Keywords
Zoonotic, morbidity, Toll-like receptor (TLR), mitogen activated protein kinases (MAPK), Gemichalcone_B
Introduction
Leptospirosis has been recognized as an important public health problem because of its epidemic proportions and increasing incidence in both developing and developed countries. The disease was first described by Adolf Weil in 1886 when he reported an acute infectious disease with enlargement of spleen, jaundice and nephritis. Leptospira was first observed in 1907from a post mortem of renal tissue slice. In 1908, Inada and Ito first identified it as the causative organism and in 1916 noted its presence in rats [1]. Leptospirosis is a rare and severe bacterial infection that occurs when people are exposed to certain environments such as contaminated food, water, soil etc. It is caused by a continuously changing number of pathogenic members of spirochetes belonging to the genus Leptospira. Leptospirosis is an infectious disease caused by Leptospira interrogans complex, which has over 20 serogroups and more than 200 serovars. The source of human leptospiral infection is infected animal urine. Leptospirosis can be transmitted by many animals such as rats, shunks, opossums, raccoons, foxes, and other vermin. Leptospires enter the body through mucous membranes of the eyes, nose or throat and via cuts or abrasions of the skin and invade the host tissues and fluids [2]. Leptospirosis is found throughout the world, because leptospira is able to survive in the hot and humid conditions. Leptospirosis can occur in any age group, but a number of factors increase the risk of developing the disease. After the organism gains entry, it multiplies in blood and tissue. The resulting leptospiremia can spread to any part of the body but particularly affects the liver and kidney causing interstitial nephritis and tubular necrosis resulting in renal failure. Leptospiral infection is confirmed by clinical signs and laboratory tests performed on blood and urine. The treatment includes high doses of antibiotics. But there are number of side effects for the above described antibiotics. In order to overcome many of the side effects natural drugs are being used. One way to achieve this is by producing and screening drug candidates more effectively and applying the molecular docking, wherein molecular modeling techniques are used to predict how a protein interacts with small molecules (Ligands) [3]. The concept of docking is important in the study of various properties associated with protein-ligand interactions such as binding energy, geometry complementarity, hydrogen bond donor acceptor properties, hydrophobicity and polarizability [4].Based on the insilico study comparative analysis between the natural and synthetic compounds is done to find the best lead compound among them for the leptospirosis.
Materials and Methods
Softwares required
Accelrys Discovery Studio Client 3.5 was used for preparation of protein and ligands for docking, Biosolve IT and GOLD 5.1 are docking softwares used for binding energy calculation.
Protein Selection and preparation
The crystal structure of human p38α MAP kinase was selected as a drug target. Its structure (PDB ID: 3HVC) has been retrieved from RCSB protein Data Bank. All water molecules were removed and on the final stage hydrogen atoms were added to the target protein molecule. Then the protein structure was subjected to energy minimization by applying CHARMm Force field in Accelrys Discovery Studio 3.5 [5].
Active site prediction
The minimized protein is further taken for binding site detection which will be very useful in active site identification for the Structure based Drug designing. This study has been used to know the important residues in the target protein which are responsible for ligand binding, present in the active site [6].
Ligand
The list of anti-inflammatory, anti-cancerous, antimicrobial compounds for both natural and synthetic products were gathered from publicly available plant medicinal database and peer published articles. The set of ligand molecules studied in this work were retrieved from PubChem and Chemicalize.org databases. 50 compounds were collected for each set of natural and synthetic from these sources for docking studies.
Drug Likeliness Evaluation
The drug likeliness properties of the selected compounds were investigated with the help of Lipinski drug filter in Discovery Studio 3.5 [7]. Lipinski rule of five is a rule of thumb to evaluate drug likeliness or determine if a chemical compound with a certain pharmacological and biological activity has properties that would make it likely orally active drug in humans.
Energy minimization of Ligand
Library of the screened compounds (SDF files) were prepared and the energy of the ligand was minimized using smart minimizer algorithm with parameters of 200 steps and at RMS gradient 0.1 by applying CHARMm force field [8] in Discovery studio 3.5.
