Cucunawangsih1*, Veronica Wiwing1, Nicolaski Lumbuun2 and Nata Pratama Hardjo Lugito3
1Microbiology Department, Faculty of Medicine, Pelita Harapan University, Tangerang, Indonesia
2Pharmacology Department, Faculty of Medicine, Pelita Harapan University, Tangerang, Indonesia
3Internal Medicine Department, Faculty of Medicine, Pelita Harapan University, Tangerang, Indonesia
*Corresponding Author:
Cucunawangsih
Microbiology Department, Faculty of Medicine, Pelita Harapan University
Jendral Sudirman Boulevard, Lippo Karawaci, Tangerang, Banten, 15811, Indonesia
Tel: +6221 54210130
E-mail: cucunawangsih.fk@uph.edu
Received date: March 03, 2016; Accepted date: March 31, 2016; Published date: April 07, 2016
Copyright: © 2016, Cucunawangsih et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Keywords
Carbapenem-resistant Aacinetobacter baumanii; Phenotypes; Tigecycline; Intensive care unit
Introduction
Acinetobacter baumanii (A.baumanii) is a Gram-negative non-fermenting bacilli, which is one of the most opportunistic pathogen in nosocomial infections, especially in critically-ill patients with poor immune response [1,2]. This bacteria frequently causes health care-associated infections, including pneumonia, meningitis, bacteremia, urinary tract infection, and wound infection with mortality rate 28-68% and prolonged length of hospital stay [3,4]. The use of medical equipment, such as mechanical ventilation, tracheostomy, and antibiotic treatment with third generation cephalosporins, fluoroquinolones, or carbapenems are a risk factors of getting acinetobacter infections, particularly MultiDrug-Resistant (MDR) A.baumanii [2,5].
In the recent years, the prevalence of MDR A.baumanii have been rapidly increasing and recognized as the most difficult antibiotic-resistant Gram-negative bacilli to treat and control [1,6].
The decreased susceptibility of various antibiotic among A.baumanii isolates have implicated in limited selection of therapeutic options for patients, mainly if the isolates were resistant to the carbapenems [1,6,7].
The production of class B Metallo-β-Lactamases (MBLs), such as Verona Integron (VIM) and Imipenemase (IMP) types, which can hydrolyze all ß-lactams, except monobactams has been assured as the major mechanism for the spreading of resistance to Acinetobacter species [8,9]. Other carbapenemase enzymes, class D Oxacillinases (OXAs) with activity against carbapenems have been reported worldwide [10]. The MBLs or OXAs were a major threat for clinicians since they were easily transferred among bacteria and induced hospital acquired infection [9,11].
The finding of these enzymes is important in the field of infection control and public health perspective [3,12]. Unfortunately, the information of these bacterias and their mechanisms resistance among hospitalized patients in Indonesia is limited. Along with this mind, our study investigate the prevalence of metallo- or OXA carbapenemase producing A. baumanii and its antibiotic susceptibility pattern from clinical isolates in secondary care healthcare facility in Tangerang, Indonesia.
Materials and Methods
During the 3 year-period of January 2013 until December 2015, 117 consecutive non-duplicate clinical isolates of A. baumanii were collected from medical records of admitted patients in the ICU of Siloam General Hospital, Tangerang, Indonesia. Antibiotic susceptibility and mechanisms of resistance to beta lactams were retrieved from data system and converted into a format which was used for analysis. Data of antimicrobial susceptibility level were presented as number. Data were analyzed using SPPS version 21.
A.baumanii identification and susceptibility testing
The clinical isolate samples were obtained as a routine examination of admitted patients in the ICU. The collection and transport of specimens were conducted according to the standard protocol from our microbiology laboratory. All clinical samples were immediately innoculated onto standard MacConkey agar prepared in house from dehydrated MacConkey powder according to manufacturer’s instructions and incubated at 37°C for 24 hours. Oxidase negative colonies morphologically similar to Acinetobacter were identified using an automated system GN-ID card from VITEX-2 Compact®. Susceptibility test was performed using the VITEX Antibiotic Susceptibility Testing (AST) N-100 card and results were interpreted based on current Clinical and Laboratory Standard Institute (CLSI) guideline [13]. Escherichia coli ATCC® 25922 and Pseudomonas aeruginosaATCC® 27853 were used as control strains, and E. coli ATCC® 35218 for β-lactam/β- lactamase inhibitor combinations [13]. Detection of metalloor OXA carbapenemase was provided by the Advance Expert System (AES) databases on phenotypes regarding Minimum Inhibitory Concentration (MICs) breakpoint of β-lactam classes [14,15].
