Keywords
SARS-CoV-2; MERS; ACE2; CK; Rhabdomyolysis; Myositis
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a novel beta-coronavirus that causes a variety of neurologic symptoms including anosmia, ageusia, seizures, stroke, confusion, encephalopathy and quadriplegia [1,2]. Up to 20% of COVID-19 patients who require intensive care unit admission are due to their neurological deficits [3]. SARS-CoV-2 virus have a high similarity to SARS-Cov1 and MERS, appears to have the capacity to injure the central and peripheral nervous systems through direct and indirect ways [4,5]. The binding of SARS-CoV-2 to angiotensin converting enzyme 2 (ACE2) receptor is a critical step in the pathophysiology [6]. The function of ACE2 in normal human physiology is to regulate blood pressure via inhibition of the angiotensin- renin-aldosterone pathways. ACE2 facilitates conversion of angiotensin II to angiotensin (1-7) [7]. Higher levels of angiotensin II are associated with vasoconstriction, kidney failure, heart disease, apoptosis, and oxidative processes [8]. This enzyme protein in the cell membranes of multiple organs to serve as the receptor for SARS-CoV-2 [9]. ACE2 has a wide distribution in multiple organs including the nose, lungs, kidneys, liver, blood vessels, immune system, skeletal muscles and brain [9]. After binding ACE2 in respiratory epithelial cells and then epithelial cells in blood vessels, SARS-CoV-2 triggers the formation of a cytokine storm [10]. High levels of these cytokines increase vascular permeability, edema, and widespread inflammation with consequent damage in multiple organs [11]. The cytokine storm also triggers hypercoagulation cascades. Combined hyperactivation of inflammatory markers, vascular injury, and coagulation factors contributes to ARDS, kidney failure, liver injury, heart failure, myocardial infarction as well as multiple neurological conditions. The cytokines activated by SARS-CoV-2 can also trigger vasculitis in and around nerves and muscles, with or without a molecular mimicry [12]. A direct invasion by the virus to the muscles can potentially occur [13]. Muscle injury and high levels of creatine kinase (CK) in COVID-19 patients in ICU can be attributed to critical care myopathy. The early time course of severe muscle weakness in COVID-19 patients may suggestive of myositis [14]. With regards to cardiac muscle, there is evidence that both myocarditis due to SARS-CoV-2 as well as myocardial infarction due to cytokine storm, hypercoagulability, and ischemia can happen at the same time [15] and reported that 19.3% of patients with severe COVID-19 had evidence of marked muscle injury [2].
Viral myositis is often self-limited but can be complicated by rhabdomyolysis [16]. Viral myositis and rhabdomyolysis are most commonly caused by influenza A and B. Approximately 3% of patients with influenza-induced myositis developed rhabdomyolysis per literature review of 300 cases [17,18]. In a study by Guan et al. 13.7% of patients with COVID-19 had CK levels above 200 U/L, but the CK levels did not correlate with the disease severity [19]. Coronavirus infections may be associated with myopathies. In published studies of COVID-19 in China, myalgia or fatigue affected 44-70% of hospitalized patients and increased creatine kinase (CK) was present in up to 33% of admitted patients [20]. As many as a third of patients infected with other coronavirus infections manifested with myalgias and elevated CKs and rhabdomyolysis [21,22]. This suggests that coronavirus infections may cause a viral myositis [23,24]. SARS-CoV, was not detected in skeletal muscle by postmortem examination [25,26]. Exact mechanism of skeletal muscle injury in COVID 19 patients are not fully recognize. Direct SARSCoV- 2 viral invasion of skeletal muscle through ACE2 receptor, hematogenous dissemination and immune-mediated skeletal muscles injury secondary to activation of immune cells and inflammatory response with cytokine storm syndrome may be possible mechanism of skeletal muscle injury, could lead to including muscle fiber proteolysis and fibrosis [27]. The available data at present suggest that COVID-19 associated muscle injury is most likely para-infectious and or immune-mediated and can manifest as dermatomyositis-like interferonopathy-1, myositis, or immune-mediated necrotizing myopathy [28]. Direct SARSCoV- 2 viral invasion of diaphragm muscle may contribute to diaphragm dysfunction and lead to difficulty to weaning from mechanical ventilation and respiratory failure [29].
