Keywords
Rate-adaptive pacemaker; Closed loop stimulation; Acceleration sensor; Chronotropic incompetence; 6- Minute walk
Introduction
Pacemaker patients often have atrial chronotropic incompetence requiring rate-adaptive pacing. Symptoms of systolic heart failure are also common in pacemaker recipients [1]. In those with both heart failure and chronotropic incompetence, multiple other mechanisms of cardiac output augmentation are impaired, including the insufficient increase in left ventricular filling, myocardial contractility, and stroke volume, leaving it up to the increase in heart rate to determine the patient’s exercise capacity [2-4].
Studies are needed to understand whether a rate-adaptive sensor capable of adequate response to all kinds of stress may better protect pacemaker patients from heart failure progression than less adequate rate-adaptive pacing.
In an earlier, randomized, crossover study comparing accelerometer sensor (AS) and closed loop stimulation (CLS) driven by myocardial contraction dynamics, Coenen et al. [5] observed that, during a 6-minute walk test, CLS resulted in higher heart rate in patients with symptomatic heart failure (NYHA class II or III) than in symptom-free patients (NYHA I).
This observation was interpreted as if CLS, unlike AS, might be able to differentiate between patients with higher exercise tolerance (NYHA I) and lower exercise tolerance (NYHA II-III), for whom walking represents a low-to-moderate exercise requiring a lower heart rate increase (NYHA I) or demanding exercise requiring a higher heart rate increase (NYHA II-III) [5]. As it was not a predefined study hypothesis, this observation required prospective validation.
We did the present study to compare pacemaker-driven heart rate during 6-minute walk test for symptomatic versus symptom-free patients. Before the comparison, the patients were randomly assigned to AS or CLS irrespective of their exercise tolerance level. We also analyzed heart failure development over 2 years of follow-up.
Materials and Methods
Study design and participants
The randomized “Closed loop stimulation: Heart Failure Indexing and Rate Modulation” (CONFIRM) study was performed at 31 investigational sites (Supplementary data). Consenting adults were enrolled if they had an accepted indication for rateadaptive pacing and were chronotropically incompetent with a heart rate <100 bpm during 6-minute walk test. Patients were excluded if they had heart failure symptoms at rest (NYHA IV), or a left ventricular ejection fraction (LVEF) <35%.
The study was done in compliance with good clinical practice guidelines and the Declaration of Helsinki, including approval of the study protocol by appropriate national and local ethics committees. Patients provided written informed consent.
Evaluations at enrolment
We evaluated the patients’ tolerance to physical activity by a 6-minute walk test. The tolerance was graded in analogy to NYHA class, irrespective of whether heart failure had been diagnosed or not (Table 1). The intention was to categorize patients as symptomatic or symptom-free, even if the limitation of their exercise ability was not caused by the cardiac function. Heart rate was measured before and immediately after the walk.
| Class |
Definition |
| Exercise tolerance evaluated at enrolment, expressed in analogy to NYHA classification irrespective of whether heart failure has been diagnosed or not |
| NYHA I-like |
No limitation of physical activity. Ordinary physical activity does not cause undue fatigue, palpitation, dyspnea, or anginal pain |
| NYHA II-like |
Slight limitation of physical activity. Ordinary physical activity results in fatigue, palpitation, dyspnea, or anginal pain |
| NYHA III-like |
Marked limitation of physical activity. Less than ordinary activity causes fatigue, palpitation, dyspnea, or anginal pain |
| NYHA IVa |
Inability to carry on any physical activity without discomfort. Symptoms of heart failure or the anginal syndrome may be present even at rest |
| AHA/ACC stages of heart failure |
| A |
Patients at high risk of developing heart failure |
| B |
Patients with cardiac structural abnormalities or remodeling who have not developed heart failure symptoms |
| C |
Patients with current or prior symptoms of heart failure |
| D |
Patients with refractory end-stage heart failure |
| None |
Patients not yet in stages A-D |
aPatient exclusion criterion at enrolment.