ADME investigation
ADME studies were executed through ADME descriptors in Discovery Studio 3.5 [9]. The Absorption, Distribution, Metabolism and Excretion (ADME) studies provide insight into the pharmacokinetic property of all natural and synthetic screened compounds. Recent years have seen a rise in the importance of computational ADMET predictions. This is because the majority of clinical trial failures have been due to ADME issues, not from a lack of efficacy. The prediction of ADME properties is incredibly very difficult task. New computational methods including consensus modeling show promise for increasing the accuracy of In-silico ADME-TOX prediction used for virtual screening in Lead optimization [10]. The ADMET is used to calculate Aqueous Solubility, Blood Brain Barrier, Cytochrome p450, Hepatotoxicity, Human Intestinal Absorption, Plasma Protein Binding.
Toxicity studies
Computational prediction of toxicity has both good and bad points, compared with prediction of ADME properties. The advantage of toxicity prediction is that it is more accurate than ADME prediction. This is because it will predict results for one, specific type of toxicity. Toxicity studies includes mutagenicity and carcinogenicity assays. Mutagenicity assay is based on the Ames test. Carcinogenicity assay predicts the ability of the compound to cause cancer to normal human cells and carcinogenicity test were carried for mouse and rat models. Toxicity prediction studies serves as a preclinical examination and helps to minimize the time and cost during clinical trials. Liver toxicity is a side effect of many drugs. Toxicity prediction studies were executed through TOPKAT in Discovery Studio 3.5 [11].
Molecular Docking Studies
Molecular docking studies were performed to investigate the binding affinities and interaction modes for the inhibitors using Biosolve-IT FlexX and Gold 5.1.
The docking algorithm in the Lead-IT suite is the FlexX-docking approach. It uses incremental buildup algorithm [12]. In Lead-IT first, the active site of the target protein is loaded. A sphere of 20Å radius in active site is selected. Then in Docking, choose the docking library is selected to load the MOL2 files of screened natural compounds and synthetic compounds separately. Finally Define FexX Docking and Apply & Dock is clicked. Using the FlexX algorithm, upto 200 poses are generated for each compound and the best pose will be selected for further studies.GOLD uses a genetic algorithm to explore the wide range of ligand conformational flexibility of the protein [13]. To run GOLD Suite 5.1 first the wizard is clicked. Then the active site of the target protein (3HVC) is loaded. Then ID is clicked, to add hydrogen atoms. The radius of protein is set 20?. Next Goldscore P450 is selected. The ADMET screened natural and synthetic ligands are added to the library. The fitness function is set as Gold score. Then Finally, Run Gold is clicked.
Results
Molecular simulation studies
CHARMM is a highly versatile and widely used molecular simulation program. CHARMM uses an empirical energy function for energy minimization. Energy minimization adjusts the structure of the molecule in order to lower the energy of the system. It is often performed in order to relieve strain in experimentally obtained or averaged structures. The results obtained after minimization of protein are shown in Table 1.
| Name |
Forcefield |
Potential Energy (kcal/mol) |
