Keywords
Apoptosis; Candida spp; Proteazom
Introduction
Candida species are a leading causes of the hospital related bloodstream infections with high morbidity and mortality. They cause serious systemic diseases especially when the normal bacterial flora is supressed by widespread use of broad spectrum antimicrobials. Increasing number of solid organ and bone marrow transplantations, invasive procedures and implantable biomaterials are also other predisposing factors [1-3].
Oral azole drugs are the first choice in the treatment of candidiasis [4]. Boric acid is one of the local treatment alternatives in resistant cases [5]. Fungicidal effect of boric acid is attributed to the blockage in the oxidative metabolism according to some authors [6]. However, there also are some boric acid analogs which effect as proteasome inhibitor and they can be local treatment alternatives. Nevertheless, the data about the use of proteasome inhibitors for therapeutic purposes on Candida species is limited. Bortezomib (Velcade), originally codenamed PS-341 (pyrazy carbonyl-Phe-Leu-boronate), is the only boric acid analogue proteasome inhibitor, which has received Food and Drug Administration (FDA) approval and is currently used parenterally [7].
Proteasomes are the major non-lysosomal protease structures found in eukaryotic cells. It provides degradation of the misconfigured and ubiquitin labelled proteins. Ubiquitinproteasome enzymatic pathway is located in the cytoplasm and is responsible for the degradation of the abnormal proteins [8]. Ubiquitin mediated degradation of regulatory proteins plays an important role in the control of metabolic processes such as cell cycle progression, signal transmission, transcriptional regulation and apoptosis. Studies on ubiquitin-proteasome system of mammalian cells and yeast species are currently ongoing to solve the complex relationship of this system with the apoptosis pathway [9].
MG-262, originally codenamed (ZL3B) Z-Leu-Leu-Leu-B(OH)2, is one of the most potent compound among boric acid analog proteasome inhibitors. It selectively and reversibly inhibits the chymotryptic activity of the proteasome which has a critical role in cell viability and proliferation [10].
In this study, the susceptibility of the active ingredient MG-262 on Candida species was detected and then possible antifungal mechanisms (oxidative metabolism inhibition, apoptosis) and the effects on some virulence factors (hyphal growth, inhibition of cell viability in biofilm condition) were analysed in vitro.
Methods
Candida strains
Standard strains; C. albicans ATCC 90028, C. krusei ATCC 6258 and a clinical isolate; C. glabrata strain were included. C. glabrata was identified via the standard methods, such as chlamydospore production, germ tube assays, micro-morphology studies in cornmeal- Tween 80 agar and biochemical tests using the commercial system ID32C (bioMérieux Marcy l'Etoile, France). Antifungal susceptibility tests were performed according to the Clinical and Laboratory Standards Institute (CLSI) M27-A3 document and interpreted in accordance with the CLSI M27-S4 suplement [11,12]. All fungal isolates were stored at -80°C in the Brain Heart Infusion Broth (Sigma Chemical Co, USA) with 20% glycerol (Sigma Chemical Co, USA) and 0.2% agar (Oxoid, UK) suspensions. Before testing, each isolate was cultured on Sabouraud Dextrose Agar (SDA) (Oxoid, UK) to ensure viability.
Stock solutions
MG-262 (Cat# I-120) was purchased from Boston Biochemical Inc. (Cambridge, USA) in 1 mg packages. The chemical compound was dissolved in DMSO (Sigma Chemical Co, USA) in order to set serial dilutions. Each of the samples was diluted in RPMI 1640 medium (Sigma Chemical Co, USA) to obtain final concentrations from 0.03 μg/mL to 200 μg/mL. Amphoterisin B (Sigma Chemical Co, USA) and Fluconazole (Ibrahim Ethem Ilaç A.S, Istanbul, Turkey) were used as control antimicrobials. Stock solutions of fluconazole and amphotericin B were prepared in distilled water and DMSO, respectively. Stock solutions of the antifungal agents (at 100 times higher concentration) were then diluted with RPMI 1640 medium buffered to pH 7.0 with 0.165 M MOPS (Sigma Chemical Co, USA). The final concentration ranges were 0.03 to 16 μg/ml for amphotericin B and 0.125 to 64 μg/ml for fluconazole. They were frozen in aliquots at –80°C until use [11].