Research Methodology
This is retrospective observational cross-sectional study. This study was done at Hamad medical cooperation (HMC), Doha, Qatar. There are designated hospitals assigned by the government to treat patients with COVID-19 with Protocol ID # MRC-01-20-523. We retrospectively analyzed 413 patients from January, 1, 2020, to January, 31, 2021, who had been diagnosed as having COVID-19. A confirmed case of COVID-19 was defined as a positive result on real-time reverse transcription polymerase chain reaction (RT-PCR) analysis of both nasopharyngeal and oropharyngeal specimens from patients were tested simultaneously. Radiologic assessments included chest x- rays and chest CT, EMG/NCS studies and all laboratory testing (CBC, CMP, PT/APPT/INR, RFT, LFT, CRP, Ferritin, LDH, D-dimer, IL-6, lactic acid, CPK, myoglobin and lumbar puncture) was performed according to the clinical care and standard clinical practice needs of the patient. Study was approved and written informed consent was waived by the Ethics Committee of HMC (MRC-01-20-523), Doha, Qatar, on June, 16, 2020, owing to the rapid emergence of the disease and the urgent need to collect data. We reviewed electronic medical records, laboratory findings, and radiologic examinations for all patients with laboratory-confirmed SARSCoV- 2 infection and collected data on age, gender, comorbidities (hypertension, diabetes, cardiac or cerebrovascular disease, malignancy, and chronic kidney disease etc.), typical symptoms from onset to hospital admission (fever, cough, anorexia, myalgia etc.), nervous system symptoms, laboratory findings and chest CT scan if available. All neurologic manifestations were reviewed and confirmed by 2 trained neurologists. Skeletal muscle injury was defined as when a patient had skeletal muscle pain and elevated serum creatine kinase level greater than 300 U/L or myoglobin >72 ng/ml and rhabdomyolysis was defined as when patients had elevated serum creatine kinase level greater than 1000 U/L. The primary objective to determine the incidence of skeletal muscle injury in COVID-19 patients and secondary objective to determine the association of skeletal muscles injury with acute myocardial injury and acute kidney injury in COVID-19 patients in tertiary care Hospitals in Qatar. The incidence of skeletal muscle injury in COVID-19 patients and to determine the association of acute myocardial injury and acute kidney injury with skeletal muscle injury in COVID-19 patients estimated and presented with 95% CI. Descriptive statistics used to summarize and determine the sample characteristics and distribution of various considered parameters related to demographic, diagnostic, clinical features, follow-up outcome measures and other related features of such patients. The normally distributed data and results reported with mean and standard deviation (SD) with corresponding 95% confidence interval (CI); the remaining results reported with median and interquartile range (IQR). Categorical data summarized using frequencies and percentages. Associations between two or more qualitative variables examined and assessed using Pearson Chi-square and Fisher Exact tests as appropriate. Unpaired t and ANOVA used to compare mean values of different quantitative parameters between two or more than two groups. Correlation between various outcomes measured evaluated quantitatively calculated by Pearson or Spearman rank-order correlation. An appropriate regression analysis performed to assess the effects of various factors and covariates on primary outcome measures. Pictorial presentations of the key results made using appropriate statistical graphs. A two-sided P value <0.05 statistically significant. All statistical analyses done using statistical packages SPSS 24.0 (SPSS Inc. Chicago, IL) and Epi Info 2000 (Centers for Disease Control and Prevention, Atlanta, GA).