Results
In the study period, a total of 954 positive bacterial cultures were collected from ICU, in which 117 (12.3%) were identified as A. baumanii. As it is shown in Table 1, most common source of infection was respiratory tract 104 (88.9%), while the least number proportion 2 (1.7%) was isolated from blood and urine samples.
| Characteristics |
Year |
Total |
| 2013 |
2014 |
2015 |
| |
n = 34 |
n = 38 |
n = 45 |
n = 117 |
| Type of specimens |
|
|
|
|
| Sputum |
31 (91.18%) |
28 (73.68%) |
41 (91.12%) |
100 (85.47%) |
| Bronchial lavage |
1 (2.94%) |
2 (5.27%) |
1 (2.22%) |
4 (3.42%) |
| Blood |
1 (2.94%) |
3 (7.89%) |
1 (2.22%) |
5 (4.27%) |
| Cerebrospinal fluid |
0 (0%) |
2 (5.27%) |
0 (0%) |
2 (1.71%) |
| Abscess swab |
0 (0%) |
3 (7.89%) |
1 (2.22%) |
4 (3.42%) |
| Urine |
1 (2.94%) |
0 (0%) |
1 (2.22%) |
2 (1.71%) |
| Phenotype of beta lactams resistance |
|
|
|
|
| Acquired penicillinase |
14 (41.17%) |
5 (13.15%) |
11 (24.44%) |
30 (25.64%) |
| Metallo- or OXA lactamase with resistantcarbapenemase (impermeability) |
19 (55.88%) |
33 (86.84%) |
28 (62.22%) |
80 (68.37%) |
| High level resistance |
2 (5.88%) |
1 (2.63%) |
4 (8.88%) |
7 (5.98%) |
| Wild (cephalosporinase) |
14 (41.17%) |
5 (13.15%) |
11 (24.44%) |
30 (25.64%) |
Table 1 Characteristics of A. baumanii isolates from clinical specimens
Our study demonstrated an increased number of MBLs or OXAs phenotypes with Outer Membrane Protein (OMP) impermeability (Table 1). The highest number of these phenotypes was in 2014 with 33 (86.8%) events. In 2013 and 2015 the number of metallo- or OXA carbapenemase producing A. baumanii was 19 (55.9% ) and 28 (62.2%) consecutively.
The isolates exhibited high sensitivity to amikacin (73.5%, 65.8%, and 66.7% in 2013, 2014, and 2015) and tigecycline (76.5%, 65.8%, and 71.1% in 2013, 2014, and 2014). Antibiotic that had moderate level of susceptibility was trimethoprimsulfamethoxazole (73.2%, 57.9%, and 66.7% in 2013, 2014, and 2015).
Meropenem, one of β-lactams class antibiotic demonstrated low sensitivity level to isolates for three consecutive years. Mostly A.baumanii isolates in this study were highly resistant to cephalosporins, especially against ceftriaxone. This study noted a relation between MBLs or class OXAs with resistant carbapenemase impermeability phenotypes and MIC ≥ 16 against meropenem. The MICs level for various antibiotics was shown in Table 2.
