Xiaolin Liu, Weiwei Nie, Jing Liang and Yan Li*
Department of Oncology, Qianfoshan Hospital, Shandong University, Jinan 250014, P.R. China
Corresponding Author:
Yan Li
Department of Oncology, Qianfoshan Hospital
Shandong University, No. 16766 Jingshi Road
Jinan 250014, Shandong Province, P.R. China
Tel: 86-531-89269316
Fax: 86-531-82963647
E-mail: qfsyyhlk@163.com
Received Date: March 06, 2017; Accepted Date: March 27, 2017; Published Date: April 03, 2017
Citation: Liu X, Nie W, Liang J, et al. Interaction of Helicobacter Pylori with Other Microbiota Species in the Development of Gastric Cancer. Arch Clin Microbiol 2017, 8:2. doi:10.4172/1989-8436.100067
Copyright: © 2017 Liu X, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Keywords
Helicobacter pylori; Gastric cancer; Microbiota
Introduction
The human gut microbiota comprises approximately 100 trillion microbial entities consisting of numerous bacteria, archaea and viruses [1]. The gastrointestinal microbiota is involved in numerous biological functions, such as energy harvest, regulation of the gut mucosal immune system, detoxification of xenobiotics and carcinogens, and protection against pathogens [2].
In this complex microbial ecosystem, Helicobacter pylori is a Gram-negative spiral bacterium that may colonize the gastric mucosa. Indeed, the bacterium is found in more than half of the world population. H. pylori is a prominent member of the human gastric microbiota. The microbe resides on the mucus layer of the gastric mucosa and is implicated in chronic gastritis, gastric and duodenal ulcers and even gastric cancer [3]. Here we aim to provide a comprehensive literature review on the roles of H. pylori and its interaction with other gastric microbes in gastric cancer, which may provide potential biomarkers for risk assessment of gastric cancer and new therapeutic targets.
Dysbiosis of Gastric Microbiota in H. pylori Associated Gastric Cancer
Gastric cancer is a multifaceted disease associated with different aetiologies, genetic changes and phenotypes. The adhesion capacity, enzymes and metabolic activity in the gastrointestinal tract could contribute largely to variation of different strains. A number of gastrointestinal conditions have been reported to be correlated with alterations in the gut microbiota, which result from changes in the life styles and dietary habits. Approximately 1000 species from relatively few phyla have been reported to be present in gut microbiota population [4]. The species are members of the phyla Firmicutes and Bacteroidetes, with very minimal number being representatives of the Proteobacteria, Fusobacteria ? Cyanobacteria, Verrucomicrobia and Actinobacteria, among other species [5].
Epidemiologic study showed that gastric cancer was the fourth most prevalent cancer and the second leading cause of cancer-resulted death [6]. H. pylori infection is considered the single most important risk factor for gastric cancer [7]. Andersson et al. showed that the stomach displayed a diverse microbiota composition when H. pylori is absent or insignificant whereas samples positive for H.pylori were predominated by this bacterium [8]. It was higher in H. pylori -infected patients compared with those uninfected individuals (P=0.005) [9]. The unweighted principal coordinate analysis showed that the communities of microbiota in gastric cancer were more diversified. Five genera of bacteria with potential carcinogic activities were enriched in patients with gastric cancer, including Lactobacillus, Escherichia-Shigella, Nitrospirae, Burkholderia fungorum, and unculturable Lachnospiraceae species. Of particular interest, Nitrospirae was present in all patients with gastric cancer but absent in patients with chronic gastritis [9].
