1Isaac, C., 1,*Nmorsi, O.P.G., 1Igbinosa, I.B., 2Umukoro, D.O. 3Imarenezor, E.P.K 2,4 Ukwandu and N.C.D.
1Tropical Disease Research Unit, Department of Zoology, Ambrose Alli University, Ekpoma, Nigeria
2Family Medicine Department, Delta State University, Abraka.
3Department of Microbiology, Ambrose Alli University, Ekpoma, Nigeria
4Department of Medical Microbiology, Ambrose Alli University, Ekpoma, Nigeria
*Corresponding Author:
Prof. O.P.G. Nmorsi
Tropical Disease Research Unit
Department of Zoology, Ambrose Alli University
Ekpoma, Nigeria
E-mail: nmorsiopg@yahoo.com
Introduction
Sleeping sickness or human African trypanosomiasis [HAT] is an endemic parasitic disease exclusively located in intertropical Africa where it is transmitted by the Glossina vector [1]. HAT if not detected and treated at the early stage could progress to the late stage [2] The control of African trypanosomiasis requires among others the contribution of variant specific glycoproteinspecific B- and T-cell responses [3,4] as well as the macrophage/ monocyte phagocyte system [5,6]. Cytokines orchestrate a type I and/or a type II immune response, which influences the outcome of African trypanosomiasis [7,8,9,10].
Trypanosome-derived products have been shown to activate the generation of pro-inflammatory mediators including IL-1a by macrophages [11]. Chronic secretion of macrophage-derived mediators is in part responsible for the pathogenic aspects of HAT [12,13]. In laboratory infected mice with T.b. brucei, an increase in the level of IL-1a has been reported [7], including upregulation of granulocyte and macrophage due to IL-1a administration in mice [14]. Synergistic role of IL-1a led to enhanced cytokine production and haematopoietic recovery [15,16].
IL-7, a type 1 cytokine is documented to be induced by IL- 1a and TNF- a [17], has been implicated in the pathology of trypanosomiasis where CD8+ predominance was reported [8]. Also, Th2 cells produced anti-inflammatory cytokines due to B-cell predominance following an increase in total cell number in spleen and lymph nodes of protozoan-infected mice treated with IL-7 [18]. Altered genes encoding IL7R alpha and liquid IL-7 in the CSF compartment have been observed in individuals with disease of the central nervous system [19].
The role of type II cytokines [IL-4, IL-10 and IL-13] in resistance to African trypanosomiasis is speculative based on contrasting reports that it could exert deleterious [20], protective [9,21] or no effect on the outcome of trypanosomiasis disease [22]. It was shown that IL-13, an anti-inflammatory cytokine, did not influence the susceptibility of Trypanosoma infection in mice and were not the main trigger to alternative macrophage activation [10]. IL-13 has been suggested to be a good indicator for the course of CSF infections [23].
Despite the relative importance of cytokines in the control of HAT and their divergent roles in the immunopathology of this disease, there is paucity of information in relation to the status of IL-1a, IL-7 and IL-13 in T.b. gambiense-infected individuals in our locality. This investigation therefore seeks to determine the profiles of these cytokines in HAT positive volunteers in Abraka, an endemic focus in Nigeria.
Materials and methods
Study area
This study was carried out in three communities [Umeghe, Urhouka and Ugono] of Abraka, a HAT endemic focus of Nigeria. The three Abraka communities surveyed are communities. Aforementioned communities lie between latitude 5°47’-6°15N and longitude 5°.42’-6°E with population of over 5,000, who are predominantly farmers.
Ethical considerations
Ethical permission was obtained from the Delta State Ministry of Health and Eku Baptist Hospital. Before the commencement of the investigation, the nature, objectives and benefits of the investigation were thoroughly explained to inhabitants of these communities. Informed consents were obtained from 474 consented volunteers who were screen for Trypanosomiasis infection using card agglutination test for trypanosomiasis [CATT] kit [24].
Population recruited
Of the 474 screened, sera of 35 seropositive subjects with T.b. gambiense infection were evaluated for interleukins [IL-1a , IL-7 and IL-13] levels. Also, 16 volunteers from the 35 seropositive subjects, demonstrated trypanosomes in their serum.