| Antibiotics |
MIC break point (µg/ml) |
| MIC range |
Sensitive |
Resistance |
| Ampicillin/sulbactam |
≤2 - ≥32 |
≤2 - 4 |
≥32 |
| Ceftazidime |
4 - ≥64 |
04-Aug |
≥64 |
| Ceftriaxone |
8 - ≥64 |
8 |
≥64 |
| Cefepime |
≤1 - ≥64 |
≤1 - 8 |
32 - ≥64 |
| Piperacillin/tazobactam |
≤4 - ≥128 |
≤4 - 16 |
≥ 128 |
| Ciprofloxacin |
≤0.25 - ≥4 |
≤0.25 - 5 |
≥4 |
| Amikacin |
≤0.25 - ≥16 |
≤0.25 - 4 |
≥16 |
| Meropenem |
≤2 - ≥64 |
≤2 - ≥16 |
≥64 |
| Trimethoprim/sulfamethoxazole |
≤20 - ≥320 |
≤2 - 40 |
160 - ≥320 |
| Tigecycline |
≤0.5 - ≥8 |
≤0.5 - 2 |
≥8 |
Table 2 Distribution of minimum inhibitory concentrations (MICs) of various antibiotics for A.baumanii isolated from ICU
Discussion
In this study, the prevalence of A.baumanii was found to be 12.3%. This result was higher than in other study, such it was made in Indonesia [16] (0.4%) and India [17] (7.5%). This study results was comparable with other study conducted by Tsakiridou et al. which found that hospitalization in ICU significantly increases the risk of A.baumanii ventilatorassociated pneumonia [18].
Factors that raised A.baumanii infections were parallel to other Gram-negative bacilli which were requiring mechanical ventilation, previous antibiotic consumption with third generation cephalosporins, fluoroquinolones, and carbapenems, or debilitated patients [5,19,20].
Almost all of isolates in our study were carbapenemresistant A.baumanii (CRAB) which was associated to the ability of various enzymes that are produced, specifically MBLs and drug impermeability [6,21,22]. Studies reported that the β-lactams class antibiotic resistance mechanism is the result of carbapenemase and OMP impermeability [4,22,23]. Previous studies from different geographical regions found that MBLs production among A.baumanii strain were detected in the range of 49-90% [6-8,21-23]. Considerably high prevalence of isolates were MBLs positive Acinetobacter (68.4%) could be the major reason of high CRAB among A.baumanii isolates in this study. The proportion of MBLs positive A.baumanii isolates in this study was higher than prior study by Peymani et al. that found the proprtion of 49%, emphasize its emergence [8]. The increasing prevalence of MBLs producing in this study was accordance with other studies in Iran [8,20]. Other predominant mechanism of CRAB, such as OXA-23 or OXA-24 carbapenemase could also explain the high prevalence of MBLs production [23]. This circumstances was also related to the increased consumption or heavy use of carbapenems and ciprofloxacin in hospitals.
In the last decades, there were emergence A.baumanii infections in ICU worldwide including Indonesia. Antibiotic sensitivity pattern in the present study showed high resistance against third generation cephalosporins, ciprofloxacin, and meropenem. Other antibiotics tested such as tigecycline, amikacin, and trimethoprim-sulfamethoxazole showed better sensitivity level. In a relatively similar study, Dent et al. reported prevalence of A.baumanii susceptibility to amikacin was 58% [24]. A study carried out by Gonlugur et al. in Turkey found that the only antibiotic susceptible against A.baumanii is amikacin [7]. Studies in Saudi Arabia and Iran found that more than 90% of A.baumanii isolates was sensitive to tigecycline [25]. Analysis of susceptibility pattern in Asia and the Middle East described that A. baumanii had high resistance to various antibiotics class, such as cephalosporins, tetracycline, β- lactam/β-lactam inhibitor combination, fluoroquinolones, aminoglycosides, and trimethoprim-sulfamethoxazole [26-28]. This not only caused the high incidence of MDR phenotype but also Extensively Drug-Resistant (XDR) phenotype which kept increasing in the past decades [21]. The reason for disparity in susceptibility pattern and phenotype of A.baumanii is presumably due to variety in clinical samples, setting of studies, and empirical antibiotics treatment in each geographical regions [27]. Our findings on antibiotic susceptibility and phenotypic characterization showed no significant variance with other regions globally (Figure 1).