Eun et al. examined the microbial composition of gastric mucosa from patients with gastric cancer in different stages of disease and revealed that the relative abundance of Helicobacteraceae family was significantly lower in gastric cancer patients than those with chronic gastritis and intestinal metaplasia and that the opposite pattern was true for the relative abundance of Streptococcaceae family [10]. Compared with the intestinal metaplasia group, the relative abundances of Epsilonproteo bacteria in the gastric cancer group were significantly decreased. On the contrary, the homogeneity and diversity of gastric microbiota in the gastric cancer group were increased. In addition, two studies showed an overall lower prevalence of H. pylori in gastric cancer patients than that in the control group [11,12]. Examination on the gastric microbiota of 63 antral mucosal and 18 corpus mucosal samples via pyrosequencing of the 16S rRNA gene revealed that the abundance of nitrate-reducing bacteria other than H. pylori was two times higher in the cancer groups than in the control groups [13] As such, H. pylori is a crucial microbe affecting the clinical manifestations and prognosis of gastric cancer [14].
Alterations of Gastric Microbiota after H. pylori Infection
Recent studies have revealed significant differences between the microbiota of individuals with and without H. pylori infection. For example, interaction between H. pylori and the normal gut microbiota was found during early stages of life using germ-free and specific pathogen free mice models [15]. In H. pylori-negative individuals, the most abundant phyla are typically Firmicutes, Bacteroidetes, and Actinobacteria [16]. The common phylotypes found in H. pylori -negative individuals include Streptococcus, Prevotella, and Gemella [8]. Consistent with these results, the composition of the microbiota between H. pylori -positive subjects and H. pylori -negative controls were different in clostridia and the total number of anaerobes [17]. H. pylori accounts for >90% of all sequence reads and greatly reduces the overall diversity of the gastric microbiota in H. pylori-colonized individuals. Proteobacteria, Firmicutes, and Actinobacteria were the most abundant phyla in H. pyloricolonized human stomachs [18]. H. pylori infection increased the relative abundance of Proteobacteria, Spirochaetes, and Acidobacteria while decreasing the that of Actinobacteria, Bacteroidetes and Firmicutes (Table1) [19].
| Group |
Microbiota |
| H.pylori-negative |
Firmicutes, Bacteroidetes, and ctinobacteria |
| H.pylori-uninfected |
Streptococcus, Prevotella, Gemella |
| H.pylori-colonized |
Proteobacteria, Firmicutes, and Actinobacteria |
Table 1: Alteration of microbiota effected by H. pylori.
Atrophy is one of the key steps in the histologic progression culminating in intestinal-type gastric cancer [19]. In the context of atrophic gastritis, non-molecular analyses have shown that in the absence of H. pylori, urease-producing members of the gastric microbiota, such as Proteus mirabilis, Klebsiella pneumonia, Staphylococcus aureus, Staphylococcus capitis, and Micrococcus species can bloom to levels that cause false positive results in H. pylori urea breath tests [20]. Specifically, the microbiota of patients with gastric cancer was dominated by species of Streptococcus, Lactobacillus, Veillonella, and Prevotella. Likewise, another study reported that Lactobacilli was increased in H. pylori- infected patients [21]. In addition, S. mitis was found to be significantly enriched in the stomach of atrophic gastritis and gastric cancer patients. Using a co-culture method, S. mitis produced and released one or more diffusible factors that directly or indirectly induce coccoid conversion of H. pylori cells [22]. Compared to peptic ulcer samples, coccoid H. pylori cells were abundant in gastrectomy specimens from adenocarcinoma [23]. The involvement of coccoid H. pylori in carcinogenesis might due to its stronger effect on proliferation and weaker effect on apoptosis of gastric epithelial cells [24]. Another bacterium commonly present in the stomach, L. fermentum could promote survival of S. mitis via secreting diffusible factors [25].
From these observations, it is tempting to hypothesize that the interactions between H. pylori and other bacteria might play important roles in H. pylori-associated diseases, especially gastric cancer.
Mechanisms of H. pylori Interaction with other Microbiota Involved in the Progress of Gastric Cancer
One critical event of gastric cancer is the rise of pH which may increase abundance of bacteria in the gastric environment. It has been acknowledged that either acute or chronic H. pylori infection can induce perturbations of acid secretion in the gastric environment [26]. Changes of gastric pH due to the decrease in acid secretion can encourage colonization of non-H. pylori bacterial species from oral mucosa, the upper-respiratory tract, or the intestines that normally cannot persist in the healthy stomach [27]. Persistent infection of the gastric mucosa by H. pylori initiates an inflammatory cascade that progress into atrophic gastritis and produces insufficient acid that increases the risk of developing gastric cancer [11,28].