Staging of HAT
Sera obtained from venous blood were used to categorize the level of infection by double serial dilution as: weakly positive [1:2-1:4] [n=9], moderately positive [1:8-1:16] [n=12] and strongly positive [≥1:32] [n=14] according to the manufacturer’s instruction [Intituut voor Tropische Geneeskunde, Antwerpen, Belgium]. Of the 35 seropositives, 16 volunteers demonstrated parasite in the blood and were further categorized into early and late stage of HAT. Volunteers categorized as early stage, demonstrated the parasite in the blood only while late stage showed the parasite both in the blood and CSF. The staging of HAT was done on the recommendation of [25]. Most of the seropositive volunteers were observed to suffer from malaise, anaemia, headache, pyrexia, weight loss and weakness following the medical history and physical examination carried out on the volunteers.
Exclusion criteria
We excluded volunteers with overt diseases like viral hepatitis B, HIV or sickle cell anaemia using standard procedures.
Interleukin assay
Sera and CSF were obtained from 35 HAT positive subjects and then assayed for IL-1a, IL-7 and IL-13 using standard enzyme linked Immunosorbent assay [ELISA] according to the manufacturer’s instruction [Abcam Plc, United kingdom]. Also, interleukin evaluation was carried out on 10 control group who were selected on the basis of being HAT negative and also not infected with the aforementioned diseases.
Data analysis
Data obtained were subjected to statistical analysis, namely Welch t-test and Tukey-analysis of variance [ANOVA] using Instat statistical package.
Results
Figure 1 shows IL-1a profile among categories of seropositive human subjects. The differences in the levels of IL-1a for weakly positive and moderately positive individuals compared with the control subjects were not significant [p>0.01]. Serum IL-1a levels were significantly higher in strongly positive volunteers than control subject [p<0.05]. The serum IL-1a concentrations for the categories of seropositive subjects were not significant [p>0.01].
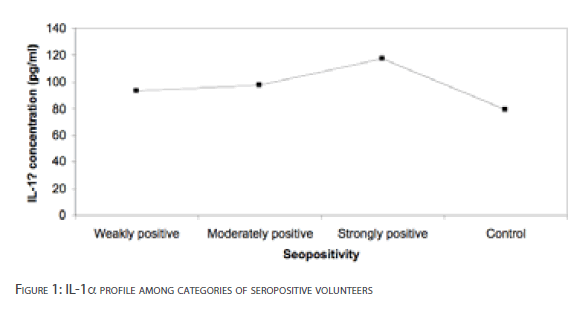
Figure 1: IL-1a profile among categories of seropositive volunteers
Table 1 presents the IL-1a concentrations in serum and CSF of HAT volunteers with early and late stages of infection. The difference in serum IL-1a concentrations between early and late stages of infection was statistically significant [p<0.001]. Significant depression of CSF IL-1a concentrations was observed for HAT late stage [p<0.001].
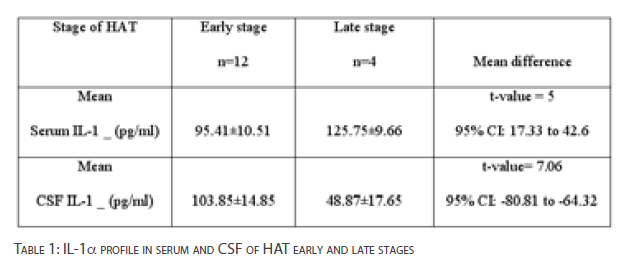
Table 1: IL-1a profile in serum and CSF of HAT early and late stages
The levels of IL-7 for weakly positive and moderately positive volunteers were not significantly depressed [p>0.05]. However, IL-7 level in strongly positive volunteers was significantly lower than the control subjects [p<0.001]. There was no significant difference in the serum IL-7 concentration among seropositive subjects [p>0.01] [Figure 2].