Antifungal susceptibility tests
Antifungal susceptibility tests of chemicals were performed according to CLSI M27-A3 guidelines by broth microdilution method. As it is defined in the document; round-bottom trays containing RPMI 1640 broth with 0.2% glucose (Sigma Chemical Co, USA) were incubated at 35°C for 48 hours with an 100 μl inoculation of 0.5 × 103 and 2.5 × 103 blastoconidia/ml (C. albicans ATCC 90028, C. krusei ATCC 6258, C. glabrata) and 100 μL aliquots from each concentration of test substances. After 24 hours of incubation Minimum Inhibitory Concentration (MIC) values were determined visually as the lowest concentration of drug that caused complete inhibition of growth relative to that of the growth control. To obtain the Minimum Fungicidal Concentration (MFC), 100 μl of each serial dilution was taken and spread on SDA. Plates were incubated at 35°C for 48 hours. The MFC was defined as the lowest drug concentration that yielded ≤ 1 colony on the agar plate. Results were demonstrated by the absence of colonies on the agar plates. Five per cent aqueous DMSO was negative control [11,13].
Germ tube test
Germ tube formation was monitored microscopically according to the conventional germ tube test protocol [14]. C. albicans ATCC 90028 strain was placed in human serum-containing media (10% v/v) with or without chemical compounds (25 μg/ml, 50 μg/ ml, 100 μg/ml and 200 μg/ml), and incubated at 37°C for 3 hours. The samples were visuliazed with 400X magnification via an optical microscope (Nikon Eclipse Ci, Japan). MG-262 untreated cells were used as control.
Corn meal agar tests
Hyphal transformation and pseudohypha production of C. albicans ATCC 90028 strain and pseudohypha production of C. krusei ATCC 6258 strain were determined via Cornmeal Agar (Oxoid, UK) with 10% Tween 80 (Oxoid, UK). Before and after incubation with MG-262 for 3 hours, Corn Meal Agar was cut deeply in the form of horizontal furrow with a loopful of suspension colonies and a flamed sterile coverslip was placed over the line of inoculum [14]. After the incubation at 22°C the results were observed both at 24 and 48 hours and documented with 400X magnification under an optical microscope (Nikon Eclipse Ci, Japan). MG-262 untreated cells were used as control.
Oxidative metabolism experiments
In our study, Candida strains were exposed to Alamar Blue stain in order to determine the effect of MG-262 on the oxidative metabolism with the method described by Seta et al. with slight modifications [6]. The Alamar Blue system is composed of an oxidation-reduction indicator, which fluoresces and turns from blue to pink secondary to oxidative metabolism and cell growth. The active ingredient of the dye is resazurin which is cell permeable, nonfluorescent and blue in color. Upon entering cells, resazurin is reduced to resorufin, which produces very bright pink fluorescence viable cells continuously convert resazurin to resorufin [15]. In order to determine the effect of chemical compound on oxidative metabolism, 45 μl samples of each strain (1 × 103 CFU/ ml) were exposed to 45 μl of serial dilutions (25 μg/ ml, 50 μg/ml, 100 μg/ml and 200 μg/ml) of MG-262 and stained with 10 μl AlamarBlue (Invitrogen, USA) dye. MG-262 untreated cells with alamarblue were used as positive control. The cells were incubated with MG-62 for 1 hour and washed gently. They were also observed for 24 hour in order to detect the colour change. After 24 hours the plate was read at 570 nm using a microtiter plate reader (Multiskan Ascent, Labsystems, Helsinki, Finland).