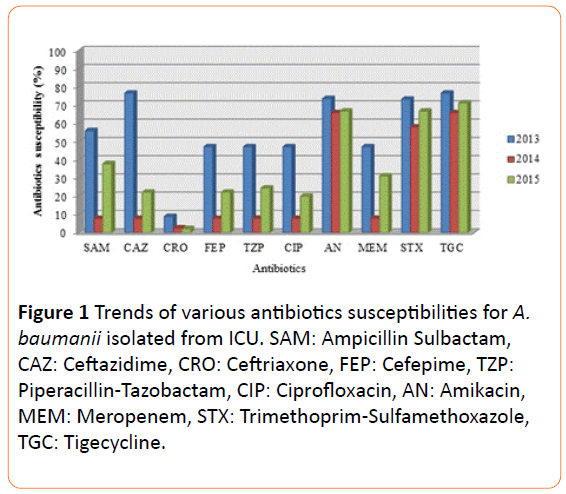
Figure 1 Trends of various antibiotics susceptibilities for A. baumanii isolated from ICU. SAM: Ampicillin Sulbactam, CAZ: Ceftazidime, CRO: Ceftriaxone, FEP: Cefepime, TZP: Piperacillin-Tazobactam, CIP: Ciprofloxacin, AN: Amikacin, MEM: Meropenem, STX: Trimethoprim-Sulfamethoxazole, TGC: Tigecycline.
Conclusion
The low number of drug susceptibility among A.baumanii strain observed in this study demonstrated MDR organism, especially CRAB. Thus, there were limited choices of antibiotic available for empirical therapy in ICU setting, such as amikacin, tigecycline, or trimethoprim-sulfamethoxazole. The significantly high level of metallo- or OXA carbapenemase phenotypes was associated with the low susceptibility against meropenem and cephalosporins.
Acknowledgements
The authors are grateful to Siloam General Hospital in Lippo Village, Tangerang, Indonesia who made this work possible. We thank to Faculty of Medicine, Pelita Harapan University for their support in this study.
8991
References
- Maragakis LL, Perl TM (2008) Acinetobacterbaumannii: epidemiology, antimicrobial resistance, and treatment options. CID 46: 1254-1263.
- Visca P, Seifert H, Towner KJ (2011) Acinetobacter infection-an emerging threat to human health. IUBMB Life 63:1048-1054.
- Dijkshoorn L, Nemec A, Harald S (2007) An increasing threat in hospitals: multidrug-resistant Acinetobacterbaumannii. Nat Rev Microbiol 5: 939-951.
- Perez F, Hujer AM, Hujer KM, Decker BK, Rather PN, et al. (2007) Global challenge of multidrug-resistant Acinetobacterbaumannii. Antimicrob Agents Chemother 51: 3471-3484.
- Brkic DV, Stanko AP, Plesko S, Tripkovic V, Bedenic B (2015) Acinetobacterbaumanii and phenotype characteristics of isolates from intensive care unit of the department of internal medicine at the university hospital centre in Zegreb over a four-year period. Signa Vitae 10:13-15.
- Safari M, Saidijam M, Bahador A, Jafari R, Alikhani MY (2013) High prevalence of multidrug resistance and metallo-beta-lactamase (MβL) producing Acinetobacterbaumanii isolated from patients in ICU wards, Hamadan, Iran. JRHS 13: 162-167.
- Gonlugur U, Bakici MZ, Akkurt I, Efeoglu T (2004) Antibiotic susceptibility patterns among respiratory isolates of Gram-negative bacilli in a Turkish university hospital. BMC Microbiology 4: 32.
- Peymani A, Nahaei MR, Farajnia S, Hasani A, Mirsalehian A, et al. (2011) High prevalence of metallo-ß-lactamase-producing Acinetobacterbaumanii in a teaching hospitals in Tabriz, Iran. Jpn J Infect Dis 64: 69-71.
- Srinivasan S, Shashikala, Devi S (2014) Mechanisms of resistance to carbapenems among Acinetobacter isolates. Int J of Biomed Res 5: 277-279.
- Hasan B, Perveen K, Olsen B, Zahra R (2014) Emergence of carbapenem-resistant Acinetobacterbaumanii in hospitals in Pakistan. J Med Microbiol 63: 50-55.