AHA/ACC: American Heart Association/American College of Cardiology; NYHA: New York Heart Association.
Table 1: Classification of patients.
The patient’s heart failure status was graded according to the AHA/ACC stages listed in Table 1. Also echocardiographic measurements of the LVEF, left atrial end-systolic and enddiastolic diameters, left ventricular end-systolic (LVESD) and enddiastolic diameters, and mitral regurgitation were undertaken.
Pacemaker
Patients received a CYLOS or CYLOS 990 pacemaker (Biotronik SE & Co. KG, Berlin, Germany) offering a choice between AS and CLS rate-adaptive pacing, without the dual-sensor option. In the AS mode, the pacemaker measures the amount and vigorousness of anterior to posterior thoracic motion and can thus respond to physical activity only.
In the CLS mode, the pacemaker couples the pacing rate to the myocardial contraction dynamics by measuring ventricular impedance variation [6-8]. As cardiac contractility is regulated by the autonomic nervous innervation, CLS is integrated into the natural cardiovascular control loop and responds to physical, mental, and emotional stress [5,7-10].
Single- and dual-chamber devices were implanted, with ventricular leads placed in the right ventricular apex and atrial leads placed in the right atrium (appendage [41%], high right atrium [37%], lateral [19%], septal [3%]).
Randomization and follow-up
After implantation, patients were randomly assigned to AS or CLS for 2 years. The randomization was done through a centralized, concealed process stratified by site. Investigators and patients were not masked to treatment allocation.
Patients underwent follow-up controls at 1, 12, and 24 months. At each control, the 6-minute walk test, the exercise tolerance grading, the AHA/ACC heart failure staging, and echocardiography were performed.
Study objectives
The study investigated the hypothesis that CLS and AS produce different heart rate depending on patients’ level of exercise tolerance. More specific, CLS was expected to produce a higher heart rate in patients with a lower exercise tolerance, in whom 6-minute walk test represents greater challenge and presumably requires higher heart rate than in stronger, symptom-free patients.
Conversely, AS was expected to increase heart rate similarly in all patients, or even more in stronger patients who walk more vigorously, although they may need less chronotropic support than weaker patients. If the hypothesis turned to be true, it would imply that CLS is not only more sensitive to various kinds of stress [5,7-10] but that it is also more specific than AS during physical exertion. The hypothesis was tested at 1 month, using symptom classification made at enrolment (NYHA I-like [higher exercise tolerance] vs. NYHA II- or III-like [lower exercise tolerance]) (Table 1).
The main secondary endpoints were the distance covered during 6-minute walk at 1 month, heart failure development over 2 years (AHA/ACC stage, echo parameters), and the percentage of atrial and ventricular pacing.
In a post-hoc analysis, we tested the primary hypothesis after exclusion of patients who had the AHA/ACC stage A or none at enrolment. This exclusion confined the analysis to the patients with structural heart disease or heart failure symptoms, whose exercise tolerance is likely controlled by the cardiac function.
Statistical analysis
The null hypotheses was that WalkHR symptomatic=WalkHR symptom-free would be fulfilled in each rate-adaptive mode. The alternative hypothesis was that WalkHR symptomatic ≠ WalkHR symptom-free would be true in at least one rateadaptive mode, where WalkHR stands for heart rate after 6- minute walk test at 1 month, symptomatic stands for NYHA II- or III-like, and symptom-free stands for NYHA I-like at enrolment.
Based on the insights from the PROVIDE study [5] with similar patient selection criteria, we made assumptions for the sample size calculation. Due to an “or” combination of the two alternative hypotheses, we used the union-intersection principle and thus a significance level of αCLS= αAS=2.5%. The calculated sample size to detect the effect size with a statistical power (1– β) of 80% was 154 patients. After accounting for a likely dropout rate of 20%, 200 patients had to be enrolled.