Biofilm assays
The effect of MG-262 on the cell viability in a biofilm condition were evaluated according to the microplate method described by Evensen and Braun with some modifications [16]. Briefly, 1 × 106 cell/ml of the samples were diluted in 100 μl Phosphate Buffered Saline (PBS, 0,05 M, pH: 7.2) (Oxoid, UK), inoculated in a 96 well plate and incubated at 37°C, in a 75 rpm shaker incubator (HVD, Austria). At the end of 48 hours, plates were washed with PBS and the wells filled with 50 μl serial dilutions of MG-262 at the concentrations 25-200 μg/ml and 100 μl Saboraud Dextrose Broth (Oxoid, UK). After 48 hours of incubation, the medium was removed and the wells were washed with PBS buffer. The plate was stained with 0.4% crystal violet (Oxoid, UK) for 15 minutes and the wells were air-dried. The crystal violet in each well was solubilized by 200 μl of 95% ethanol. The plate was read at 595 nm using a (Multiskan Ascent, Labsystems, Helsinki, Finland). MG-262 untreated cells were used as positive control.
Proteasome activity
Proteasome activity tests were performed according to the method described by Evensen and Braun [16]. In brief, 1 × 106 cell/ml of yeast cells were suspended in 100 μl PBS and kept in 96 well microtiter plate with various concentrations (200 μg/ml, 100 μg/ml, 50 μg/ml, 25 μg/ml) of MG-262 at 35°C. After 2 hours, 2 μl of 20 μM fluorogenic peptide substrate (Calbiochem); Suc- Leu-Leu-Val-Tyr-AMC (indicator of proteosomal chymotrypsinlike activity) was added and the final volume was adjusted to 200 μl with PBS-KCl. After 30 minutes of incubation, the proteasome enzyme activities were measured at 595 nm via spectrophotometer (Fluoroskan Ascent, Microplate Fluorometer, Thermoscientific). MG-262 untreated cells with fluorogenic peptides were used as controls.
Apoptosis (DNA ladder assay)
In order to detect the apoptosis effect of MG-262 on test strains, different concentrations of the compound (25 μg/ml, 50 μg/ ml, 100 μg/ml and 200 μg/ml) were tested on 0.5 × 103 - 2.5 × 103 CFU/ml of the yeast cells. After 3 hours of incubation with MG-262, DNA was extracted according to the protocol described by Suman et al. [17,18]. DNA fragmentation was visualized using a 50-10000 bp (base pair) Hi-Lo DNA marker/mass ladder (Bionexus, ABD) in the 1% agaroz gel via the gel electrophoresis method at 25 V for 10 min and at 50 V for 30 min. Untreated cells were used as positive control. The results were documented via Gel Logic 2000 gel magnification system (Kodak, USA).
Statistical analysis
All experiments were performed three times in triplicate. Chisquare test was used to compare filamentous growth, germ tube and DNA ladder assays. Arithmetic mean of absorbance values of oxidative metabolism, biofilm and proteasome activity experiments were calculated. The absorbance values of the negative control wells (containing no cells) were subtracted from the values of the test wells in order to eliminate any background absorbance and compared with the absorbance of the MG-262 untreated cell absorbance results. In the comparison of groups that provide the normal distribution independent samples T test was used while Mann-Whitney U test was used to compare the groups in which normality assumption was not provided. Analyses were performed, using the SPSS for Windows (Version 11.3.8.0) pocket program. Descriptive statistics were shown as mean ± standart deviation. A probability value of p less than 0.05 was considered statistically significant.
Results
Antifungal susceptibility tests
MG-262 exhibited minimum inhibitory activity at 25 μg/ml concentration for C. albicans ATCC 90028 and at 50 μg/ml concentration for C. krusei ATCC 6258 and C. glabrata. MFC of all species were 50 μg/ml. MIC of Fluconazole was 1 μg/ml for C. albicans whereas MFC of C. krusei and C. glabrata were ≥ 64 μg/ ml (interpreted as resistant) and MIC of Amphotericin B was 0.5 μg/ml for all strains. Negative control did not have any inhibitory effect on any of the Candida species tested.
Corn meal agar and germ tube tests
The serial dilutions of MG-262 at 25 μg/ml concentration, virulence factors; such as filamentous growth and germ tube formation were still continuing. However at 50 μg/ml, 100 μg/ml and 200 μg/ml concentrations, they were all inhibited. Figure 1 shows the results of germination and germ tube formation of C. albicans at 25 μg/ml and 100 μg/ml concentrations of MG-262.
