a MHC class-I bound peptide to interact with their ligand. This requirement for a MHCbound peptide might avoid inhibition of the NK cells by some decoy MHC class-I which do not form complex with a peptide.
6594
References
- Rajalingam R(2012) Overview of the killer cell immunoglobulin-like receptor system.Methods MolBiol 882: 391-414.
- Chan CJ , Smyth MJ, Martinet L (2014) Molecular mechanisms of natural killer cell activation in response to cellular stress.Cell Death Differ 21: 5-14.
- Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S (2008) Functions of natural killer cells.Nat Immunol 9: 503-510.
- Moretta A , Bottino C, Vitale M, Pende D, Cantoni C, et al. (2001) Activating receptors and coreceptors involved in human natural killer cell-mediated cytolysis.Annu Rev Immunol 19: 197-223.
- Shinkai Y, Rathbun G, Lam KP, Oltz EM, Stewart V, et al. (1992) RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement.Cell 68: 855-867.
- Mombaerts P, Iacomini J, Johnson RS, Herrup K, Tonegawa S, et al. (1992) RAG-1-deficient mice have no mature B and T lymphocytes.Cell 68: 869-877.
- Cheent KS, Jamil KM, Cassidy S, Liu M, Mbiribindi B, et al. (2013) Synergistic inhibition of natural killer cells by the nonsignaling molecule CD94.ProcNatlAcadSci U S A 110: 16981-16986.
- Parham P, Abi-Rached L, Matevosyan L, Moesta AK, Norman PJ, et al. (2010) Primate-specific regulation of natural killer cells.J Med Primatol 39: 194-212.
- Pegram HJ, Andrews DM, Smyth MJ, Darcy PK, Kershaw MH (2011) Activating and inhibitory receptors of natural killer cells.Immunol Cell Biol 89: 216-224.
- Ljunggren HG, Kärre K (1990) In search of the 'missing self': MHC molecules and NK cell recognition.Immunol Today 11: 237-244.
- Watzl C(2003) The NKG2D receptor and its ligands-recognition beyond the "missing self"?Microbes Infect 5: 31-37.
- DiefenbachA , Jensen ER, Jamieson AM, Raulet DH (2001) Raeand H60 ligands of the NKG2D receptor stimulate tumour immunity.Nature 413: 165-171.
- Lanier LL, Corliss B, Phillips JH (1997) Arousal and inhibition of human NK cells.Immunol Rev 155: 145-154.
- Groh V, Rhinehart R, Randolph-Habecker J, Topp MS, Riddell SR, et al. (2001) Costimulation of CD8alphabeta T cells by NKG2D via engagement by MIC induced on virus-infected cells.Nat Immunol 2: 255-260.
- Taveirne S (2011) Inhibitory receptors specific for MHC class I educate murine NK cells but not CD8alphaalpha intestinal intraepithelial T lymphocytes. Blood 118: 339-347.
- Moretta A, Bottino C, Vitale M, Pende D, Biassoni R, et al. (1996) Receptors for HLA class-I molecules in human natural killer cells.Annu Rev Immunol 14: 619-648.
- Moretta A (2002) Natural killer cells and dendritic cells: rendezvous in abused tissues.Nat Rev Immunol 2: 957-964.
- Zappacosta F, Borrego F, Brooks AG, Parker KC, Coligan, JE (1997) Peptides isolated from HLA-Cw*0304 confer different degrees of protection from natural killer cell-mediated lysis. Proceedings of the National Academy of Sciences of the United States of America 94, 6313-6318.
- Storkus WJ, Salter RD, Cresswell P, Dawson JR (1992) Peptide-induced modulation of target cell sensitivity to natural killing.J Immunol 149: 1185-1190.
- Storkus WJ, Salter RD, Alexander J, Ward FE, Ruiz RE, et al. (1991) Class I-induced resistance to natural killing: identification of nonpermissive residues in HLA-A2.ProcNatlAcadSci U S A 88: 5989-5992.
- Petersen JL, Morris CR, Solheim JC (2003) Virus evasion of MHC class I molecule presentation.J Immunol 171: 4473-4478.
- Harel-Bellan A, Quillet A, Marchiol C, DeMars R, Tursz T, et al. (1986) Natural killer susceptibility of human cells may be regulated by genes in the HLA region on chromosome 6.ProcNatlAcadSci U S A 83: 5688-5692.
- Storkus WJ, Howell DN, Salter RD, Dawson JR, Cresswell P (1987) NK susceptibility varies inversely with target cell class I HLA antigen expression.J Immunol 138: 1657-1659.
- StorkusWJ, Alexander J, Payne JA, Dawson JR, Cresswell P (1989) Reversal of natural killing susceptibility in target cells expressing transfected class I HLA genes. Proceedings of the National Academy of Sciences of the United States of America 86, 2361-2364.