Van der Waals Energy (kcal/mol) |
Electrostatic Energy (kcal/mol) |
Final RMS Gradient (kcal/(mol x A) |
Minimization Criteria |
| 3HVC_calculate Energy |
CHARMm |
-20597.3635 |
-2343.3068 |
-20771.1674 |
1.04189 |
CONJUG> Minimization exiting with number of steps limit(200) exceeded. |
Table 1: Energy of protein (3HVC) after minimization
| S. No |
Descriptor |
A: Sodium curcuminate |
B:Curcumin |
C:Gemichalcone_B |
D:Diosmetin |
E:Genistein |
| 1 |
ADME.2D.FPSA |
94.092 |
94.092 |
126.793 |
97.607 |
88.677 |
| 2 |
A LOG P98 |
3.554 |
3.554 |
6.011 |
2.394 |
2.14 |
| 3 |
AQ Sol LEV |
3 |
3 |
2 |
3 |
3 |
| 4 |
BBB LEV |
3 |
3 |
4 |
3 |
3 |
| 5 |
ADMET ABSORPTION LEV |
0 |
0 |
2 |
0 |
0 |
| 6 |
CYP2D6 PROB |
0.004904 |
0.004904 |
4.70E-05 |
0.003038 |
0.60832 |
| 7 |
HEPATOX PROB |
0.027637 |
0.027637 |
1.40E-05 |
0.616312 |
0.999233 |
| 8 |
PPB LOG |
0.318579 |
0.318579 |
0 |
0.000229 |
0.143509 |
Table 2: ADME values of Natural compounds
| S. No |
Descriptor |
A:Cefazolin |
B:SB203580 |
C: SB202190 |
D:Ofloxacin |
E: PD0325901 |
| 1 |
ADME.2D.FPSA |
147.019 |
54.877 |
54.182 |
70.741 |
93.482 |
| 2 |
A LOG P98 |
-1.992 |
3.883 |
2.23 |
-1.374 |
2.578 |
| 3 |
AQ Sol LEV |
2 |
2 |
2 |
4 |
2 |
| 4 |
BBB LEV |
1 |
1 |
2 |
4 |
3 |
| 5 |
ADMET ABSORPTION LEV |
0 |
0 |
0 |
1 |
0 |
| 5 |
CYP PROB |
0 |
0 |
0.000821 |
0 |
0.000543 |
| 6 |
HEPATOX PROB |
0 |
0.010398 |
0.001907 |
0 |
0.089519 |
| 7 |
PPB LOG |
0.00347 |
0.397867 |
0 |
0.599006 |
0.015771 |
Table 3: ADME values of Synthetic compounds
| S. No |
Natural Compounds |
NTP Carcinogenicity Call (Female Rat) (v3.2) |
FDA Carcinogenicity Male Rat Non vsCarc (v3.1) |
FDA Carcinogenicity Female Rat Single vsMult (v3.1) |
Ames Mutagenicity (v3.1) |
Rat Oral LD50 (v3.1) Log (1/Moles) |
Skin Irritation (v6.1) |
| 1 |
Sodium curcuminate |
0.000 |
0.000 |
0.996 |
0.012 |
1.738 |
0.000 |
| 2 |
curcumin |
0.000 |
0.000 |
0.996 |
0.012 |
1.738 |
0.000 |
| 3 |
Gemichalcone B |
1.000 |
0.000 |
0.002 |
0.000 |
1.831 |
0.001 |
| 4 |
Genistein |
0.000 |
0.000 |
0.000 |
0.946 |
2.865 |
0.000 |
| 5 |
Diosmetin |
0.002 |
1.000 |
0.000 |
0.995 |
3.200 |
0.000 |
Table 4: TOPKAT values of Natural compounds
| Sl. No |
Natural Compounds |
NTP Carcinogenicity Call (Male Rat) (v3.2) |
NTP Carcinogenicity Call (Female Rat) (v3.2) |
FDA Carcinogenicity Female Rat Non vsCarc (v3.1) |
Ames Mutagenicity (v3.1) |
Rat Oral LD50 (v3.1) Log (1/Moles) |
Skin Irritation (v6.1) |
| 1 |
SB203580 |
0.000 |
0.000 |
1.000 |
0.995 |
(1/Moles) 1.861 |
1.000 |
| 2 |
PD0325901 |
0.000 |
0.000 |
0.000 |
0.000 |
2.622 |
0.000 |
| 3 |
Cefazolin |
0.031 |
0.000 |
0.000 |
0.000 |
2.852 |
0.000 |
| 4 |
SB202190 |
1.000 |
0.000 |
0.344 |
1.000 |
2.249 |
1.000 |
| 5 |
Ofloxacin |
0.031 |
0.000 |
0.000 |
0.000 |
1.782 |
1.000 |
Table 5: TOPKAT values of Synthetic compounds
Active site prediction
Based on the receptor cavity method using “eraser algorithm” we identified 9 active sites for the target protein. The amino acids of the first site were selected as active site for docking study. Number of amino acids are 48.(ARG189, ALA190, PRO191,GLU192, ILE193, MET194, LEU195, ASN196, TRP197, MET19 8, HIS199, TYR200, ASN201, GLN231, LEU232, LYS233, VAL239, GLY240, THR241, PRO242, GLY243, ALA244, GLU245,LEU246, LEU247, LYS248,LYS249,ILE250,SER251,SER252,GLU253,SER25 4,ALA255,ARG256,ASN257,TYR258,ILE259,GLN260,S ER261,LEU262,LEU289,VAL290,LEU291,ASP292,SER2 93,ASP294,LYS295,ARG296).