- Thomson JM, Bonomo RA (2005) The threat of antibiotic resistance in Gram-negative pathogenic bacteria: beta-lactams in peril.CurrOpinMicrobiol 8: 518-524.
- Park KH, Shin JH, Lee SY, Kim SH, Jang MO, et al. (2013) The clinical characteristics, Carbapenem resistance, and outcome of Acinetobacter bacteremia according to Genospecies. PLOS One 8: 65026.
- Winstanley T, Courvalin P (2011) Expert system in clinical microbiology. ClinMicrobiol Rev 24: 515-556.
- Sanders CC, Peyret M, Moland ES, Cavalieri SJ, Shubert C, et al. (2001) Potential impact of VITEX 2 system and the advanced expert system on the clinical laboratory of a university-based hospital. J ClinMicrobiol 39: 2379-2385.
- Radji M, Fauziah S, Aribinuko N (2011). Antibiotic sensitivity pattern of bacterial pathogens in the intensive care unit of Fatmawati Hospital, Indonesia. Asian Pac J Trop Biomed 1: 39-42.
- Gokale SK, Sonth SB (2015) Characterization and antibiotic sensitivity pattern of Acinetobacter species from various clinical samples in a tertiary care hospital. Int J CurrMicrobiol App Sci 4: 934-937.
- Tsakiridou E, Makris D, Daniil Z, Manoulakas E, Chatzipantazi V, et al. (2014) Acinetobacterbaumanii infection in prior ICU bed occupants is an independent risk factor for subsequent cases of ventilator-associated pneumonia. BioMed Res Int.
- Cerqueira GM, Peleg AY (2011) Insights into Acinetobacterbaumanniipathogenicity. IUBMB Life 63: 1055-1060.
- Nhu NTK, Lan NPH, Campbell JI, Parry CM, Thompson C, et al. (2014) Emergence of carbapenem-resistant Acinetobacterbaumanii as the major cause of ventilator-associated pneumonia in intensive care unit patients at an infectious disease hospital in southern Vietnam. J Med Microbiol 63: 1386-1394.
- Noori M, Karimi A, Fallah F, Hashemi A, Alimehr S, et al. (2014) High prevalence of metallo-beta-lactamase producing Acinetobacterbaumanii isolated from two hospitals of Tehran, Iran. Arch Pediatr Infect Dis 2: e15439.
- Widyatmoko L, Rahayu, Anandani A, Isbandrio B, Wahjono H (2014) The antibiotic resistance mechanisms of Acinetobacterbaumanii isolated from intensive care unit, dr. Kariadi hospital Semarang-Indonesia based on advance expert system VITEX-2 compact software. JCMID 1: 33-39.
- Mohanty S, Maurya V, Gaind R, Deb M (2013) Phenotypic characterization and colistin susceptibilities of carbapenem-resistant of Pseudomonas aeruginosa and Acinetobacter spp. J Infect DevCtries 7: 880-887.
- Dent LL, Marshal DR, Pratap S, Hulette RB (2010) Multidrug resistant Acinetobacterbaumanii: a descriptive study in a city hospital. BMC Infectious Diseases 10:196.
- Baadani AM, Thawadi SI, El-Khizzi NA, Omrani AS (2013) Prevalence of colistin and tigecycline resistance in Acinetobacterbaumannii clinical isolates from 2 hospitals in Riyadh Region over a 2-year period. Saudi Med J 3: 248-253.
- Morovat T, Bahram F, Mohammad E, Setareh S, Mohamad Mehdi F (2009) Distribution of different carbapenem resistant clones of Acinetobacterbaumannii in Tehran hospitals. New Microbiol 32:265-271.
- Ghajavand H, Esfahani BN, Havaei SA, Moghim S, Fazeli H (2015) Molecular identification of Acinetobacterbaumanii isolated from intensive care units and their antimicrobial resistance patterns. Adv Biomed Res 4: 110.
- Wang SH, Sheng WH, Chang YY, Wang LH, Lin HC, et al. (2003) Healthcare-associated outbreak due to pan-drug resistant Acinetobacterbaumanii in a surgical intensive care unit. J Hosp Infect 53:97-102.