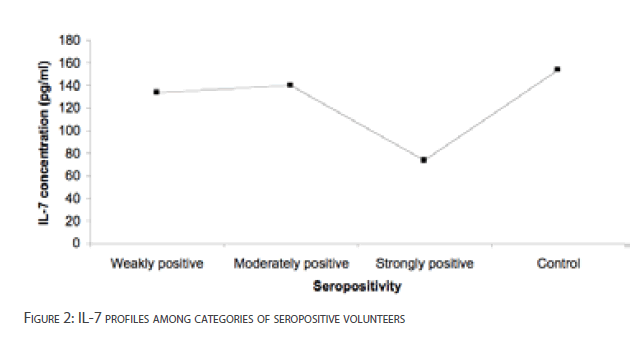
Figure 2: IL-7 profiles among categories of seropositive volunteers
Table 2 shows IL-7 concentrations in serum and CSF of individuals with HAT early and late stages of infection. The mean difference in serum IL-7 concentrations between early stage and late stage of infection was statistically significant [p<0.05].The CSF IL-7 concentration was significantly higher in volunteers with early stage of HAT than late stage [p<0.05].
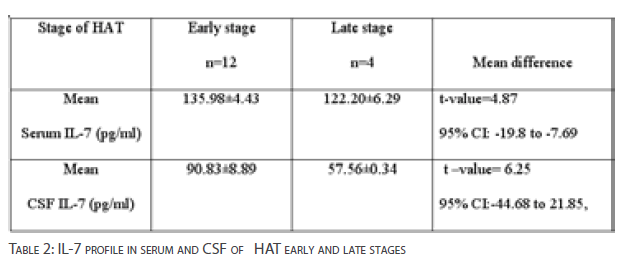
Table 2: IL-7 profile in serum and CSF of HAT early and late stages
The mean differences of IL-13 levels between weakly positive, moderately positive and strongly positive volunteers compared with HAT negative volunteers were not significant [p>0.05] [Figure 3]. The differences in the levels of IL-13 among the categories of seropositive volunteers were also not significant [p>0.01].
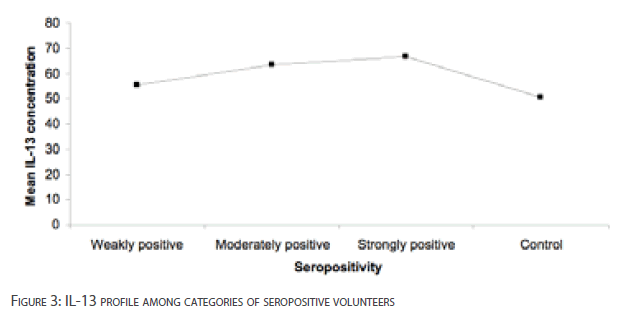
Figure 3: IL-13 profile among categories of seropositive volunteers
Table 3 shows IL-13 concentrations in serum and CSF of volunteers with early and late stages of HAT. The mean difference in serum IL-13 concentrations between HAT early stage and late stage of infection was not statistically significant [p>0.05].There was also no significant difference in the mean CSF IL-13 concentration between HAT early stage and late stage [p>0.05].
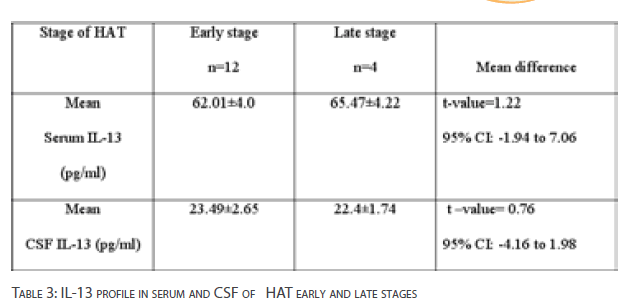
Table 3: IL-13 profile in serum and CSF of HAT early and late stages
Discussion
Our data demonstrated elevated level of serum IL-1a among the strongly seropositive volunteers. Similarly, serum IL-1a levels of HAT late stage patients were increased compared to early stage. In contrast, we also showed depressed concentration of CSF IL- 1a in HAT late stage. The elevated levels of serum IL-1a supports the report of [11] in which derived variant surface glycoprotein [VSG] from T. brucei, elicit IL-1a. This suggests that IL-1a may be implicated in the immunopathogenesis of HAT infection. Also, we report depressed levels of CSF IL-1a in HAT late stage. We observed that the profile of IL-13, an anti-inflammatory cytokine was unaltered in the CSF of HAT late stage. This data therefore suggests that the imbalance between IL-13 and IL-1a could be due to the inability of IL-13 to immunomodulate IL-1a in HAT late stage. This partly contradicts the hypothesis of a switch from dominant type I to a predominant type II cytokine [26, 27]. However, we hypothesize that other anti-inflammatory cytokines such as IL-10 may be implicated in the down regulation of this cytokine in the CSF [28].