ADME studies
In this study, various pharmacokinetic and pharmacodynamics properties of 45 Natural and 38 synthetic compounds were analyzed, among which are Aq.Solubility, Human Intestinal Absorption, Plasma protein binding (PPB), Blood- Brain-Barrier (BBB) penetration, cytochrome p450 inhibition and hepatotoxicity levels.
ADME.2D.FPSA: ADME 2D Fast Polar Surface Area, ALOGP98: Hydrophobhicity Parameter, AQ.Sol.LEV: Predicts Aqueous solubility level, BBB LEV: Predicts blood-brain-barrier penetration level, ADMET ABSORPTION LEV: predicts the human intestinal absorption, CYP2D6: Predicts inhibition or non-inhibition of CYP450 2D6 enzyme, HEPATOTOX: Predicts hepatotoxicity or nonhepatotoxicity, PPB: Plasma Protein Binding.
Docking Studies
Docking studies were accomplished by employing Lead-IT and Gold 5.1 softwares to predict the affinity, activity, binding orientation of Ligands to the target protein 3HVC. The results of Lead-IT interaction between the target active site1 and top 3 natural and synthetic compounds can be seen in the Table 6.
| Natural Compounds |
| S. No |
COMPOUND NAME |
LEAD-IT |
| LEAD-IT SCORE |
H- BOND |
AMINO ACID |
AMINO ACID ATOM |
LIGAND ATOM |
H-BOND LENGTH |
| 1 |
Sodium_curcuminat e |
-25.6515 |
6 |
ASN196 |
HN_ |
O3 |
1.94813 |
| SER251 |
HG_ |
O4 |
2.11509 |
| SER252 |
HN_ |
O4 |
2.31651 |
| LEU291 |
O_ |
O6 |
3.00857 |
| MET198 |
SD |
H46 |
2.28514 |
| LEU291 |
O |
H47 |
2.20197 |
| 2 |
Curcumin |
-22.8393 |
5 |
GLU192 |
OE1_ |
O19 |
3.11623 |
| LEU291 |
O_ |
O19 |
2.82449 |
| SER293 |
HN_ - |
O19 |
1.9604 |
| SER29 |
HG_ |
O21 |
2.16459 |
| LEU291 |
O_ |
H40 |
1.90474 |
| 3 |
Gemichalcone_B |
-19.1219 |
5 |
LEU232 |
HN_ |
O6 |
1.96179 |
| LEU291 |
O_ |
O7 |
2.65086 |
| SER293 |
HN_ |
O7 |
2.0986 |
| SER252 |
OG_ |
H49 |
2.47718 |
| LEU291 |
O_ |
H62 |
1.79217 |
| Synthetic Compounds |
| 1 |
SB203580 |
-21.3518 |
5 |
GLN231 |
HE21 |
N6 |
2.3556 |
| VAL239 |
HN_ |
N5 |
2.11636 |
| THR241 |
HN_ |
O3 |
1.99065 |
| THR241 |
HG1_ |
O3 |
1.86805 |
| LEU232 |
O_ |
H2 |
1.81446 |
| 2 |
PD0325901 |
-19.3497 |
8 |
ARG189 |
O_ |
O6 |
2.81272 |
| ARG189 |
O_ |
O7 |
2.87114 |
| GLN231 |
HE21 |
O7 |
1.91117 |
| LYS233 |
O_ |
F4 |
2.00595 |
| GLY240 |
O_ |
H3 |
2.16259 |
| LEU232 |
O_ |
H3 |
1.7227 |
| ARG189 |
O_ |
H39 |
1.96117 |
| ARG189 |
O_ |
H40 |
1.92468 |
| 3 |
Cefazolin |
-17.8486 |
8 |
GLY243 |
O_ |
O4 |
2.83549 |
| GLY243 |
HN_ |
O6 |
2.12442 |
| ALA244 |
HN_ |
N11 |
1.60758 |
| ALA244 |
HN_ |
N14 |
1.99694 |
| GLU245 |
HN_ |
O4 |
1.85581 |
| LEU291 |
HN_ |
N12 |
1.84261 |
| LEU291 |
HN_ |
N15 |
2.35349 |
| LYS295 |
HZ2_ |
O5 |
2.05646 |
Table 6: Lead-IT docking interactions of Natural compounds Synthetic compounds
| S. No. |
Natural Compound |
Synthetic Compound |
| Compound name |
Lead-IT (score) |
Gold (score) |
TOPKAT |
Compound name |
Lead-IT (score) |
Gold (score) |
TOPKAT |
| 1 |
Sodium curcuminate |
-25.6515 |
76.1886 |
5 |
SB203580 |
-21.3518 |
60.2911 |
7 |
| 2 |
Curcumin |
-22.8393 |
71.1196 |
5 |
PD0325901 |
-19.3497 |
50.7694 |
4 |
| 3 |
Gemichalcone B |
-19.1219 |
69.449 |
5 |
Cefazolin |
-17.8486 |
63.3892 |
4 |
Table 7: Comparison of docking results between Natural and Synthetic Compounds
Discussion