H. Pylori uses different mechanisms to inhibit acid secretion. H. pylori can indirectly inhibit parietal cell function as a result of changes in cytokines as well as hormone levels, such as interleukin 1β (IL-1β), and tumor necrosis factor-α [29]. CagApositive H. pylori strains have been shown to affect the secretion of several hormones, including 5-HT, ghrelin, dopamine, and gastrin, and altered levels of these hormones might be the cause of the psychological disorders of functional dyspepsia patients [30]. Our study has indicated that H. pylori eradication was associated with long-term disturbance in three hormones (active amylin, PP and total PYY) both pre- and post-prandially [31]. Indeed, as the gastric acidity reduced in late stages of chronic H. pylori infection, other bacterial species appeared to outgrow H. pylori [32]. Thus, the beneficial effects of antibiotic eradication regimens could be explained in part by the effects of interbacterial competition. The gastrointestinal tract is a complex and dynamic network that includes interactions between intestinal mucosal cells, the immune system, food particles, and the commensal microbiota. An intricate and mutualistic symbiosis modulates relationship between the host and the gut microbiota. The interaction of H. pylori and the gut microbiota needs to be further studied.
The risk of developing gastric cancer is associated with H. pylori strains, variations in host responses governed by genetic diversity, and/or specific interactions between host, microbial, and environmental determinants [20]. It has been shown that the carcinogenic effects of H. pylori infection have been mainly linked to the cag pathogenicity island (cag PAI) and the vacuolating cytotoxin gene A (vacA) [33]. The cytotoxinassociated gene A (cagA) is the most investigated gene of the cag PAI. CagA protein, encoded by cagA, could be delivered into mammalian cells and affect cytoskeletal and tissue structure, as well as cell proliferation. Infection with cagA-positive H. pylori strains is associated with high risk of peptic ulcers and gastric carcinoma. H. pylori strains that harbor the cag PAI (Cag+ strains) are associated with a significantly increased risk of gastric cancer compared to cag–strains. Non-phosphorylated CagA also exerts effects within host cells that affect oncogenesis. Following translocation, phospho-CagA by Src and Abl kinases subsequently interacts with and activates several host cell proteins, result in morphological alterations such as cell scattering and elongation.
CagA can activate the transcription factor NF-1 and stimulate expression of IL-8, leading to neutrophil infiltration into the gastric mucosa [34]. In addition, CagA also induces DNA damage in vitro and in rodent models of infection which have been validated in human subjects colonized with H. pylori cag+ strains. Cag+ strains contract with host cells and thereby activate multiple signaling pathways that may increase the risk for malignant transformation during prolonged colonization. H. pylori CagA induce TWIST1 expression and EMT in gastric cancer cells by regulating PDCD4 [35].
VacA is another H. pylori component linked to the development of gastric cancer. The toxin is secreted by H. pylori as an 88 kDa monomer (p88) consisting of two domains (p33 and p55) [36]. Infection of epithelial target cells by H. pylori has been demonstrated to be associated with both increased and reduced levels of apoptosis [37]. VacA causes multiple alterations in human cells, including autophagy, vacuolation, altered plasma and mitochondrial membrane permeability and apoptotic cell death. All H. pylori strains possess vacA and produced marked variation in the 5’ region of VacA sequences, which encodes the signal. VacA and CagA can mutually regulate each other to affect host cell responses [38]. CagA antagonizes VacA-induced apoptosis, which activates the survival pathway mediated by the MAPK ERK [39]. CagA also activates EGFR signaling inhibited by VacA [40]. Recently, it was shown that the opposing effects of CagA and VacA may be cell-lineage specific.