Results
A total of 413 hospitalized patients with confirmed SARS CoV- 2 infection were included in this study. Their demographic in Table 1 and clinical characteristics are shown in Table 2. Their Age mean (SD-14) was 52 (range from 22 to 86 year) years, and 94% were men (389) and 6% were female (25). 57% were less than 55 years age, 43% were greater than 55 years age and 5.6% were greater than 75 years of total patients. Different nationality with majority were 20% Indian (84), 12.5% Bangladeshi (52), 10% Qatari (42), 9% Nepali (38) and 8.7% (36) Filipino and Pakistanis. Of these all patients, following underlying risk factors including 47.6% hypertension (197), 46.9% diabetes (194), 21% obesity (88), 10% newly diagnosed (42), 10% chronic kidney disease (43), 9.7% ischemic heart disease (40), 6.8% smoker (28), 3.6% asthma (15) and 2.91% malignancy (12). These are presented in Table 3. Neurologic manifestations were categorized into three major categories: Central nervous system (CNS), peripheral nervous system (PNS) and skeletal muscles. Skeletal muscle injury was 47.8% and rhabdomyolysis was 15.7% (Figures 1-5). CNS manifestations were 26.7%, PNS were 4.3%. 48.5% patients (201) had nervous system symptoms: the most common neurological symptoms at onset of illness including myalgia were 28% shown in bar chart (116), headache was 10.4% (43), dizziness was 5.8% (24), anosmia was 2.9% (12) and dysgeusia were 1.4% (6). In patients with skeletal muscle injury the most common reported symptoms including were 28% myalgia. 29% (120) patients admitted in MICU were intubated and ventilated while 16.2% (67) patients had noninvasive ventilation. 8.5% (35) patients died in MICU with COVID complication and septic shocks. 66.7% (276) patients received steroids, 21.5% (89) took convalescent plasma. Multiorgan systems involvement of COVID-19 patients (Figures 6-9) including 47.8% (198) had skeletal muscle injury (serum creatine kinase >300 U/L or myoglobin >72 ng/ml), 15.7% (65) had rhabdomyolysis (serum creatine kinase >1000U/L), 36.7% (152) had acute kidney injury, 27.5% (114) had acute liver injury (>200 ALT or AST levels), 23.9% (99) acute myocardial injury (high tropT hs, male>30, female>20ng/l), 11.4% (47) had acute heart failure (high proBNP >1800pg/ml), 11.1% (46) had acute pancreatitis (serum lipase >180 u/l), 5.8% (24) had previous uncontrolled blood sugar (with HbA1c>12) and 3.1% (13) had post ICU critical illness neuromyopathy supported by clinical and NCS/EMG study. 11% (46) had AKI associated with rhabdomyolysis, 9.9% (41) had cytokine storm syndrome associated with rhabdomyolysis, 9.2% (38) had acute liver injury associated with rhabdomyolysis, 6.7% (28) had acute myocardial injury associated with rhabdomyolysis. Table 4 showed the laboratory, Echocardiography and Nerve conduction study / Electromyographic findings. Laboratory features associated with severe COVID-19 including 39.4% (163) had > 300 u/L serum creatine kinase, 35.7% (148) had serum myoglobin >72 ng/ml, 15.7% (65) had rhabdomyolysis (serum creatine kinase >1000 U/L), 71% (295) had >500 mcg/L ferritin level, 66.2% (274) had LDH >245 units/L, 61% (253) had >100 mg/l C-reactive protein levels, 53.9% (223) had >1000 ng/mL D-dimer, 37% (153) had >70 pg/m of IL-6, 22.2% (90) had >2 ng/ ml procalcitonin, 6.3% (26) had >4 mmol/l lactic acid. 7.7% (32) patients had echo showed 3.4% (14) were mild low EF, 1.7% (7) were severe low EF, 0.5% (2) were moderate low EF. 2.7% (11) patients had NCS/EMG studies showed 1.7% (7) patients were CINM and 0.7% (3) patients were CIM.
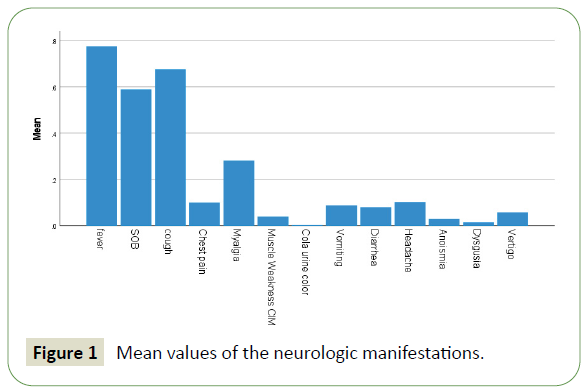
Figure 1: Mean values of the neurologic manifestations.