Natural compounds have played an important role in treating and preventing human diseases than synthetic drugs which are being used, as they are more toxic and cause more side effects. The primary goal of the project is to unearth natural chemical compounds that can be used as a drug for the treatment of Leptospirosis in comparison with the synthetic drugs. Computational strategies for structure based drug discovery offer a valuable alternative to the costly and time consuming process of random screening [14]. According to Manjula Sritharan [1], Karen V Evangelista and Jenifer Coburn [15] there is a strong evidence of pathogen involved in the leptospirosis is Leptospira interrogans. CW Yang [16], suggested that Leptospira outer membrane proteins (OMPs) may elicit tubular injury and inflammation through Toll-like receptors (TLRs) dependent pathway causing tubular interstitial nephritis and necrosis. Durga devi M et al. [17] have taken p38α, a non-receptor serine/threonine kinase, plays an essential role in cell proliferation, cell differentiation and tumor genesis. Over expression of p38α enhances the production of cytokines, leading to inflammation causing cancers. Therefore, the protein p38α selected as a target for the inhibition of progression of inflammatory cancers. The above study helped us for the selection of Human p38α as a drug target for the synthesis of natural drugs for the disease. The 3D structure of the target protein has been retrieved from PDB with (PDB-ID: 3HVC). Energy minimization and active site prediction was done by using Accelrys Discovery Studio 3.5. The ligands for the study have been collected from many research articles. Lipinski’s rule of five was calculated for all the 50 Natural and 50 Synthetic ligand molecules that satisfy the rule of 5 and it was found that 48 Natural and 38 Synthetic computationally designed ligands were pre-filtered for their drug like properties. Similarly ADMET are key determinants whether the molecule can be taken further for drug development. Among these screened ligands top five have shown comparatively greater binding stability to the human p38α protein in both Lead-IT and GOLD suites. The five natural ligands are Sodium curcuminate, Curcumin, Gemichalcone_B, Diosmetin, Genistein showed comparatively most stable complex with human p38α protein followed by five synthetic ligands Cefazolin, SB203580, SB202190, Ofloxacin, PD0325901 than other screened compounds.
According to CHS Venkataramana et al, [18] aqueous solubility helps to predict the solubility of the compound in water. In this context, the compounds are observed to have good solubility so that they can have complete oral absorption for effective dosage. Gade Deepak Reddy et al, [19] concluded that Blood Brain penetration level shows the penetrating efficacy of compound towards the brain. In the present study, it is observed that all 5 natural compounds were fallen outside the 99% ellipse. Hence the compounds may not be able to penetrate the blood brain barrier. So, the chances of CNS side effects are low or absent [17]. But in case of 5 synthetic compounds in the table 4 with their BBB scores of Cefazolin, SB203580, SB202190, Ofloxacin, PD0325901 have shown variable penetrating efficacy. Sodium curcuminate, Curcumin, Diosmetin, Genistein scored 0 which implies these compounds do not inhibit CYP2D6 enzymes when they undergo metabolism via cytochrome p450 (CYP) enzymes for Gemichalcone_B which scored 1. The PPB (plasma protein binding) level shows whether the compound binds to carrier proteins in the blood [20]. Our result shows that most of the compounds have good binding capacity to cross the membrane and bind to plasma protein. Hepatotoxicity level predicts organ toxicity of the molecule and it falls in two levels. 0 for non-toxic and 1 for toxic. Based on these levels, the results suggest that the compounds are non-toxic so these can be used for further studies.