The as-treated (per-protocol) principle was used. Missing NYHA class was imputed by the last observation carried forward. Data are shown as mean ± SD (continuous variables) or as absolute values and percentages (categorical variables).
Comparisons were made using the two-sided Mann-Whitney U-test (continuous variables) and the Fisher’s exact test (categorical variables). A two-sided P-value <0.025 for the primary endpoint and <0.05 for other endpoints was considered statistically significant. For multiple comparisons of patient baseline characteristics, the Holm-Bonferroni method was applied. All statistical analyses were conducted with the IBM SPSS 23 software for Windows (IBM Corporation, Armonk, New York).
Results
Patients
From September 26, 2007, to October, 28, 2009, 205 patients were randomly assigned to the AS or CLS rate-adaptive mode.
Two patients randomized to AS received CLS at hospital discharge, and four patients randomized to CLS received AS, resulting in an early crossover rate of 2.9%. All subsequent analyses were done for the programmed mode. The analysis population comprised 104 AS and 101 CLS patients (Figure 1).
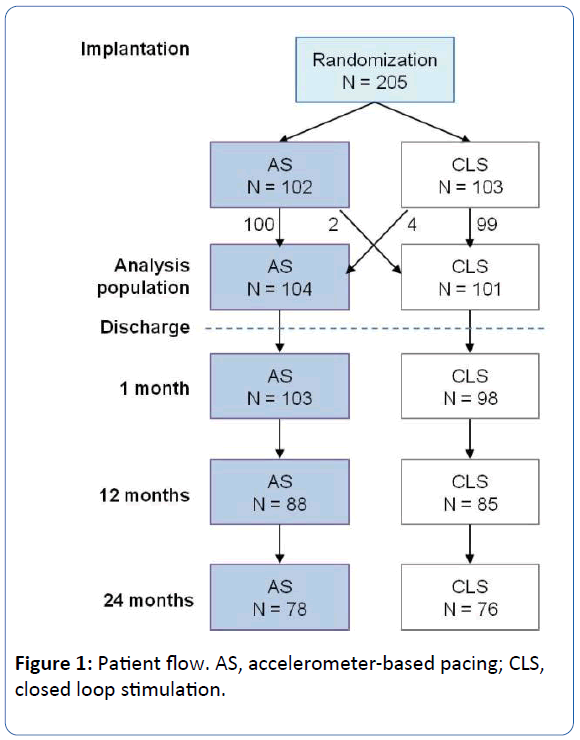
Figure 1: Patient flow. AS, accelerometer-based pacing; CLS, closed loop stimulation.
Patient characteristics at baseline were reasonably well balanced between the study groups (Table 2). Cardiac function was generally preserved, as only one in six patients had a LVEF <50%. Sixty percent of patients were symptomatic (NYHA II- or III-like) during the exercise tolerance test.