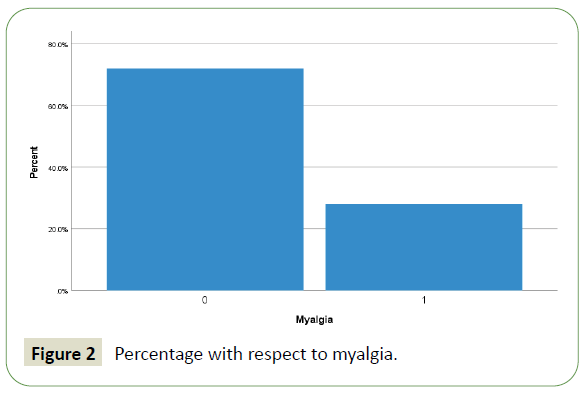
Figure 2: Percentage with respect to myalgia.
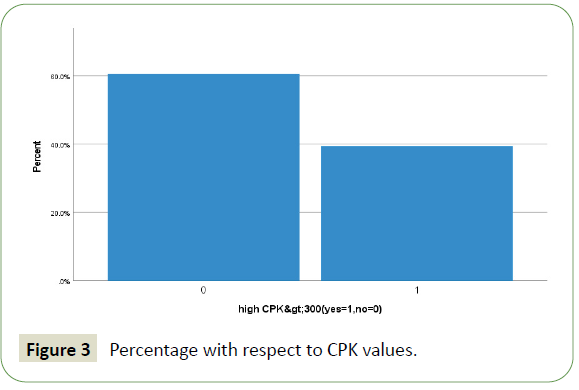
Figure 3: Percentage with respect to CPK values.
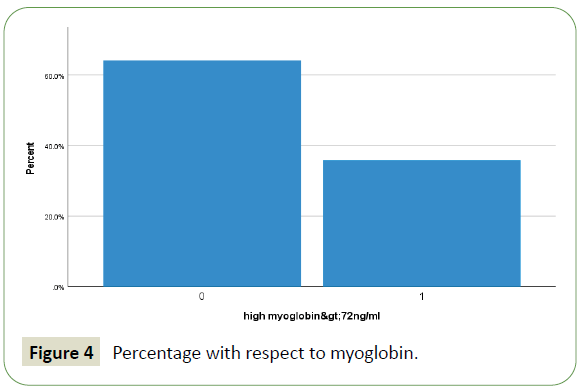
Figure 4: Percentage with respect to myoglobin.
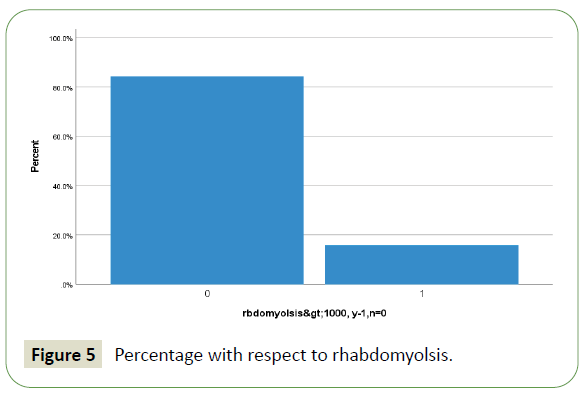
Figure 5: Percentage with respect to rhabdomyolsis.