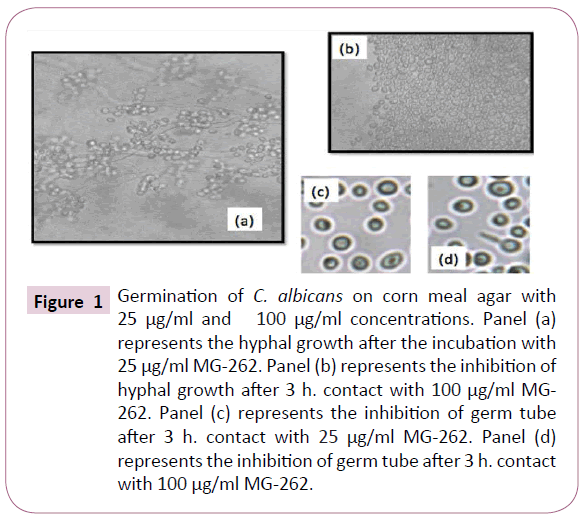
Figure 1: Germination of C. albicans on corn meal agar with 25 μg/ml and 100 μg/ml concentrations. Panel (a) represents the hyphal growth after the incubation with 25 μg/ml MG-262. Panel (b) represents the inhibition of hyphal growth after 3 h. contact with 100 μg/ml MG- 262. Panel (c) represents the inhibition of germ tube after 3 h. contact with 25 μg/ml MG-262. Panel (d) represents the inhibition of germ tube after 3 h. contact with 100 μg/ml MG-262.
Oxidative metabolism inhibition
The control group and 25 μg/ml of MG-262 treated cells developed a pink colour, which is a visual evidence of reduction of AlamarBlue. However, 50 μg/ml, 100 μg/ml and 200 μg/ ml treated cells showed no colour change during 24 hour after the addition of the dye. The statistical analysis results of the absorbance values are given both as table (Table 1) and as figure (Figure 2) in order to be more visually descriptive. When values were analyzed statistically, it was observed that MG-262 has a significant effect on the oxidative metabolism of Candida isolates at 50 μg/ml and higher concentrations. At 50 μg/ml concentrations, p values for C. albicans, C. krusei ve C. glabrata isolates were 0.000, 0.046 and 0.000, respectively. At 100 μg/ml concentration, p values for C. albicans, C. krusei ve C. glabrata isolates were 0.000, 0.043 and 0.000, respectively. Finally at 200 μg/ml concentrations, p values for C. albicans, C. krusei ve C. glabrata were found to be 0.003, 0.046 and 0.001 respectively.
| |
|
|
t- test |
p value |
| Biofilm |
C. albicans |
200-Unt |
-177,455 |
0.000* |
| 100-Unt |
-145,797 |
0.000* |
| 50-Unt |
-52,699 |
0.000* |
| 25-Unt |
0,707 |
0.519 |
| |
|
Mann-Whitney U |
p value |
| C. crusei |
200-Unt |
-1,964 |
0.05 |
| 100-Unt |
-1,993 |
0.46* |
| 50-Unt |
-1,964 |
0.05 |
| 25-Unt |
-0,886 |
0.376 |
| C. glabrata |
200-Unt |
-1,964 |
0.05 |
| 100-Unt |
-1,993 |
0.46* |
| 50-Unt |
-1,964 |
0.05 |
| 25-Unt |
-0,655 |
0.513 |
| |
|
|
t- test |
p value |
| Oxidative Metabolism |
C. albicans |
200-Unt |
-18,051 |
0.003* |
| 100-Unt |
-41,763 |
0.000* |
| 50-Unt |
-42,079 |
0.000* |
| 25-Unt |
1,782 |
0.198 |
| |
|
Mann-Whitney U |
p value |
| C. crusei |
200-Unt |
-1,993 |
0.046* |
| 100-Unt |
-2,023 |
0.043* |
| 50-Unt |
-1,993 |
0.046* |
| 25-Unt |
-0,221 |
0.825 |
| |
|
t- test |
p value |
| C. glabrata |
200-Unt |
-22,103 |
0.001* |
| 100-Unt |
-114,099 |
0.000* |
| 50-Unt |
-40,588 |
0.000* |
| 25-Unt |
-0,131 |
0.903 |
| |
|
|
T test |
p value |
Proteasome
Inhibition |
C. albicans |
200-Unt |
-61,724 |
0.000* |
| 100-Unt |
-39,735 |
0.000* |
| 50-Unt |
-23,927 |
0.000* |
| 25-Unt |
-0,979 |
0.426 |
| C. crusei |
200-Unt |
-69,987 |
0.000* |
| 100-Unt |
-46,555 |
0.000* |
| 50-Unt |
-24,867 |
0.000* |
| 25-Unt |
-0,436 |
0.686 |
| |
|
Mann-Whitney U |
p value |
| C. glabrata |
200-Unt |
-1,993 |
0.046* |
| 100-Unt |
-1,993 |
0.046* |
| 50-Unt |
-1,993 |
0.046* |
| 25-Unt |
-0,664 |
0.507 |
*statistically significant
Table 1: Statistical analysis results of biofilm reduction, oxidative metabolism and proteasome inhibition experiments.