| Patient characteristics |
AS group |
CLS group |
| (n=104) |
(n=101) |
| Age, years |
73.1 ± 9.1 |
73.6 ± 8.3 |
| Male gender |
53 (51%) |
60 (59%) |
| Exercise tolerancea |
| Symptom free (NYHA I-like) |
40 (38%) |
40 (40%) |
| Moderately symptomatic (NYHA II-like) |
46 (44%) |
43 (43%) |
| Highly symptomatic (NYHA III-like) |
18 (17%) |
18 (18%) |
| AHA/ACC stagea |
| None |
35 (34%) |
28 (28%) |
| A |
14 (13%) |
14 (14%) |
| B |
20 (19%) |
29 (29%) |
| C |
35 (34%) |
30 (30%) |
| LVEF <50% |
17 (17%) [n=101] |
15 (16%) [n=96] |
| Atrial rhythm |
| Sinus bradycardia |
47 (45%) |
43 (43%) |
| Brady-tachy syndrome |
24 (23%) |
19 (19%) |
| Atrial fibrillation |
32 (31%) |
34 (34%) |
| High-grade AV block (2nd or 3rd degree) |
24 (23%) |
17 (17%) |
| Any bundle branch block |
18 (17%) |
20 (20%) |
| Intrinsic QRS duration |
101 ± 27 [n=92] |
103 ± 24 [n=85] |
| Hypertension |
69 (66%) |
65 (64%) |
| Ischemic disease |
21 (20%) |
30 (30%) |
| Symptoms |
| Shortness of breath |
53 (51%) |
53 (52%) |
| Fatigue/weakness |
46 (44%) |
35 (35%) |
| Peripheral edema |
11 (11%) |
7 (7%) |
| Syncope |
29 (28%) |
29 (29%) |
| Diabetes mellitus |
21 (20%) |
23 (23%) |
| Renal insufficiency |
15 (14%) |
24 (24%) |
| Chronic pulmonary disease |
4 (4%) |
11 (11%) |
| Medication |
[n=100] |
[n=98] |
| Antiarrhythmic |
13 (13%) |
9 (9%) |
| Beta-blocker |
63 (63%) |
47 (48%) |
| ACE inhibitor |
55 (55%) |
57 (58%) |
| Diuretic |
50 (50%) |
43 (44%) |
| Anticoagulant |
72 (72%) |
75 (77%) |
| 6-minute walk test |
| Distance walked, m |
329 ± 130 |
333 ± 115 |
| Heart rate after walk, bpm |
72.2 ± 15.0 |
74.0 ± 13.7 |
| Heart rate before walk, bpm |
59.3 ± 10.7 |
60.1 ± 12.1 |
| Implanted pacemaker |
| Dual-chamber |
85 (82%) |
80 (79%) |
| Single-chamber (ventricular) |
19 (18%) |
21 (21%) |
aAs defined in Table 1.
Data are mean ± SD or number [n]. Data is available in all patients unless stated otherwise. There were no statistically significant differences between study groups after multiplicity correction.
AHA/ACC: American Heart Association/American College of Cardiology; ACE: Angiotensin-Converting Enzyme; AS: Acceleration Sensor; AV: Atrioventricular; bpm: Beats per Minute; CLS: Closed Loop Stimulation; LVEF: Left Ventricular Ejection Fraction; NYHA: New York Heart Association.
Table 2: Patient characteristics at enrolment.
Follow-up
Three quarters of patients had regular study termination after 24 months (Figure 1). The rest (26 AS, 25 CLS) had premature study termination for the following reasons. Eleven patients died, from multi-organ failure (1 AS, 2 CLS), worsened heart failure (1 AS), ventricular tachycardia (1 CLS), pneumonia and lung edema (1 CLS), uterine cancer (1 CLS), or unknown causes (2 AS, 2 CLS). Other premature terminations were due to loss to follow-up (10 AS, 6 CLS), change of pacemaker mode (5 AS, 4 CLS), moving away (5 AS, 3 CLS), withdrawal of consent (1 AS, 2 CLS), change of pacemaker system (1 AS, 2 CLS), and upgrade to biventricular pacing (1 CLS).
Programmed pacemaker parameters
Most pacemakers were programmed to the dual-chamber mode (81 AS, 74 CLS), with a basic rate of 59.8 ± 3.1 bpm (AS) and 59.8 ± 2.8 bpm (CLS). The rate-responsive parameters were programmed at the physician’s discretion. Factory settings were largely preferred.
Primary endpoint
Of the 201 patients who underwent 6-minute walk test at 1 month, 179 had heart rate measured before and immediately after the walk, and are therefore included in the primary endpoint analysis (Table 3).
There was a tendency in the expected direction for both sensors. In the CLS group, WalkHR tended to be higher in symptomatic than symptom-free patients (median, 89.5 vs. 86 bpm; P=0.33), and in the AS group the trend was the opposite (84 vs. 87 bpm; P=0.95). As no statistical significance was reached, the null hypothesis must be maintained. Similarly, the increase in heart rate during the walk (DeltaHR) tended to be greater in symptomatic than symptom-free patients for CLS (median, 21.5 vs. 15 bpm; P=0.06), but not for AS (16 vs. 20 bpm; P=0.47).