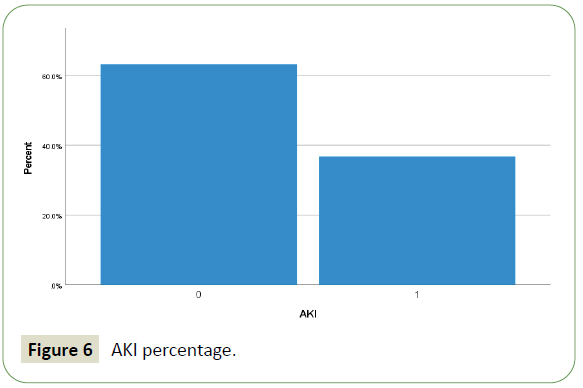
Figure 6: AKI percentage.
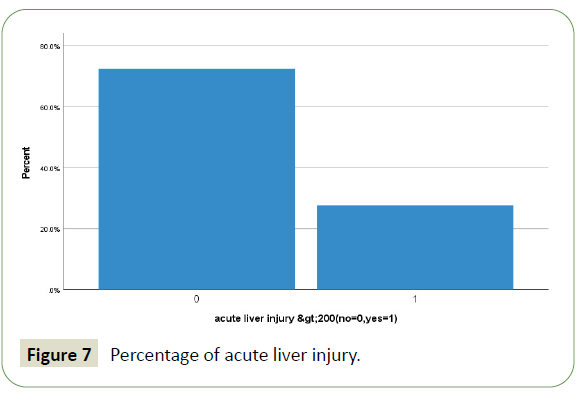
Figure 7: Percentage of acute liver injury.
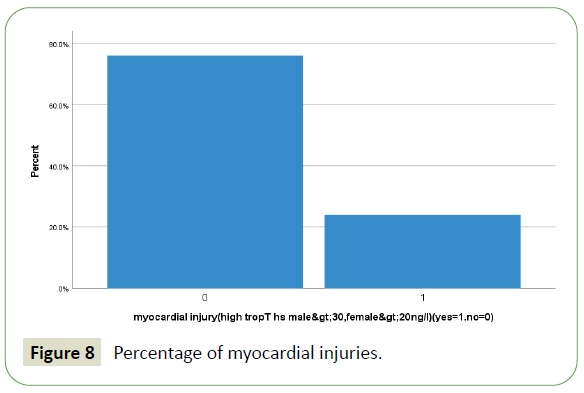
Figure 8: Percentage of myocardial injuries.
Figure 9: Percentage of acute pancreatitis.
| Age (Years) |
| Variables |
Frequency |
Percentage (%) |
| <54 |
234 |
56.7% |
| 55-64 |
107 |
25.9 |
| 65-74 |
49 |
11.9 |
| 75-84 |
21 |
5.1 |
| >85 |
2 |
0.5 |
| Gender |
| Male |
389 |
94.0% |
| Female |
23 |
6.0 |
| Nationality |
| Afghan |
3 |
0.7 |
| American |
1 |
0.2 |
| Bangladesh |
52 |
12.5 |
| Cameron |
1 |
0.2 |
| Egyptian |
29 |
7.0 |
| Filipino |
36 |
8.7 |
| Greek |
1 |
0.2 |
| Indian |
84 |
20.3 |
| Indonesian |
2 |
0.5 |
| Iranian |
11 |
2.7 |
| Iraqi |
2 |
0.5 |
| Jordanian |
8 |
1.9 |
| Kenyan |
2 |
0.5 |
| Malaysian |
1 |
0.2 |
| Moroccan |
3 |
0.7 |
| Nepalese |
38 |
9.2 |
| Nigerian |
1 |
0.2 |
| Pakistani |
36 |
8.7 |
| Palestinian |
12 |
2.9 |
| Qatari |
42 |
10.1 |
| Somali |
1 |
0.2 |
| Sri Lankan |
12 |
2.9 |
| Sudanese |
13 |
3.1 |
| Syrian |
14 |
3.3 |
| Thai |
2 |
0.5 |
| Tunisian |
1 |
0.2 |
| Turkish |
1 |
0.2 |
| Yemeni |
5 |
1.2 |
Table 1: Demographics.
