Aamir Shahzad1, 2*, Randall J Cohrs1
1Department of Neurology, University of Colorado Denver, Anschutz Medical Campus, Aurora, CO, USA.
2Department of Structural and Computational Biology, University of Vienna, Vienna, Austria
- *Corresponding Author:
- Aamir Shahzad
Department of Neurology, University of Colorado Denver, Anschutz Medical Campus, Aurora, CO, USA
E-mail: aamir.shahzad@univie.ac.at
Objectives: The aim of this study was to determine the in vitro anti-viral effectof honey on varicella zoster virus.
Methods: Manuka and clover honeys were used at concentrations ranging from0-6% wt/vol. A clinical VZV isolate was obtained from a zoster vesicle and used atlow passage. Various concentrations of manuka and clover honey were added to thetissue culture medium of VZV-infected human malignant melanoma (MeWo) cells.
Results: Both types of honey showed antiviral activity against varicella zoster viruswith an approximate EC50 = 4.5 % (wt/vol).
Conclusions: Our results showed that honey has significant in vitro anti-VZVactivity. As, honey is convenient for skin application, is readily available and inexpensive,honey may be an excellent remedy to treat zoster rash in developingcountries where antiviral drugs are expensive or not easily available.
Keywords
Varicella Zoster, Shingles, Zoster, Honey, Antivirals, Translational Medicine.
Introduction
Herpes zoster (shingles) is the reactivation of varicella zoster virus from latency in cranial, autonomic and dorsal root ganglia and characterized by rash and severe pain in affected dermatomes. [1] Zoster is a disease affecting more than one million people per year in United States. [2] Zoster is especially common in developing poor countries possibly due to malnutrition, chronic diseases, and ineffective immunization programs. Patients affected by zoster in poor countries are more prone to develop complications such as postherpetic neuralgia (PHN), herpes zoster ophthalmicus, vasculitis, meningitis, and myelitis. [3] Antiviral VZV therapeutic agents effective for treating VZV reactivation such as acyclovir, famcyclovir and valacyclovir are very expensive in developing countries and are not readily available. There is great need for a remedy which is inexpensive and easily available in developing countries.
Since antiquity, honey has been used to treat many diseases. [4-8] Currently, honey has been shown to have excellent antibacterial activity for many wound pathogens. [9, 10] Honey has excellent antibacterial activity against Methicillin-resistant Staphylococcus aureus (MRSA) and various species of pseudomonas commonly associated with wound and burn infections. [11-14]Honey dressings are used commonly to manage skin and burn wounds infections. [15]Honeys also possess antifungal activity. [16] Honey has antiviral activity against Rubella virus [17], and honey is used topically to treat recurrent herpes simplex lesions. [18]In this study we screened two types of honey for antiviral activity against a clinical isolate of varicella zoster virus.
Materials & Method
Cell culture
Human malignant melanoma cells (MeWo) were grown at 37oC and 5% CO2 in Dulbecco’s minimal essential medium supplemented with 10% fetal bovine serum (DMEM, Life Technologies, Carlsbad CA). The MeWo cell line was established from a skin biopsy of malignant melanoma, and is the standard cell line used to propagate VZV.[19]
Virus stock
Varicella zoster virus (VZV) obtained from a zoster vesicle and shown to be of the European strain was used before the 20th in vitro passage [20] Since VZV is highly cell-associated, virus was propagated by co-cultivation of infected cells with uninfected cells at ratios ranging from 1:4 to 1:100 depending on use. VZV stocks were prepared using infection ratios of 1:100 and dose response experiments were performed at infection ratios of 1:4. [21].
Treatment with honey
Commercially obtained pure clover and Manuka honey were diluted to a final concentration ranging from 0% to 6% (wt/ vol) in culture medium and filter sterilized.
Cell viability assay
Cell viability in various honey concentrations was determined by neutral red uptake assay. [22] Briefly, after 3 days in culture MeWo cells were incubated for 3 hrs with 40 ug/ml neutral red (Sigma, St. Louis, MO) in DMEM, fixed in 0.5% formaldehyde, 1% CaCl2 for 1 min, dissolved in 50% ethanol, 1% acetic acid for 5 min, and optical density at 560 nm determined on triplicate samples.