A growing body of evidence has suggested that inflammation is a critical component of tumor progression in stomach. For example, many cancers arise from sites of infection, chronic irritation and inflammation [41]. H. pylori induced chronic inflammation is believed to be one of the mechanisms in the development of gastric cancer. It is revealed that colonization with both H. pylori and complex intestinal flora resulted in a greater inflammatory response, manifested by higher proinflammatory gene expression levels compared with those treated with H. pylori alone or with restricted Altered Schaedler's Flora [42]. Various studies have demonstrated that the interaction between H. pylori and the microbial community in the stomach may stimulate inflammatory signals, which contribute to the development and progression of cancer. Overgrowth of some bacterial species may enhance inflammatory responses accelerating atrophy, metaplasia,and cancer [43]. The alterations of gastric environment, hormones levels, immunity and inflammatory response might attribute to H. pylori-associated gastric cancer.
Production of IL-1, which may inhibit gastric acid secretion, is increased in the gastric mucosa of infected individuals in comparison with uninfected controls [20]. Polymorphisms within the IL-1 gene cluster that increase IL-1 production are associated with significant increases in risk for hypochlorhydria, gastric atrophy, and distal gastric adenocarcinoma compared with lowexpression IL-1 genotypes [44]. Virulent strains of H. pylori in a genetically susceptible person further increase the risk for gastric cancer. High-expression IL-1 alleles infected with H. pylori cagA+ or vacA-type strains increases in risk for gastric cancer compared to uninfected persons at 25-fold or 87-fold [20].
Diet may also be a factor influencing gastric cancer development. Iron depletion accelerates the development of gastric dysplasia and cancer by promoting assembly and activity of the H. pylori cagPAI [45]. H. pylori-induced expression of TLR-2 and TLR-5 can qualitatively shift cag PAI- ependent to cag PAIindependent pro-inflammatory signaling pathways with possible impact on the outcome of H. pylori -associated diseases [29]. H. pylori predominantly activates the NLRP3 inflammasome through a process that requires TLR2-dependent licensing. H. pylori can activate the inflammasome and caspase-1 in antigenpresenting and other cells, in result to the processing and release of caspase-1-dependent cytokines [46]. It is reported that activation of the TLR2/NLRP3/caspase-1 axis is required for acid adaptation (Figure 1).
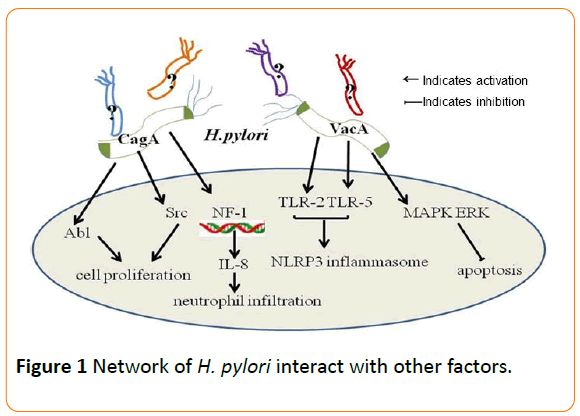
Figure 1: Network of H. pylori interact with other factors.
Conclusions
Gastric cancer is still a major challenge worldwide; because detection is frequently made only at an advanced stage, mortality remains high. Gastric cancer prevention programmes via H.pylori eradication have been shown to benefit high-risk populations [47]. The best results from gastric cancer prevention strategies are obtained when H. pylori eradication is performed before advanced atrophic gastritis with changes becomes established and thus implementation of H. pylori screening and treatment in early adulthood is required. H. pylori infection could decrease acid secretion by the production of ammonia and bicarbonate from urea. Additionally, these two products could serve as substrates for other bacteria and the gastric microbiota changed [35].
In contrast to studies comparing the composition of the gastric microbiota between H. pylori-infected and -uninfected subjects, few studies have examined differences in microbial composition and outcomes from diseases such as gastric cancer [48]. The composition of the gastric microbiota in wellcharacterized human populations with and without gastric cancer, are needed further clearly studied. Simultaneously, the alteration of gastric microbiota during H. pylori infection might in turn exert a dramatic effect on H. pylori itself and thus promote carcinogenesis. This will help to find more personalized therapies and potential approaches to treat gastric cancer.