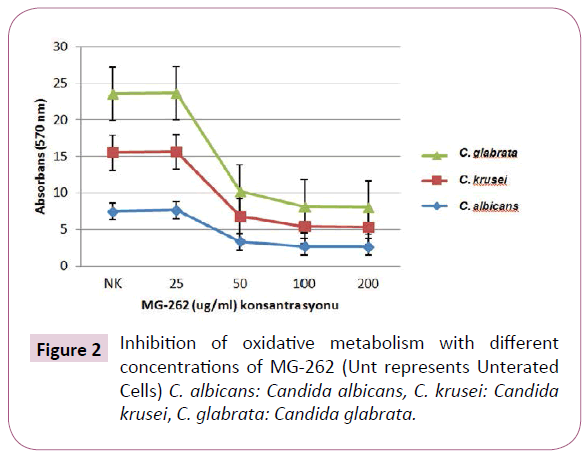
Figure 2: Inhibition of oxidative metabolism with different concentrations of MG-262 (Unt represents Unterated Cells) C. albicans: Candida albicans, C. krusei: Candida krusei, C. glabrata: Candida glabrata.
Biofilm assays
The inhibitory effect of MG-262 on cell viability of Candida strains in biofilm condition was observed to be enhanced with increasing concentrations (Figure 3). The ability of MG-262 to inhibit the cells in biofilm was found to be significantly increased at 50 μg/ml and higher concentrations. The most prominent effect was observed at 200 μg/ml concentration. At 50 μg/ml concentrations, p values for C. albicans, C. krusei ve C. glabrata were 0.000, 0.05 and 0.05 respectively. At 100 μg/ml concentrations, p values for C. albicans, C. krusei ve C. glabrata were 0.000, 0.046 and 0.046 respectively. At 200 μg/ml concentrations, p values for C. albicans, C. krusei ve C. glabrata were 0.000, 0.05 and 0.05 respectively (Table 1).
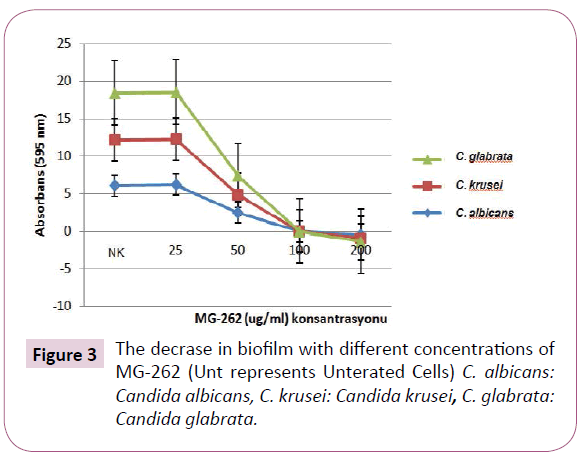
Figure 3: The decrase in biofilm with different concentrations of MG-262 (Unt represents Unterated Cells) C. albicans: Candida albicans, C. krusei: Candida krusei, C. glabrata: Candida glabrata.