In the post-hoc analysis of the primary endpoint confined to patients with heart failure or structural heart disease at enrolment, both WalkHR and DeltaHR were significantly higher in symptomatic than symptom-free patients in the CLS group (median, 92 vs. 84 bpm [P=0.021] and 23 vs. 15 [P=0.018], respectively). The opposite trends in the AS group did not reach statistical significance (Table 4).
The fact that the mean WalkHR with pacemaker (Table 3) and without pacemaker (Table 2) differed by 16 bpm for both rateadaptive modes suggests a large prevalence of paced heart beats after the walk at the 1-month follow-up.
Secondary endpoints
No statistically significant difference between AS and CLS was observed for any secondary endpoint, including the 6-minute walk test distance at 1 month (373 ± 116 m [AS] vs. 371 ± 109 m [CLS]) or at 24 months (415 ± 154 vs. 387 ± 120 m), the 24 month percentual distribution of AHA/ACC stages none/A/B/C/D (36/14/24/21/0 [AS] vs. 33/16/25/22/1 [CLS]) or NYHA-like classes I/II/III/IV (54/38/6/0 [AS] vs. 41/50/8/0 [CLS]), and the 24-month echo parameters (AS vs. CLS: LVEF [57 ± 10% vs. 58 ± 11%], LVESD [34 ± 7 vs. 34 ± 7 mm]).
Moreover, no significant worsening of any echo parameter was observed from 1 to 24 months in either study group or in pooled data. For example, pooled LVEF was 59 ± 10% (n=179) at 1 month and 58 ± 10% (n=126) at 24 months, while pooled LVESD was 33 ± 7 mm (n=172) and 34 ± 7 mm (n=123), respectively.
The percentage of ventricular pacing was similar for AS (51 ± 42%; median 54 [n=81]) and CLS (52 ± 39%; 55 [n=67]). The percentage of atrial pacing tended to be higher for CLS (71 ± 22% vs. 59 ± 33%; median 74 vs. 67), which did not reach statistical significance (P=0.11).
Discussion
The hypothesis that CLS produces higher exercise heart rate in symptomatic than symptom-free patients did not reach statistical significance although the trend was as expected (P=0.06 for DeltaHR). When the analysis was confined to the subset of patients with heart failure or structural heart disease, the effect was statistically significant (WalkHR [P=0.021], DeltaHR [P=0.018]).
A larger prevalence of mitral insufficiency (64% vs. 55%) and atrial fibrillation (34% vs. 25%) in patients with NYHA II-III than in symptom-free patients compromises stroke volume and shifts more emphasis on heart rate to increase cardiac output during exercise. However, in the presence of heart failure, exercise heart rate should optimally not exceed ~85% (~123 bpm) of the age-predicted maximum (~145 bpm=220-patient age) [11]. With a mean WalkHR of ~93 bpm with CLS, a significant reserve of 30 bpm (123 minus 93) still remains to protect patients from an adverse outcome potentially caused by excessive tachycardia coming from inappropriate rate-adaptive pacing.
Increasing stoke volume to improve cardiac output during exercise is mainly not possible in heart failure patients. The main mechanism to increase the cardiac output in this patient population is to accelerate the heart frequency. This study confirmed the potential of CLS sensor realizing a moderate but rapidly increase of heart rate during exercise, more effective compared to the simple accelerometer.
The likely explanation for a higher WalkHR in NYHA II-III with CLS is the increased sympathetic drive of the autonomic nervous system, sensed as an increase in cardiac contractility. This effect is likely less pronounced in NYHA II-III patients with an AHA/ACC stage lower than B, since their symptoms are less of cardiac origin and hence less likely to modify sympathetic drive and contractility. Therefore, the restriction of the post-hoc analysis to patients with an AHA/ACC stage B or C appears justified.