Infectivity assay
MeWo cells infected with VZV and uninfected MeWo cells were incubated in culture medium containing various honey concentrations. After plaque development (3 days post-infection) cultures were fixed in 4% formaldehyde, permeabilized for 10 minutes in methanol/acetone (50:50; v/v) and extensively washed with Tris-buffered saline (TBS; 20 mM Tris-HCl, pH 8.0, 150 mM NaCl). VZV plaques were identified by immunostaining. Briefly, monolayers were blocked for 60 minutes in 3% blocked for 60 minutes in 3% BSA in TBS, incubated for 60 minutes with primary antibody (rabbit anti- IE63: 1:1,000 dilution) [23] followed by 60 minutes incubation with alkaline phosphatase conjugated secondary antibody (goat anti-rabbit IgG; 1:10,000 dilution; Abcam, Cambridge, MA). Immunostaining was visualized with NBT/BCIP (Pierce, Rockford IL). Between all incubations, the cultures underwent extensive washing in TBS. Plaques were counted with the aid of a dissecting microscope (4 x magnifications).
Results & Discussion
Both clover and Manuka honey are readily soluble in water, and at low concentrations inhibit neutral red uptake to equal amounts. MeWo cells in both honey concentrations < 3.75% showed equal survival slopes (Figure 1). This is most likely due to nonspecific osmotic actions of the sugars. Both types of honey have concentration dependent in vitro anti-VZV activity with half maximal effective concentration (EC50) approximately 4.5% (wt/vol) (Figure 2). Manuka honey showed a slightly less EC50 as compared to clover honey. At the EC50 concentration, cells remained viable. Higher honey concentrations resulted in significant reduction in VZV plaque size. (Figure 3)
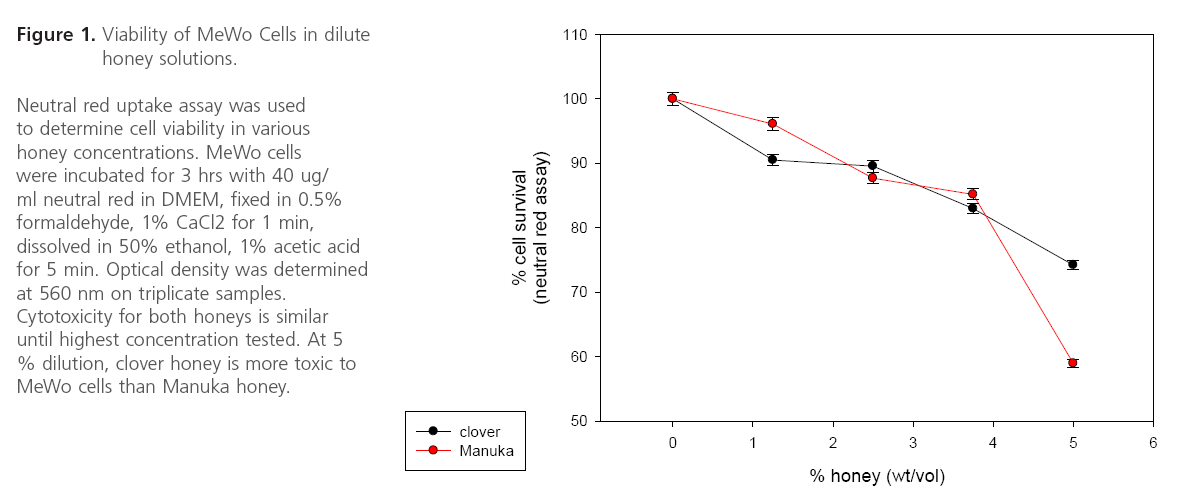
Figure 1: Viability of MeWo Cells in dilute honey solutions.
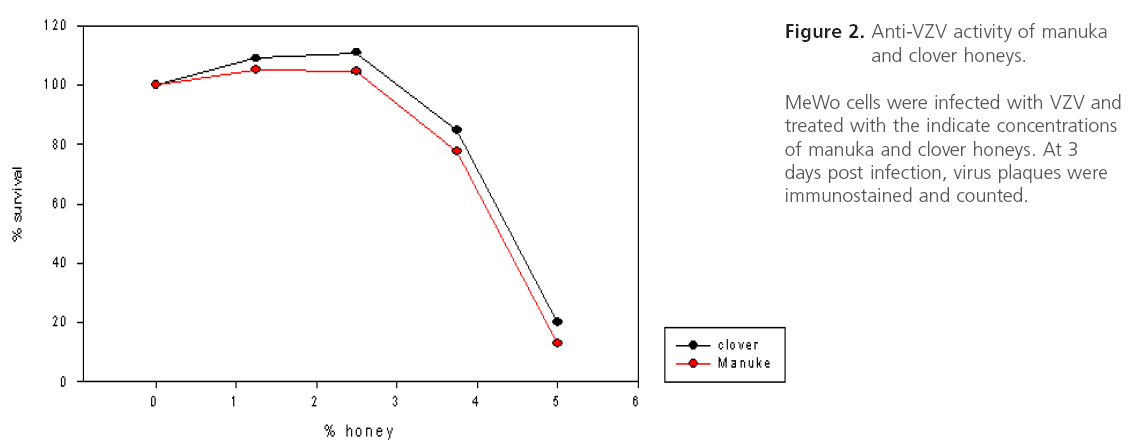
Figure 2: Anti-VZV activity of manuka and clover honeys.