Proteasome activity
We observed a prominent decrease in the proteasome activity with increasing concentrations of MG-262. The statisticall analysis of absorbance results are given both in (Table 1 and Figure 4). When analysed statistically, this effect was significant at 50 μg/ ml and higher concentrations. At 50 μg/ml concentrations, p values for C. albicans, C. krusei ve C. glabrata were 0.000, 0.000 and 0.046 respectively. At 100 μg/ml concentrations, p values for C. albicans, C. krusei ve C. glabrata were 0.000, 0.00 and 0.046 respectively. At 200 μg/ml concentrations, p values for C. albicans, C. krusei ve C. glabrata were 0.000, 0.000 and 0.046 respectively.
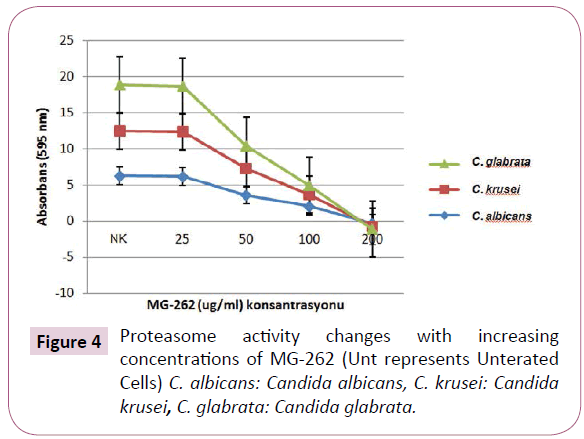
Figure 4: Proteasome activity changes with increasing concentrations of MG-262 (Unt represents Unterated Cells) C. albicans: Candida albicans, C. krusei: Candida krusei, C. glabrata: Candida glabrata.
Apoptosis
DNA damage was detected only at 200 μg/ml concentration. The DNA ladder image obtained with 200 mg/ml MG-262 on gel electrophoresis is given in Figure 5.
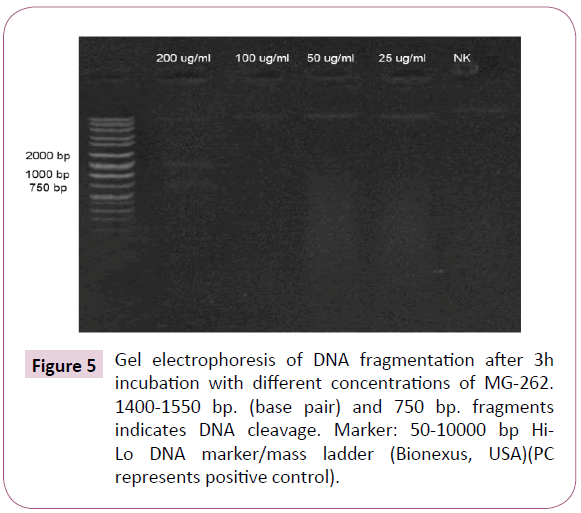
Figure 5: Gel electrophoresis of DNA fragmentation after 3h incubation with different concentrations of MG-262. 1400-1550 bp. (base pair) and 750 bp. fragments indicates DNA cleavage. Marker: 50-10000 bp Hi- Lo DNA marker/mass ladder (Bionexus, USA)(PC represents positive control).
Discussion
The aim of our study was to investigate the antifungal activity of MG-262 in for Candida infections. We determined that one of the most potent proteasome inhibitors, MG-262 inhibits virulence factors, and also cause apoptosis by proteasome inhibition and blockage of oxidative metabolism.
The researches on the apoptosis of the yeast give opinion about the apoptotic process of the eukaryotic cells and are promising in competing against unicellular eukaryotic pathogens. New drug molecules are required for life threatening resistant C. albicans and non-albicans Candida isolates. Agents performing fungal-programmed cell death can be a model for future novel antifungals [18].