In the AS group, no notable difference was observed between symptomatic and non-symptomatic patients, with P-values ranging from 0.47-0.95. Overall, in symptom-free patients, the mean WalkHR was nearly identical for AS and CLS, while in symptomatic patients, it was higher in the CLS group by 4.4 bpm (all patients) and 6.6 bpm (AHA/ACC stage B-C), which is expected to translate into a higher cardiac output with CLS.
Heart failure progression did not differ significantly between AS and CLS. In pooled data for all patients, worsening of echo parameters over 2 years was negligible. The low progression of heart failure is likely the consequence of a preserved LVEF (≥50%) in 83% and no AHA/ACC stage C in 68% of patients at baseline, and a moderate percentage of right ventricular pacing during the study (mean ~50%) [12]. The mean LVEF (60%) was at the upper edge of the range reported from larger pacemaker studies (47%-58%) [1,12]. Deleterious long-term effects of rateadaptive pacing are more likely in patients with poor baseline cardiac condition [13].
The distance covered during 6-minute walk did not differ significantly between AS and CLS in CONFIRM or in previous studies [5,14]. Although AS is specifically designed to support physical activity associated with thoracic movement, CLS is not inferior for this kind of activity and is superior to AS during other forms of exercise, like cycling, mental exertion, or emotional stress [5-10,14]. In the randomized crossover PROVIDE study, twice as many chronotropically incompetent patients preferred CLS over AS [5].
Perspectives: CLS in advanced heart failure
Chronotropic incompetence affects nearly 50% of patients with advanced congestive heart failure and is an independent predictor of mortality [15]. Those with advanced heart failure and a wide QRS complex are eligible for cardiac resynchronization therapy (CRT). By counteracting the chronotropic incompetence, rate-adaptive CRT devices may allow more aggressive use of beta-blockers for improved outcomes through a lower resting heart rate [2-4,13]. So far, only AS-driven CRT pacing has been studied, with an inconsistent effect on exercise capacity due to blunted forcefrequency response and other complex interactions [2-4,16].
By a sensitive and selective response to all kinds of stress, CLS might reduce sympathetic tone and cardiac contractility peaks, potentially offering better protection from cardiac overstress and disease progression. In a randomized, crossover (intraindividual), double-blind pilot study, the newly available CLS-based CRT has been compared with fixed-rate CRT in patients with severe chronotropic incompetence (<70% of agepredicted maximum heart rate). Major endpoints are the minute ventilation/carbon dioxide production slope (ventilatory efficiency), maximal oxygen uptake, the oxygen uptake efficiency slope, pulmonary end-tidal carbon dioxide, 6-minute walk test distance, biomarkers, and quality of life. Study results are expected in 2018.
Limitations
Like in many pacemaker studies the mean left ventricular function of the patient population of this study is good to preserved. Many compensation mechanisms cover the positive or negative hemodynamic effects of pacing.
The observation period is not long enough to prove any heart failure developments.
The patients have not blinded regarding the treatment group, but there are only objective parameters (heart rate), which cannot really influenced by the chronotropic incompetent patient.
Conclusion
In chronotropically incompetent patients with NYHA class II-III symptoms, CLS resulted in higher heart rate during exercise than AS, potentially improving cardiac output without excessive tachycardia. In symptom-free patients, AS and CLS showed equivalent performance. Heart failure progression over 2 years was insignificant overall, and not differing between AS and CLS, in the study cohort with a relatively preserved LVEF (mean 60%).
Acknowledgments
The study was funded by Biotronik SE & Co. KG, Berlin, Germany. The authors are thankful to Bernd Bruesehaber, PhD, for contributions to study design, study coordination, data analysis, and statistical calculations; to Jürgen Schrader, PhD, Beate Wenzel, PhD, and Jochen Proff for scientific input; to Dejan Danilovic, PhD, for scientific input and linguistic editing; and to Dean J. MacCarter, MS, PhD, for the cardiopulmonaryrelated discussion.