Docking studies
The concept of docking is important to determine the properties associated with proteinligand interactions such as binding energy, electron distribution, hydrogen bond donor acceptor properties and hydrophobicity. Two different scoring functions, Lead-IT score and Gold score were used. The Lead-IT suite provides the FLexX-scoring function, which was used to find the best poses. The best score from the best pose for each compound was taken and compound to the scores of the other compounds. The compounds which shows highest negative Lead-IT score shows that it has the capacity to bind strongly with the protein. Likewise GOLD is the most straightforward method of evaluating the accuracy of docking procedure to determine how closely the binding conformation is predicted by the scoring functions of the docking program. Higher binding capacity of the molecule to the protein is indicated by the positive gold score. GOLD and Lead-IT is employed to study the docking molecules within active site region of 3HVC and Accelrys, DS visualizer 3.5 is used to study the Hbond interaction. At the end of each run, docked orientations are saved and the resultant molecules are checked for geometry and number hydrogen bonds. Figure 1 shows the interaction modes of 3 selected Natural and Synthetic compounds with 3HVC receptor site.
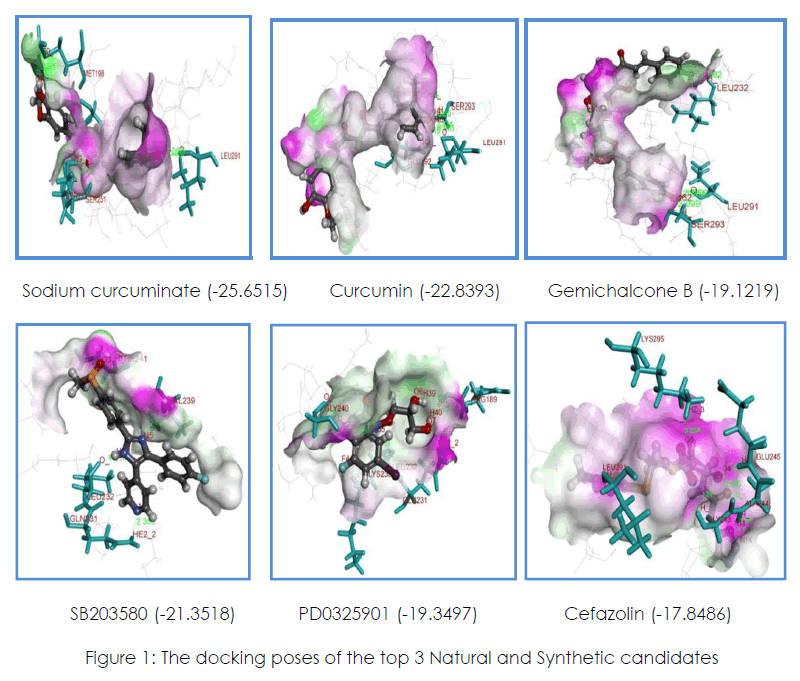
Figure 1: The docking poses of the top 3 Natural and Synthetic candidates
Then the selected compounds are subjected to toxicity prediction using TOPKAT. In NTP carcinogenicity call (Female rat) (v3.2), FDA carcinogenicity Male Rat Non vs. Carc (v3.1) all the compounds are devoid of any carcinogenicity, as all the compounds have got negative discriminant score. However compound A, B, C is decisively non-mutagenic. The Rat Oral LD50 values of all the compounds are within the Optimum Prediction Space (OPS). These high LD50 values suggest higher safety of these compounds [19]. None of the compounds showed skin irritation effect. This information’s concluded that the five compounds had good ADMET properties and can be taken further for docking studies.
According to Durga devi M et al. [17] inhibitory synthetic drug molecules of human p38α reported till date are in preclinical stages. In these Clinical studies, the drug molecules had shown side effects such as liver toxicity, development of lung tumors. The analysis and comparison of the docking results allows us to know the efficiency of the natural bioactive compound to control the Leptospirosis over Synthetic compounds. From the results obtained, it will be essential to understand the important structural features required to augment inhibitory compounds.