Chymotrypsin-like region of the 20S proteasome is a well conserved region both in human and fungus cells. MG-262 is a peptide boronic acid. It inhibits proteasome by binding to chymotrypsin-like region of the 20S proteasome in human cells. The properties such as being a more potent agent than other proteasome inhibitors and being resistant to be easily inactivated by oxidation may make MG-262 a good candidate for a novel antifungal drug [10]. The previous in vitro sensitivity analysis have demonstrated that C. krusei and C. glabrata are more resistant than other Candida strains and MIC values of these two subspecies have shown to be higher than other species [19]. In our study, these two strains which are believed to have a Multi Drug Resistance (MDR) potential were also 2-fold more resistant to MG-262.
Unlike the fungistatic effects of the clinically used local boric acid preparation, MG-262 showed fungicidal activity [6]. The concentration for lethal effect of MG-262 was 2 times higher than for inhibitory effect only for C. albicans, for the 2 other strains it was the same concentration. Previous studies have revealed that proteosome inhibitor Bortezomib shows inhibitory effect on both P. falciparum and human cells in nM concentrations [7,20]. In our study MIC and MFC of the MG-262 were in μM concentration range and far above nM levels. We suggest that this can be due to the mechanical strength of the cell wall which consists of β1,3- glucan and chitin [21]. On the other hand, when compared with the other proteasome inhibitors, it showed efficacy at much lower concentration on the yeast probably secondary to being a more potent agent [22].
In addition to the effective concentration, the effects on virulence factors are also of great importance when an agent is being investigated as a potential novel antimicrobial. In our study, the effects of MG-262 on germ tube, hyphe, pseudohypha and cell viability in biofilm condition were examined. Candida infections generally originate from implants such as prosthetic valves, endotracheal tubes and joint prostheses. The major virulence factors are biofilm formation and adhesion to the biomaterial surface [23]. The hyphal growth and germ tube development capabilities of the fungus cells are also important in the formation of cell damage and deeply located infections [24]. MG-262 inhibited hyphal growth, germ tube and pseudohypha formation at 50 μg/ml and higher concentrations. The effects on cell viability in biofilm were more prominent with increasing MG- 262 concentrations (Figure 3). The antimicrobial resistance of the biofilm-producing microorganisms can be up to 1000-fold greater than planctonic cells [25]. Therefore, the inhibition at higher concentration and the most efficacy at 200 μg/ml concentration can not to be regarded as a surprising finding.
Proteaosome inhibitors cause apoptosis in almost all cells at high concentrations [10]. Our study was in line with this literature, indicating that MG-262 provided a significant proteasome inhibition only at 50 μg/ml and higher concentration.
The breakdown of the oxidative metabolism in cells and associated oxidative stress are the most substantial signals portending the death due to apoptosis [26]. In the oxidationreduction experiments of our study, the color had changed to pink both in control strains and 25 μg/ml MG-262 treated strains in about 1 hour whereas the blue color had not changed in the following 24 hours beginning from 50 μg/ml concentration. These results showed that oxidative metabolism was blocked in increasing concentrations from 50 μg/ml. It has been reported that the effect of some protease inhibitors is reversible [26].
19780
References
- Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP et al. (2004) Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis 39: 309-317.
- Martins N, Ferreira IC, Barros L, Silva S, Henriques M (2014) Candidiasis: predisposing factors, prevention, diagnosis and alternative treatment.Mycopathologia 177: 223-240.
- Williams DW, Kuriyama T, Silva S, Malic S, Lewis MA (2011) Candida biofilms and oral candidosis: treatment and prevention. Periodontol 55: 250-265.
- Pappas PG, Kauffman CA, Andes DR, Clancy CJ, Marr KA, et al. (2016) Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin Infect Dis 62: e1-50.
- das Neves J, Pinto E, Teixeira B, Dias G, Rocha P, et al. (2008) Local Treatment Of Vulvovaginal Candidosis:general and practical considerations. Drugs 68:1787-1802.
- Seta FD, Schmidt M, Essmann BV, Larsen B (2009) Antifungal mechanisms supporting boric acid therapy of Candida vaginitis. J Antimicrob Chemother 63: 325-336.
- Reynolds JM, El Bissati K, Brandenburg J, Günzl A, Mamoun CB (2007) Antimalarial activity of the anticancer and proteasome inhibitor bortezomib and its analog ZL3B. BMC Clin Pharmacol 7: 13.