It is reported that H. pylori eradication from the residual gastric mucosa after the endoscopic treatment of early gastric cancer may result in an inhibitory effect on the occurrence of metachronous gastric cancer [49]. Triple therapy (proton-pump inhibitors+amoxicillin+clarithromycin) is the first-line regimen for the eradication of H. pylori. Quadruple therapy (protonpump inhibitors+bismuth subcitrate potassium+metronidazole +tetracycline) remains the best second-choice treatment. Conversely, several meta-analyses have shown that the progression of atrophic gastritis and intestinal metaplasia to gastric cancer can indeed occur following H. pylori eradication, suggesting that other factors contribute to the progression of pre-neoplastic lesions. Thus, the actual role of H. pylori eradication in the prevention of gastric cancer is still a matter of wide debate.
In conclusion, it can be seen that various stages of the development of gastric cancer involve the progression from H. pylori superficial gastritis to atrophic gastritis and then the further progression to development of dysplasia and cancer. Numerous co-factors are involved in promoting or inhibiting this progression. However, H. pylori infection does appear to be an obligatory co-factor in most cases of non-cardia gastric cancer. Consequently, prevention of H. pylori infection or its eradication at an early stage should markedly reduce the incidence of this common and usually fatal cancer.
18664
References
- Walsh CJ, Guinane CM, O'Toole PW, Cotter PD (2014) Beneficial modulation of the gut microbiota. FEBS letters 588:4120-4130.
- He C, Yang Z, Lu N(2016) Imbalance of Gastrointestinal Microbiota in thePathogenesis of Helicobacter pylori-Associated Diseases. Helicobacter 21: 337-348.
- Titov SE, Panasyuk GV, Ivanov MK, Dymshits GM (2011) Detection ofHelicobacter pylori in bioptates of gastric mucosa of patients with gastritisand gastric ulcers using real-time PCR. Mol Genet MicrobiolVirol26:126-131.
- Kemperman RA, Gross G, Mondot S, Possemiers S, Marzorati M, et al. (2013)Impact of polyphenols from black tea and red wine/grape juice on a gutmodel microbiome. Food ResInt 53:659-669.
- Nelson KE (2015) An Update on the Status of Current Research on the Mammalian Microbiome. ILAR journal/National Research Council, Institute ofLaboratory Animal Resources 56:163-168.
- Chen B, Luo RC, Cui F, Qian XY (2006) Association of HER-2/neu expression with prognosis of gastric cancer. J South Med Univer26:344-347.
- Conteduca V, Sansonno D, Lauletta G, Russi S, Ingravallo G, et al.(2013) H. pylori infection and gastric cancer: state of the art (review). IntJOncol 42: 5-18.
- Andersson AF, Lindberg M, Jakobsson H, Bäckhed F, Nyrén P, et al.(2008)Comparative analysis of human gut microbiota by barcoded pyrosequencing. PloSOne 3:e2836.
- Wang L, Zhou J, Xin Y, Geng C, Tian Z, et al.(2015) Bacterial overgrowth anddiversification of microbiota in gastric cancer. EurJGastroenterolHepatol28: 261-266.
- Eun CS, Kim BK, Han DS, Kim SY, Kim KM, et al.(2014) Differences in gastricmucosal microbiota profiling in patients with chronic gastritis, intestinalmetaplasia, and gastric cancer using pyrosequencing methods. Helicobacter 19:407-416.
- Dicksved J, Lindberg M, Rosenquist M, Enroth H, Jansson JK, et al.(2009) Molecular characterization of the stomach microbiota in patients with gastriccancer and in controls. J MedMicrobiol58:509-516.
- Hashemi MR, Rahnavardi M, Bikdeli B, DehghaniZahedani M (2006) H pyloriinfection among 1000 southern Iranian dyspeptic patients. World J Gastroenterol12:5479-5482.
- Jo HJ, Kim J, Kim N, Ji HP, Nam RH,et al.(2016) Analysis of Gastric Microbiotaby Pyrosequencing: Minor Role of Bacteria Other Than Helicobacter pylori inthe Gastric Carcinogenesis. Helicobacter 21: 364-374.