Conclusion
The Protein-Ligand interaction plays a significant role in structure based drug designing. In the present work the natural and synthetic ligands were docked with the target protein p38α [PDB ID: 3HVC]. Till date there is no work undertaken on in-silico analysis of natural compounds on human p38α protein, the present study analysed that the natural compounds Sodium curcuminate (Lead-IT score -25.6515) and curcumin (Lead-IT score - 22.8393) which belongs to same family of curcuma longa as well as Gemichalcone_B (Lead-It score -19.1219) are the probable compounds for leptospirosis. Natural compounds have shown good results when compared to synthetic compounds in terms of Lead-IT and GOLD docking softwares. As these compounds showed good effect on leptospirosis target, it can be taken for further preclinical and clinical studies.
7293
References
- Manjula Sritharan. Insights into Leptospirosis, a Neglected Disease, Zoonosis, 2012; 167-192.
- P Vijayachari, AP Sugunan and ANShriram. Leptospirosis: an emerging global public health problem. Journal of Bioscience. 2008; 33:557-569.
- Roy K and Paul S. Docking and QSAR studies of protoporphyringen oxidase inhibitor 3H-pyrazolo [3,4-d][1,2,3] triazin-4-one derivatives. J Mol Model. 2010; 16:137-153.
- Girija CR, Karunakar P, Poojari CS, Begum NS and SYED AA. Molecular docking studies of curcumin derivatives with multiple protein targets for procarcinogen activating enzyme inhibition. J Proteomics Bioinform, 2010; 3:200- 203.
- Xiaojin Xu, Xueyong Zhu, Raymond AD, James Stevens, Ian AW. Structural Characterization of the Influenza Virus H1N1 Neuraminidase. Journal of virology. 2008; 82(21): 10493-10501.
- Venkatachalam CM, Jiang X, Oldfield T, Waldman M. Ligand Fit: a novel method for the shape-directed rapid docking of ligands to protein active sites. J Mol. Graph. 2003; 21:289- 307.
- CA Lipinski, I Franco, BW Dominy, PJ Feeney. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev. 1997; 23: 3-25.
- Mayo SL, Olafson BD, Goddard WA. DREIDING: A Generic Force Field foe molecular simulations. J. phys. Chem. 1990; 94: 8897-8909.
- Egan WJ, Lauri G. Prediction of intestinal permeability. Adv. Drug Del. 2002; 54:273.
- Gregory M Bankil. ADME-TOX prerdiction: The more, the merrier, Current Drug Discovery. 2004.
- Xia X, Maliski EG, Gallant P, Rogers D. Classification of kinase inhibitors using a Bayesian model. J. Med. Chem. 2004; 47: 4463- 4470.
- Rarey M, Kramer B, Lengauer T, Klebe G. A Fast Flexible Docking Method using an Incremental construction Algorithm. J. Mol. Biol. 1996; 261: 470-489.
- G Jones, P Wilett, R C Glen, A R Leach, R Taylor. Development and validation of a genetic algorithm for flexible docking. J. Mol. Biol. 1997; 267:727-748.
- D Jayasimha Rayalu, D Muralidhara rao and DS Rao. Phytochemical screening and insilico approach for the identification of anti-stress compounds from medicinal plants. Mar 2013; 4:324-334.
- Karen V Evangelista and Jenifer Coburn. Leptospira as an emerging phathogen: a review of its biology, Pathogenesis and host immune responses. Further Microbiology. 2010; 5:1413-25.
- CW Yang. Leptospirosis renal disease initiation: Understanding the initiation by Toll-like receptors. Kidney International. 2007; 72:918-925.
- Durga Devi M, Dibyabhaba Pradhan, Manne Munikumar and Amineni Umamaheswari. Implementation of computational methods for designing potential inhibitors against human p38α protein. Nature precedings. 2010.
- CHS Venkataramana, KM Ramya Sravani, S Swetha Singh and V Madhavan. In-silico ADME and toxicity studies of some novel indole derivatives. Journal of Applied Pharmaceutical Science 2011; 1:159-162.
- Gade Deepak Reddy, KNV Pavan Kumar, N Duganath, Raavi Divya, Kancharla Amitha. ADMET, Docking studies & binding energy calculation of some Novel ACE-inhibitors for the treatment of Diabetic Nephropathy. International Journal of Drug Development & Research. 2012; 4: 268-282.
- SL Dixon, KM Merz. One dimensional molecular representations and similarity calculations: methodology and validation. Journal of Medicinal Chemistry. 2001; 44:3795-3809.