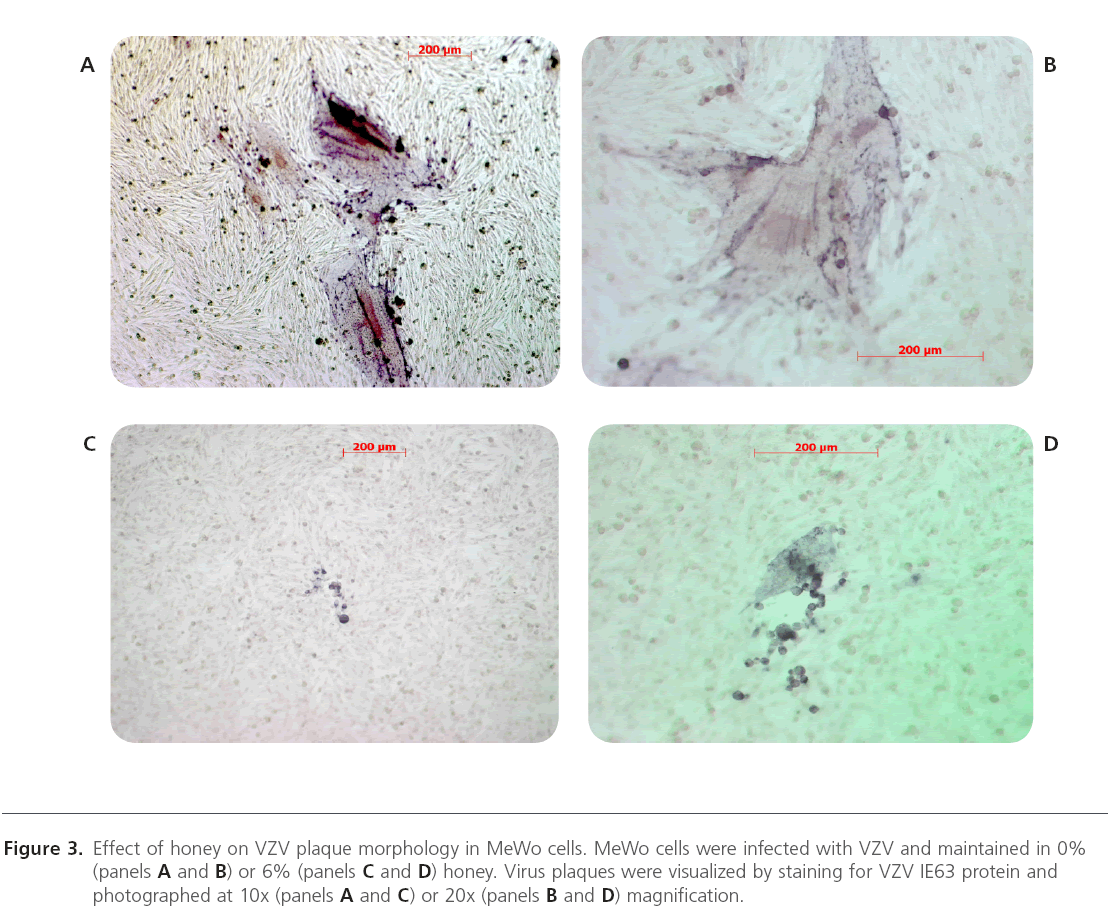
Figure 3: Effect of honey on VZV plaque morphology in MeWo cells. MeWo cells were infected with VZV and maintained in 0% (panels A and B) or 6% (panels C and D) honey. Virus plaques were visualized by staining for VZV IE63 protein and photographed at 10x (panels A and C) or 20x (panels B and D) magnification.
For centuries, honey has been used in traditional medicine. In recent past, honey has gain significant attention from the scientific community to explore its potential applications to treat various clinical conditions. Honey has wide range of therapeutic properties including anti-inflammatory, antibacterila, antifungal and antineoplastic activity. [24, 25] Our results suggest the presence in honey of compounds possessing anti- VZV activity, the identity of which is yet to be determined. Honey is convenient for application on skin, readily available and inexpensive, and potentially an excellent remedy to treat zoster rash in developing countries where antiviral drugs are expensive or not easily available.
Acknowledgements
This work was supported in part by Public Health Service grant AG032958 (R.J.C.) from the National Institutes of Health (NIH).
2493
References
- Kleinschmidt-DeMasters BK, Gilden DH: Varicella-Zoster virus infections of the nervous system: clinical and pathologic correlates. Arch Pathol Lab Med 2001, 125(6):770-780.