- Qiu HB, Zhang LY, Keshari RP, Wang GQ, Zhou ZW, et al.(2010) Relationshipbetween H.pyloriinfection and clinic-pathological features and prognosis ofgastric cancer. BMC Cancer10:1-9.
- Org E, Parks BW, Joo JW, Emert B, Schwartzman W, et al.(2015) Genetic andenvironmental control of host-gut microbiota interactions. Genome Res25:281-294.
- Abreu MT, Peek Jr RM (2014)Gastrointestinalmalignancy and the microbiome. Gastroenterol146: 1534-1546.
- Ottman N, Smidt H, de Vos WM, Belzer C (2012) The function of ourmicrobiota: who is out there and what do they do? Front CellInfectMicrobiol 2:608-616.
- Yang I, Nell S, Suerbaum S (2013) Survival in hostile territory: the microbiota of the stomach. FEMS MicrobiolLett 37:736-761.
- Maldonadocontreras A, Goldfarb KC, Godoyvitorino F, Karaoz U, Contreras M, et al. (2011) Structure of the human gastric bacterial community in relation to Helicobacter pylori status. Isme Journal 5:574-579.
- Abreu MT, Peek RM (2014) Gastrointestinal Malignancy and the Microbiome -Gastroenterology. Gastroenterol146:1534-1546.
- Tam YH, Lee KH, To KF, Chan KW, Cheung ST (2009) Helicobacter pylori-positiveversus Helicobacter pylori-negative idiopathic peptic ulcers in children with their long-term outcomes. J PediatrGastroenterolNutr 48:299-305.
- Faghri J, Poursina F, Moghim S, Zarkesh EH, Nasr EB, et al. (2014) Morphological and Bactericidal Effects of Different Antibiotics on Helicobacterpylori. Jundishapur JMicrobiol 7:e8704-e8704.
- Conteduca V, Sansonno D, Lauletta G, Russi S, Ingravallo G, et al. (2012) H. pylori infection and gastric cancer: state of the art (review). IntJoncol 42:5-18.
- Li N, Han L, Chen J, Lin X, Chen H, et al. (2013) Proliferative and apoptoticeffects of gastric epithelial cells induced by coccoidHelicobacter pylori. J Basic Microbiol 53:147–155.
- ForsgrenBrusk U, Husmark U, Grahn HE, Rönnqvist D (2012) Biologically pure strain of Lactobacillus fermentum , strain Ess-1.U.S. Patent8,222,020.
- Cong H, Zhen Y, Lu N (2016) Imbalance of Gastrointestinal Microbiota in thePathogenesis of Helicobacter pylori -Associated Diseases. Helicobacter 21: 337-348.
- Kim E, Yang J, Kim HO, An Y, Lim EK, et al. (2013) Hyaluronic acid receptor- targetable imidazolizednanovectors for induction of gastric cancer cell deathby RNA interference. Biomaterials 34:4327-4338.
- Abdulrahman IS, Alhwiesh AK (2012) Gastroduodenal lesions in peritonealdialysis patients: the role of Helicobacter pylori infection. Nephrology Reviews4: 1
- Kumar PS, Brandt S, Madassery J, Backert S (2011) Correction: Induction of TLR-2 and TLR-5 Expression by Helicobacter pylori Switches cagPAI- Dependent Signalling Leading to the Secretion of IL-8 and TNF-α. PloSOne 6:e19614.
- Meng WP, Wang ZQ, Deng JQ, Liu Y, Deng MM, et al. (2016) The Role of H. pyloriCagA in Regulating Hormones of Functional Dyspepsia Patients.Gastroenterol Res Pract4:1-10.
- Yap WC, Leow HR, Azmi AN, Francois F, Perezperez GI, et al. (2015): Changes in Metabolic Hormones in Malaysian Young Adults following Helicobacter pylori Eradication. PloSOne 10:e0135771.