- Wojcik C (2002) Regulation of apoptosis by the ubiquitin and proteasome pathway. J Cell Mol Med d 6: 25-48.
- Wojcik C (2002) Regulation of apoptosis by the ubiquitin and proteasome pathway. J Cell Mol Med 6: 25-48.
- Kisselev AF, van der Linden WA, Overkleeft HS (2012) Proteasome inhibitors: an expanding army attacking a unique target. Chem Biol 19: 99-115.
- Clinical and Laboratory Standards Institute (CLSI) (2008) Reference method for broth dilution antifungal susceptibility testing of yeasts: approved standard. 3rd edn. CLSI document M27-A3. Wayne, PA: Clinical and Laboratory Standards Institute 22: 156.
- Clinical and Laboratory Standards Institute (CLSI) (2012) Reference method for broth dilution antifungal susceptibility testing of yeasts, 4th informational supplement. CLSI document M27-S4.
- Oro D, Heissler A, Rossi EM, Scapin D, Malheiros PS, et al. (2015) Antifungal activity of natural compounds against Candida species isolated from HIV-positive patients. Asian Pac J Trop Biomed 5: 781-784.
- Mahmoudi Rad M, Zafarghandi S, Abbasabadi B, Tavallaee M (2011) The epidemiology of Candida species associated with vulvovaginal candidiasis in an Iranian patient population. Eur J Obstet Gynecol Reprod Biol 155: 199-203.
- Rampersad SN (2012) Multiple Applications of Alamar Blue as an Indicator of Metabolic Function and Cellular Health in Cell Viability Bioassays. Sensors (Basel, Switzerland) 12: 12347-12360.
- Evensen NA, Braun PC (2009) The effects of tea polyphenols on Candida albicans: inhibition of biofilm formation and proteasome inactivation. Can J Microbiol 55: 1033-1039.
- Suman S, Pandey A, Chandna S (2012) An improved non-enzymatic "DNA ladder assay" for more sensitive and early detection of apoptosis. Cytotechnology 64: 9-14.
- Madeo F, Herker E, Wissing S, Jungwirth H, Eisenberg T, et al. (2004) Apoptosis in yeast. Curr Opin Microbiol 7: 655-660.
- Pfaller MA, Diekema DJ, Sheehan DJ (2006) Interpretive breakpoints for fluconazole and Candida revisited: a blueprint for the future of antifungal susceptibility testing. Clin Microbiol Rev 19: 435-447
- Shabaneh TB, Downey SL, Goddard AL (2013) Molecular basis of differential sensitivity of myeloma cells to clinically relevant bolus treatment with bortezomib. PLoS One 8: e56132.
- Klis FM, Mol P, Hellingwerf K, Brul S (2002) Dynamics of cell wall structure in Saccharomyces cerevisiae. FEMS Microbiology Reviews 26: 239-256.
- Evensen NA, Braun PC (2009) The effects of tea polyphenols on Candida albicans:inhibition of biofilm formation and proteasome inactivation. Can J Microbiol 55: 1033-1039.
- Seneviratne CJ, Jin L, Samaranayake LP (2008) Biofilm lifestyle of Candida: a mini review. Oral Dis 14: 582-590.
- Calderone RA, Fonzi A (2001) Virulence factors of Candida albicans. Trends in Microbiology 9: 327-335.
- Mah TF, O'Toole GA (2001) Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol 9: 34-39.
- Kannan K, Jain SK (2007) Oxidative stress and apoptosis. Pathophysiology 7: 153-163.
- Rahbar SY, Saeidi N, Zununi VS, Barzegari A, Barar J (2015) An update to DNA ladder assay for apoptosis detection. Bioimpacts 5: 25-28.
- Zörnig IK, Baum M, Hajibagheri B, James N, Chittenden C, et al. (1997) Human Bak induces cell death in Schizosaccharomyces pombe with morphological changes similar to those with apoptosis in mammalian cells. Molecular and Cellular Biology 17: 2468-2474.