- Malnati MS, Peruzzi M, Parker KC, Biddison WE, Ciccone E, et al. (1995) Peptide specificity in the recognition of MHC class I by natural killer cell clones.Science 267: 1016-1018.
- Moretta A, Pende D, Locatelli F, Moretta L (2009) Activating and inhibitory killer immunoglobulin-like receptors (KIR) in haploidenticalhaemopoietic stem cell transplantation to cure high-risk leukaemias. Clinical and experimental immunology 157: 325-33.
- Moretta A, Sivori S, Vitale M, Pende D, Morelli L, et al. (1995) Existence of both inhibitory (p58) and activatory (p50) receptors for HLA-C molecules in human natural killer cells.J Exp Med 182: 875-884.
- Biassoni R, Cantoni C, Falco M, Verdiani S, Bottino C, et al. (1996) The human leukocyte antigen (HLA)-C-specific "activatory" or "inhibitory" natural killer cell receptors display highly homologous extracellular domains but differ in their transmembrane and intracytoplasmic portions.J Exp Med 183: 645-650.
- Colonna M, Samaridis J (1995) Cloning of immunoglobulin-superfamily members associated with HLA-C and HLA-B recognition by human natural killer cells.Science 268: 405-408.
- Long EO(2008) Negative signaling by inhibitory receptors: the NK cell paradigm.Immunol Rev 224: 70-84.
- Geluk A, van Meijgaarden KE, Ottenhoff TH (1997) Flexibility in T-cell receptor ligand repertoires depends on MHC and T-cell receptor clonotype.Immunology 90: 370-375.
- Boyington JC, Sun PD (2002) A structural perspective on MHC class I recognition by killer cell immunoglobulin-like receptors.MolImmunol 38: 1007-1021.
- Parham P (2005) MHC class I molecules and KIRs in human history, health and survival.Nat Rev Immunol 5: 201-214.
- Peruzzi M, Parker KC, Long EO, Malnati MS (1996) Peptide sequence requirements for the recognition of HLA-B*2705 by specific natural killer cells.J Immunol 157: 3350-3356.
- Rajagopalan S,LongEO (1997)The direct binding of a p58 killer cell inhibitory receptor to human histocompatibility leukocyte antigen (HLA)-Cw4 exhibits peptide selectivity. The Journal of experimental medicine 185:1523-1528.
- Boyington JC, Motyka SA, Schuck P, Brooks AG, Sun PD (2000) Crystal structure of an NK cell immunoglobulin-like receptor in complex with its class I MHC ligand.Nature 405: 537-543.
- Cassidy SA, CheentKS ,Khakoo SI2 (2014) Effects of Peptide on NK cell-mediated MHC I recognition.Front Immunol 5: 133.
- Maenaka K (1999) Killer cell immunoglobulin receptors and T cell receptors bind peptide-major histocompatibility complex class I with distinct thermodynamic and kinetic properties. The Journal of biological chemistry 274: 28329-28334.
- Fadda L, Borhis G, Ahmed P, Cheent K, Pageon SV, et al. (2010) Peptide antagonism as a mechanism for NK cell activation.ProcNatlAcadSci U S A 107: 10160-10165.
- Rajagopalan S, Long EO (2010) Antagonizing inhibition gets NK cells going.ProcNatlAcadSci U S A 107: 10333-10334.
- Barber LD, Percival L, Valiante NM, Chen L, Lee C, et al. (1996) The inter-locus recombinant HLA-B*460has high selectivity in peptide binding and functions characteristic of HLA-C.J Exp Med 184: 735-740.
- Mandelboim O (1996) Protection from lysis by natural killer cells of groupand 2 specificity is mediated by residue 80 in human histocompatibility leukocyte antigen C alleles and also occurs with empty major histocompatibility complex molecules. The Journal of experimental medicine 184:913-922.
- Adams EJ (2007) Structural elucidation of the m157 mouse cytomegalovirus ligand for Ly49 natural killer cell receptors. Proceedings of the National Academy of Sciences of the United States of America 104: 10128-10133.
- Berry R,Rossjohn J, Brooks AG3 (2014) The Ly49 natural killer cell receptors: a versatile tool for viral self-discrimination.Immunol Cell Biol 92: 214-220.
- Correa I, Raulet DH (1995) Binding of diverse peptides to MHC class I molecules inhibits target cell lysis by activated natural killer cells.Immunity 2: 61-71.
- Roth C, Kourilsky P, Ojcius DM (1994) Ly-49-independent inhibition of natural killer cell-mediated cytotoxicity by a soluble major histocompatibility complex class I molecule. European journal of immunology 24: 2110-2114.
- Parham P, Norman PJ, Abi-Rached L,Guethlein LA (2012) Human-specific evolution of killer cell immunoglobulin-like receptor recognition of major histocompatibility complex class I molecules. Philosophical transactions of the Royal Society of London. Series B, Biological sciences 367: 800-881.