- Sheh A, Fox JG (2013) The role of the gastrointestinal microbiome inHelicobacter pylori pathogenesis. Gut Microbes 4:505-531.
- Correa P, Piazuelo MB (2012) Evolutionary History of the Helicobacter pyloriGenome: Implications for Gastric Carcinogenesis. Gut and Liver 6:21-28.
- Ren S, Higashi H, Lu H, Azuma T, Hatakeyama M (2006) Structural basis andfunctional consequence of Helicobacter pylori CagAmultimerization in cells.JBiolChem 281:32344-32352.
- Yu H, Zeng J, Liang X, Wang W, Zhou Y, et al. (2014) Helicobacter pylori promotes epithelial-mesenchymal transition in gastric cancer by down regulating programmed cell death protein 4 (PDCD4). PloSOne 9:e105306.
- Gangwer KA, Mcclain MS, Eli IM, Chambers MG, Ohi MD, et al. (2010)Reconstitution of Helicobacter pylori VacA Toxin from Purified Components. Biochem 49:5743-5752.
- Liu H, Liu W, Fang D, Wang Z, Liang H, et al. (1998) Effect of Heilicobacter Pylori on Gastric Epithelial Cell Proliferation and Apoptosis.Chinese JDigest Endoscopy 15:91-93.
- Rhead JL, Letley DP, Mohammadi M, Hussein N, Mohagheghi MA, et al. (2007)A New Helicobacter pylori VacuolatingCytotoxin Determinant, theIntermediate Region, Is Associated With Gastric Cancer. Gastroenterol133:926-936.
- Grivennikov SI, Wang K, Mucida D, Stewart CA, Schnabl B, et al. (2012) Adenoma-linked barrier defects and microbial products drive IL-23/IL-17-mediated tumour growth. Nature 491:254-258.
- Backert S, Tegtmeyer N (2010) The versatility of the Helicobacter pylorivacuolatingcytotoxinvacA in signal transduction and molecular crosstalk. Toxins 2:69-92.
- Lertpiriyapong K, Whary MT, Muthupalani S, Lofgren JL, Gamazon ER, et al.(2013) Gastric colonisation with a restricted commensal microbiota replicatesthe promotion of neoplastic lesions by diverse intestinal microbiota in theHelicobacter pylori INS-GAS mouse model of gastric carcinogenesis. Gut 63: 763-766.
- Krzysiek-Maczka G, Targosz A, Ptak-Belowska A, Korbut E, Szczyrk U, et al.(2013) Molecular alterations in fibroblasts exposed to Helicobacter pylori: amissing link in bacterial inflammation progressing into gastric carcinogenesis? J PhysiolPharmacol 64: 77-87.
- Zhang D, Zheng H, Zhou Y, Tang X, Yu B, et al. (2006) Association of IL-1beta gene polymorphism with cachexia from locally advanced gastric cancer. BMC Cancer 7:1-7.
- Noto JM, Gaddy JA, Lee JY, Piazuelo MB, Friedman DB, et al. (2013) Iron deficiency accelerates Helicobacter pylori–induced carcinogenesis in rodents and humans. J ClinInvest 123: 479-492.
- Koch KN, Muller A (2015) Helicobacter pylori activates the TLR2/NLRP3/caspase-1/IL-18 axis to induce regulatory T-cells, establishpersistent infection and promote tolerance to allergens. Gut Microb 6:382-387.
- Ford AC, Forman D, Hunt RH, Yuan YH, Moayyedi P (2014) Helicobacter pylori eradication therapy to prevent gastric cancer in healthy asymptomatic infected individuals: systematic review and meta-analysis of randomised controlled trials. BMJ348: 3174.
- Sheh A, Fox JG (2013) The role of the gastrointestinal microbiome inHelicobacter pylori pathogenesis. Gut Microb 4:505-531.
- Conteduca V, Sansonno D, Lauletta G, Russi S, Ingravallo G, et al. (2013) H. pylori infection and gastric cancer: state of the art (review). IntJOncol 42:5-18.