We observed depressed levels of IL-7 with severity of infection. These findings contradicts the report that human chronic trypanosomiasis was associated with elevated IL-7 production [8]. It has been documented that natural killer cells are recognized as major effectors of innate resistance to protozoan infections through the control of the growth of this pathogens by indirectly producing cytokines [29]. In a report, IL-7 deficient patients were observed to be deficient of T and natural killer [NK] cells [30]. This study therefore suggests that depressed IL-7 concentration with disease progression may be associated with the pathogenic conditions in HAT positive patients which could be due to a regulatory role of anti-inflammatory cytokine in the CSF as documented by [28].
We reported unaltered IL-13 levels in seropositive volunteers and serum/CSF IL-13 levels in the early and late stages of HATinfected human subjects. We therefore suggest that IL-13 is not a mediator to host immune response of HAT infection. This observation is in consonance with the report that IL-13 in trypanosome susceptible mice was not the main trigger of alternative macrophages because IL-13 signaling occurred independently of an anti-inflammatory cytokine [IL-4], corroborating the natural propensity of animals to develop alternatively activated macrophages [10, 31]. The clearance of trypanosome parasites through innate immunity involves macrophage/monocyte phagocyte system [5,6].
This investigation showed elevated IL-1a among the strongly positive volunteers. Conversely, depressed levels of IL-7 were observed in HAT late stage. These data suggests that IL-1a and IL-7 could be major mediators in the immunopathology of T. b. gambiense infection. Additionally, we propose that depressed levels of CSF IL-1a and IL-7 should serve as markers of HAT late stage in our locality.
282
References
- Vicherman K [1985] Developmental cycles and biology of pathogenic trypanosomes. Brit Med Bull 41: 105– 114.
- Greenwood BM, Whittle HCC [1980] The pathogenesis of sleeping sickness. Trans R Soc Trop Med Hyg 74: 716-725.
- Hertz CJ, Filutowicz H, Mansfield JM [1998] Resistance to the African trypanosomes is IFN- dependent. J Immunol 161:6775-6783
- ReinitzDM, Mansfield JM [1990] T-cell-independent and T-cell-dependent B-cell responses to exposed variant surface glycoprotein epitopes in trypanosome-infected mice. Infect Immun 58:2337-2342.
- Dempsey WL, Mansfield JM [1983] Lymphocyte functions in experimental African trypanosomiasis. V. Role of antibody and the mononuclear phagocyte system in variant-specific immunity. J Immunol 130:405-411.
- Kaushik RS, Uzonna JE, Gordon JR, Tabel H [1999] Innate resistance to Trypanosomacongolense infections: differential production of nitric oxide by macrophages from susceptible BALB/c and resistant C57BL/6 mice. ExpParasitol 92:131-143.
- SileghemMR, Darji A, Hamers, R, De Baetselier P [1998] Modulation of IL-1a production and IL-1a release during experimental trypanosome infections. Immunol
- FonescoSG, Reis MM, Coelho V, Nogueira LG, Monteiro SM, Mairena EC, Bacal F, Bocchi E, Guiherma L, Zheng XX, Liew FY, Higuchi ML, Kalil J, Cunha-Neto E [2007] Locally produced survival cytokines IL-15 and IL-7 may be associated to the predominance of CD8+ T cells at heart lesions of human chronicchagas disease cardiomyopathy. Scand. J Immunol 66[2-3]: 362-71.
- Bakhiet M, Jansson L, Busche P, Holmdahl R, Kristensson K, Olsson T [1996] Control of parasitemia and survival during Trypanosomabruceibrucei infection is related to strain-dependent ability to produce IL-4. J Immunol 157:3518-3526.
- Noel W, Hassanzadeh G, Raes G, Namangala B,Deams I, Brys L, Brombacher F, De Baetseiler P, Beschin A [2002] Infection-stage dependent modulation of macrophage activation in Trypanosomacongolense-resistant and susceptibility mice. Infect Immun 70: 6180-6187.