| Symptoms |
| Variables |
Frequency |
Percentage (%) |
| Fever |
321 |
77.5 |
| Shortness of breath |
243 |
58.7 |
| Cough |
279 |
67.4 |
| Chest pain |
41 |
9.9 |
| Myalgia |
116 |
28.0 |
| Muscle Weakness |
16 |
3.9 |
| Cola urine colour |
1 |
0.2 |
| Headache |
43 |
10.4 |
| Anosmia |
12 |
2.9 |
| Dysuria |
6 |
1.4 |
| Vertigo |
24 |
5.8 |
Table 2: Clinical manifestations.
| Underlying risk factors |
| |
Frequency |
Percentage (%) |
| Hypertension |
197 |
47.6 |
| Diabetes |
194 |
46.9 |
| Obesity |
88 |
21 |
| Newly diagnosed |
42 |
10 |
| Chronic Kidney disease |
43 |
10 |
| Ischemic heart disease |
40 |
9.7 |
| Smoker |
28 |
6.8 |
| Asthma |
15 |
3.6 |
| Malignancy |
12 |
2.91 |
Table 3: Underlying risk factors.
| Laboratory features |
| Variables |
Frequency |
Percentage (%) |
| Serum CK>300u/L |
163 |
39.4 |
| Serum myoglobin >72 ng/dl |
148 |
35.7 |
| Serum CK>1000 U/L causing Rhabdomyolysis |
65 |
15.7 |
| Serum Ferritin>500mcg/L |
295 |
71 |
| LDH >245 units/L |
274 |
66.2 |
| C-reactive protein levels |
253 |
61 |
| D-Dimer >1000 ng/ml |
223 |
53.9 |
| IL6 >70 pg/ml |
153 |
37 |
| Procalcitonin > 2 ng/ml |
90 |
22.2 |
| Lactic acid > 4 mml/l |
26 |
6.3 |
| Echocardiography |
| Mildly low Ejection Fraction |
32 |
7.7 |
| Severely low Ejection Fraction |
7 |
1.7 |
| Moderately low Ejection Fraction |
11 |
2.7 |
| Nerve conduction/Electromyographic studies |
| CINM |
7 |
1.7 |
| CIM |
3 |
0.7 |
Table 4: Laboratory, echocardiography and nerve conduction study/electromyographic findings.
Discussion
This is the first report on skeletal muscles injury and rhabdomyolysis of the hospitalized patients with COVID-19 from Qatar. From 1, January, 2020 to 31, January, 2021, total 413 patients were included in this study. Majority of patient were less than 55 years age (57%) with different nationality. The most common risk factors were found Hypertension, Diabetics and Obesity. Total of 413 patients, 48.5% (201) had various neurologic symptoms that involved CNS, PNS, and skeletal muscles. Most common neurological symptoms including myalgia, headache and dizziness in order of descending frequency. The most common neurological involvement was skeletal muscle injury (47.8%). In addition to respiratory system, other extra-pulmonary multiorgan systems involvement of COVID patients including skeletal muscle injury (47.8%), acute kidney injury (36.7%), acute liver injury (27.5%), acute myocardial injury (23.9%), rhabdomyolysis (15.7%), acute heart failure (11.4%), acute pancreatitis (11.1%) and post ICU critical illness neuromyopathy (3.1%) that supported by clinical and NCS/ EMG study. Multiorgan system involvements association with rhabdomyolysis including AKI, cytokine storm syndrome, acute liver injury and acute myocardial injury in order of descending frequency. Exact mechanisms of skeletal muscle injury in COVID 19 patients are not fully recognize. Direct SARS-CoV-2 viral invasion of skeletal muscle including diaphragm through ACE2 receptor, hematogenous dissemination and immune-mediated skeletal muscles injury secondary to activation of immune cells and inflammatory response with cytokine storm syndrome may lead to pathological changes in skeletal muscle tissue including muscle fiber proteolysis and fibrosis. Dystrophic myopathies like Duchenne muscular dystrophy with severe COVID-19 infection are at higher risk of respiratory failure due to diaphragm dysfunction. Rhabdomyolysis have been reported in COVID-19 patients that can lead to acute kidney injury, compartment syndrome and disseminated intravascular coagulation.