- Lua PJ, Euler GL, Jumaan AO, Harpaz R: Herpes zoster vaccination among adults aged 60 years or older in the United States, 2007: Uptake of the first new vaccine to target seniors. Vaccine 2009, 27(6):882-887.
- Weinberg JM: Herpes zoster: epidemiology, natural history, and common complications. J Am Acad Dermatol 2007, 57(6 Suppl):S130-135.
- Mcinerney RJF: Honey - a Remedy Rediscovered. J Roy Soc Med 1990, 83(2):127-127.
- Chiba M, Idobata K, Kobayashi N, Sato Y, Muramatsu Y: [Use of honey to ease the pain of stomatitis during radiotherapy]. Kangogaku Zasshi 1985, 49(2):171-176.
- English HKP, Pack ARC, Molan PC: The effects of manuka honey on plaque and gingivitis: a pilot study. J Int Acad Periodontol 2004, 6(2):63-67.
- Molan PC: Why honey is effective as a medicine. I. Its use in modern medicine. Bee World 1999, 80(2):80-92.
- Molan P: Why honey is effective as a medicine - 2. The scientific explanation of its effects. Bee World 2001, 82(1):22-40.
- Al-Waili NS: Investigating the antimicrobial activity of natural honey and its effects on the pathogenic bacterial infections of surgical wounds and conjunctiva. J Med Food 2004, 7(2):210-222.
- Molan PC: The role of honey in the management of wounds. J Wound Care 1999, 8(8):415-418.
- Hazrati M, Mehrabani D, Japoni A, Montasery H, Azarpira N, Hamidian-shirazi AR, Tanideh N: Effect of Honey on Healing of Pseudomonas aeruginosa Infected Burn Wounds in Rat. J Appl Anim Res 2010, 37(2):161-165.
- Lusby PE, Coombes AL, Wilkinson JM: Bactericidal activity of different honeys against pathogenic bacteria. Arch Med Res 2005, 36(5):464- 467.
- Nejabat M, Astaneh A, Eghtedari M, Mosallaei M, Ashraf MJ, Mehrabani D: Effect of Honey in Pseudomonas aeruginosa Induced Stromal Keratitis in Rabbits. J Appl Anim Res 2009, 35(2):101-104.
- Maeda Y, Loughrey A, Earle JA, Millar BC, Rao JR, Kearns A, McConville O, Goldsmith CE, Rooney PJ, Dooley JS et al: Antibacterial activity of honey against community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA). Complement Ther Clin Pract 2008,
- 14(2):77-82. 15. Dunford C, Cooper R, Molan P: Using honey as a dressing for infected skin lesions. Nurs Times 2000, 96(14 Suppl):7-9.
- Irish J, Carter DA, Shokohi T, Blair SE: Honey has an antifungal effect against Candida species. Med Mycol 2006, 44(3):289-291.
- Zeina B, Othman O, al-Assad S: Effect of honey versus thyme on Rubella virus survival in vitro. J Altern Complement Med 1996, 2(3):345-348.
- Al-Waili NS: Topical honey application vs. acyclovir for the treatment of recurrent herpes simplex lesions. Med Sci Monit 2004, 10(8):MT94- 98.
- Grose C, Brunel PA: Varicella-zoster virus: isolation and propagation in human melanoma cells at 36 and 32 degrees C. Infect Immun 1978, 19(1):199-203.
- Cohrs RJ, Barbour MB, Mahalingam R, Wellish M, Gilden DH: Varicella-zoster virus (VZV) transcription during latency in human ganglia: prevalence of VZV gene 21 transcripts in latently infected human ganglia. J Virol 1995, 69(4):2674-2678.
- Cohrs RJ, Barbour M, Gilden DH: Varicella-zoster virus gene 21: transcriptional start site and promoter region. J Virol 1998, 72(1):42- 47.
- Repetto G, del Peso A, Zurita JL: Neutral red uptake assay for the estimation of cell viability/cytotoxicity. Nat Protoc 2008, 3(7):1125- 1131.
- Mueller NH, Bos NL, Seitz S, Wellish M, Mahalingam R, Gilden D, Cohrs RJ: Recombinant Monoclonal Antibody Recognizes a Unique Epitope on Varicella-Zoster Virus Immediate-Early 63 Protein. J Virol 2012.
- Swellam T, Miyanaga N, Onozawa M, Hattori K, Kawai K, Shimazui T, Akaza H: Antineoplastic activity of honey in an experimental bladder cancer implantation model: in vivo and in vitro studies. Int J Urol 2003, 10(4):213-219.
- Jaganathan SK, Mandal M: Antiproliferative effects of honey and of its polyphenols: a review. J Biomed Biotechnol 2009, 2009:830616.