Conflicts of interest
Norbert Klein, Maika Klein, and Dietrich Pfeiffer received consulting fees from Biotronik and St. Jude Medical. The other authors report no conflicts of interest.
20083
References
- Thackray SD, Witte KK, Nikitin NP, Clark AL (2003) The prevalence of heart failure and asymptomatic left ventricular systolic dysfunction in a typical regional pacemaker population. Eur Heart J 24: 1143-1152.
- Tse HF, Siu CW, Lee KL, Fan K (2005) The incremental benefit of rate-adaptive pacing on exercise performance during cardiac resynchronization therapy. J Am Coll Cardiol 46: 2292-2297.
- Witte KK, Cleland JG, Clark AL (2006) Chronic heart failure, chronotropic incompetence, and the effects of beta blockade. Heart 92: 481-486.
- Witte KK, Clark AL (2006) Chronotropic incompetence in heart failure. J Am Coll Cardiol 48: 595-596.
- Coenen M, Malinowski K, Spitzer W, Schuchert A, Schmitz D, et al. (2008) Closed loop stimulation and accelerometer-based rate adaptation: Results of the PROVIDE study. Europace 10: 327-333.
- Occhetta E, Bortnik M, Marino P (2011) Usefulness of hemodynamic sensors for physiologic cardiac pacing in heart failure patients. Cardiol Res Pract 2011: 925653.
- Griesbach L, Gestrich B, Wojciechowski D, Weyers G (2003) Clinical performance of automatic closed loop stimulation systems. PACE 26: 1432-1437.
- Santini M, Ricci R, Pignalberi C, Biancalana G (2004) Effect of autonomic stressors on rate control in pacemakers using ventricular impedance signal. PACE 27: 24-32.
- Chandiramani S, Cohorn LC, Chandiramani S (2007) Heart rate changes during acute mental stress with Closed Loop Stimulation: Report on two single-blinded, pacemaker studies. PACE 30: 976-984.
- Proietti R, Manzoni G, Di Biase L, Castelnuovo G, Lombardi L, et al (2012) Closed loop stimulation is effective in improving heart rate and blood pressure response to mental stress: Report of a single-chamber pacemaker study in patients with chronotropic incompetent atrial fibrillation. PACE 35: 990-998.
- Kindermann M, Schwaab B, Finkler N, Schaller S, Böhm M, et al. (2002) Defining the optimum upper heart rate limit during exercise. A study in pacemaker patients with heart failure. Eur Heart J 23: 1301-1308.
- Stockburger M, Boveda S, Moreno J, Da Costa A, Hatala R, et al. (2015) Long-term clinical effects of ventricular pacing reduction with a changeover mode to minimize ventricular pacing in a general pacemaker population. Eur Heart J 36: 151-157.
- Nagele H, Rodiger W, Castel MA (2008) Rate-responsive pacing in patients with heart failure: Long-term results of a randomized study. Europace 10: 1182-1188.
- Abi-Samra FM, Singh N, Rosin BL, Dwyer JV, Miller CD, et al. (2013) Effect of rate-adaptive pacing on performance and physiological parameters during activities of daily living in the elderly: Results from the CLEAR (Cylos Responds with Physiologic Rate Changes during Daily Activities) study. Europace 15: 849-856.
- Elhendy A, Mahoney DW, Khandheria BK, Burger K, Pellikka PA (2003) Prognostic significance of impairment of heart rate response to exercise: Impact of left ventricular function and myocardial ischemia. J Am Coll Cardiol 42: 823-830.
- Van Thielen G, Paelinck BP, Paul B, Vrints CJ, Conraads VM (2008) Rate response and cardiac resynchronisation therapy in chronic heart failure: Higher cardiac output does not acutely improve exercise performance: A pilot trial. Eur J Cardiovasc Prev Rehabil 15: 197-202.