Limitations
All data were retrieved from the electronic medical records retrospectively and mostly from dedicated COVID facility for male patients. During COVID 19 spike waves times and high admissions rate of patients infected with COVID-19, neuroimaging like CT scan or magnetic resonance imaging and diagnostic procedures such as EMG/NCS were limited to reduce the risk of cross infection. Therefore, most of neuromuscular symptoms of patients were subjective descriptions.
Conclusion
In conclusion, the most common neuromuscular manifestation of COVID-19 patients were skeletal muscle injury and followed by other multi-organ dysfunctions including renal, hepatobiliary, cardiac and venous thromboembolism. The patients with severe COVID-19 infection with concomitant dystrophic myopathies are at higher risk for severe skeletal muscle injury including diaphragm. Skeletal muscles injury with SARS-CoV-2 infection manifest as myalgia, generalized fatigue, myositis, rhabdomyolysis, dermatomyositis-like interferonopathy 1, immune-mediated necrotizing myopathy and diaphragmic or respiratory failure. Thus, we postulated that skeletal muscles injury may be peculiarly affected by SARS-CoV-2 via direct viral invasion through ACE2 receptor, immune-mediated cytokines and proinflammatory signaling molecules and hematogenous dissemination. Further studies are requiring with focus on long term outcomes of skeletal muscles and neuromuscular health of patients recovering from COVID-19 and benefits of using post COVID rehabilitation programs.
Ethical Approval
Protocol ID MRC-01-20-523 entitled “Incidence of skeletal muscle injury with SARS-CoV-2 infection in tertiary care hospitals in Qatar.” has been approved by MRC.
Conflict of Interest
There is no conflict of interest.
Funding Disclosure
Nil.
Author’s Contributions
Liaquat Ali: Data collection, Data analysis, Manuscript writing & Review literature.
Adnan Khan: Manuscript writing, Manuscript review.
Mohammad Alhatou; Manuscript writing & review literature.
Osama Elalamy: Data analysis, Manuscript review
Beatriz Canibano: Data analysis, Manuscript writing & review
Golam Adeli: Data analysis, Manuscript writing & review
Ahmed Mohammad: Data collection, data analysis
Khawaja H Haroon: Manuscript writing, manuscript review
Suhail H: Data analysis, manuscript writing
Suha Elmaki: Data analysis, manuscript writing
Yahya Imam: Data collection, data analysis
Ambreen Iqrar: Manuscript writing, manuscript review.
38739
References
- Coronavirus Disease (COVID-19) Naming the Coronavirus Disease and the virus that causes it.
- Mao L, Jin H, Wang M, Hu Y, Chen S, et al. (2020) Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol 77 (6): 683-690.
- Phua J, Weng L, Ling L, Egi M, Lim CM, et al. (2020) Intensive care management of coronavirus disease 2019 (COVID-19): Challenges and recommendations. Lancet Respir Med 5 (8): 506–517.
- Nath A (2020) Neurologic complications of coronavirus infections. Neurology 94 (19): 809-810.
- Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, et al. (2020) Genomic characterization and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet 395 (10224): 565–574.
- Verdecchia P, Cavallini C, Spanevello A, Angeli F (2020) The pivotal link between ACE2 deficiency and SARS-cov-2 infection. Eur J Intern Med 76 (17): 14-20.
- Kai H, Kai M (2020) Interactions of coronaviruses with ACE2, angiotensin II, and RAS inhibitors—lessons from available evidence and insights into COVID-19. Hypertens Res 43: 648-654.
- Vaduganathan M, Vardeny O, Michel T, Mc Murray JJV, Pfeffer MA, et al. (2020) Renin–angiotensin–aldosterone system inhibitors in patients with Covid-19. N Engl J Med 382: 1653–1659.
- Li MY, Li L, Zhang Y, Wang XS (2020) Expression of the SARS-cov-2 cell receptor gene ACE2 in a wide variety of human tissues. Infect Dis Poverty 9 (45).
- Mehta P, Mcauley DF, Brown M, Sanchez E, Tatter RS, (2020) COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 395 (102290): 1033–1034.
- Spiezia L, Boscolo A, Poletto F, Cerruti L, Tiberio I, et al. (2020) COVID-19-related severe hypercoagulability in patients admitted to intensive care unit for acute respiratory failure. Thromb Haemost 120 (6): 998-1000.
- Ang CW, Jacobs BC, Laman JD (2004) The Guillain-Barre syndrome: A true case of molecular mimicry. Trends Immunol 25 (2): 61–66.
- Toscano G, Palmerini F, Ravaglia S, Ruiz L, Invernizzi P, et al. (2020) Guillain–Barre Syndrome associated with SARS-cov-2. N Engl J Med 382: 2574-2576
- Guidon AC, Amato AA (2020) COVID-19 and neuromuscular disorders. Neurology 94 (22).
- Basu-Ray I, Soos MP, Almaddah NK, Adeboye A (2020) Cardiac manifestations of coronavirus (COVID-19). In statpearls.
- Agyeman P, Duppenthaler A, Heininger U, Aebi C (2005) Influenza-associated myositis in children. Infection. (2004) 32: 199-203.
- Pancheri E, Lanzafame M, Zamo A, Angheben A, Sartoriset S, et al. (2019) Benign acute viral myositis in African migrants: a clinical, serological, and pathological study. Muscle Nerve 60: 586-590.
- Chen LL, Hsu CW, Tian YC, Fang JT (2005) Rhabdomyolysis associated with acute renal failure in patients with severe acute respiratory syndrome. Int J Clin Pract 59:1162-1166.
- Guan WJ, Ni ZY, Hu Y (2020) Clinical characteristics of coronavirus disease 2019 in China . N Engl J Med 382: 1708-1720.
- Wang D, Hu B, Hu C, Zhu F, Liu X, et al. (2020) Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus infected pneumonia in Wuhan, China. JAMA Epub 323 (11): 1061-1069
- Fan CK, Yieh KM, Peng MY, Lin JC, Wang NC, et al. (2006) Clinical and laboratory features in the early stage of severe acute respiratory syndrome. J Microbiol Immunol Infect 3(1): 45- 53.
- Tsai LK, Hsieh ST, Chao CC, Chen YC, Lin YH, et al. (2004) Neuromuscular disorders in severe acute respiratory syndrome. Arch Neurol. 61 (11): 1669-1673.
- Leung TW, Wong KS, Hui AC, To KF, Lai ST, et al. (2005) Myopathic changes associated with severe acute respiratory syndrome: A postmortem case series. Arch Neurol 262: 1113-1117.
- Arabi YM, Deeb AM, Al-Hameed F, Mandourah Y, Almekhlafi GA, et al. (2019) Macrolides in critically ill patients with Middle East Respiratory Syndrome. Int J Infect Dis 81: 184-190.
- Torbic H, Duggal A (2019) Neuromuscular blocking agents for acute respiratory distress syndrome. J Copyright © 2020 Wolters Kluwer Health, Inc. Unauthorized reproduction of this article is prohibited. Crit Care 49: 179-184.
- Ding Y, He L, Zhang Q, Huang Z, Che X, et al. (2004) Organ distribution of severe acute respiratory syndrome (SARS) associated coronavirus (SARS-CoV) in SARS patients: Implications for pathogenesis and virus transmission pathways. J Pathol 203 (2): 622- 630.
- Disser NP, Micheli AJ, Schonk MM, Konnaris MA, Piacentini AN, et al. (2020) Musculoskeletal Consequences of COVID-19. The Journal of Bone and Joint Surgery 102 (14): 1197-1204.
- Margeta M (2021) Department of Pathology, University of California, San Francisco, CA, USA. Neuromuscular Disease 2: 3-3
- Shi Z, De Vries HJ, Vlaar APJ, Hoeven OV, Boon RA, et al. (2021) Diaphragm pathology in critically Ill patients with COVID-19 and postmortem findings from 3 medical centers. JAMA Intern Med 181 (1): 122–124.