- Tachado SD, Schofield L [1994] Glycosylphatidylinositol toxin of Trypanosomabrucei regulates IL-1a and TNF- a expressions in macrophages by protein tyrosine kinase-mediated signal transduction. BiochemBiophys Res Commun 205: 984-991.
- RhindSG, Sabiston, BH, ShekPN, Buguet A, Muanga G, Stanghelli A, Dumas M, Radomski, WM [1997] Effect of Melarsoprol treatment on circulating IL-10 and TNF-_ levels in human African trypanosomiasis. ClinImmunolImmunopathol 83: 185-189.
- Okomo-Assoumon, MC, Daulouede S, Lemesre J, Mouanda-N’Zila A, VincendeanP [1995] Correlation of high serum levels of tumor necrosis factor-_ with disease severity in human African trypanosomiasis. Am J Trop Med Hyg 53[5]: 539- 543.
- Hestdal, K., Jacobsen, S.E., Ruscetti, F.W., Dubois,C.M., Longo, D.H., Chizzonite, R., Oppenheim, J.J. and Keller, J.R. [1992] In vivo effect Interleukin-1 alpha on hematopoiesis role of colony-stimulating factor receptor modulation. Blood, 80[10]: 2486-2494.
- Pang G, Couch L, Batey R, Clancy R, Cripps A [1994] GM-CSF, IL-a, IL-1a, IL-6, IL-8, IL-10, ICAM-1 and VCAM-1 gene expression and cytokine production in human duodenal fibroblasts stimulated with lipopolysaccharide, IL-1_ and TNF-_. ClinExpImmunol 96: 437-443.
- Kovacs CJ, Kerr JA, Daly BM, Evans MJ, JohnkeRM[1998] Interleukin 1 alpha [IL- 1a] and macrophage colony-stimulating factor [M-CSF] accelerate recovery from multiple drug-induced myelosuppression. Anticancer Res 18[3A]: 1805-1812.
- Weitzmann NM, Cenci S, Ritas L, Brown C, Pacifici RC [2000] Interleukin-7 stimulates osteoclastogenic formation by up-regulating T-cell production of soluble osteoclastogenic cytokines. Immunobiol96[5]: 1873-1878.
- Gessner A, Will A, Vieth M, Schroppel K, Rollinghoff M [1995] Stimulation of Bcelllymphopoiesis by interleukin-7 leads to aggravation of murine leishmaniasis[1995]. Immunol 84[3]: 416-422.
- Landmark F, Duvefelt K, Lacobaeus E, kockum I, Wallstrom E et al [2007] Variation in Interleukin-7 receptor alpha chain [IL-7R] influences risk of multiple sclerosis. Nat Genet 39[9]: 1108-1113.
- Uzonna, JE, Kaushik RS, Gordon JR, Tabel H [1998] Immunoregulation in experimental murine Trypanosomacongolense infection: anti-IL-10 antibodies reverse trypanosome-mediated suppression of lymphocyte proliferation in vitro and moderately prolong the lifespan of genetically susceptible BALB/c mice. Parasite Immunol 20:293-302.
- Inoue N, Inoue M, Kuriki K, Yamaguchi H, NagasawaH et al [1999] Interleukin 4 is a crucial cytokine in controlling Trypanosomabruceigambiense infection in mice. Vet Parasitol 86:173-184.
- 22.Schopf, LR, Filutowicz H, Bi XJ, Mansfield JM [1998] Interleukin-4-dependent immunoglobulin G1isotype switch in the presence of a polarized antigen-specific Th1-cell response to the trypanosome variant surface glycoprotein. Infect Immun66:451-461.
- Mukodzi S, Muller C, Ahrens N, Kuno S, Stenger R-D [2002]. CSF cytokines [IL-6, IL-12, and IL-13]: the right marker for shunt reimplanation after infections? Critical Care, 6[Suppl.1]: P64.
- Magnus E, Vervoot T, Van Meirvenne NA [1978]A card agglutination test with stained diagnosis of T.b.gambiense. Annales de la societebelge de MedecineTropicale58: 169-76.
- World Health Organization [1998]. Control and Surveillance of African trypanosomiasis.Report of a WHO expert committee.World Health Organization.Geneva,Switzerland. Technical Report.Series.No., 8881, 114.
- Goerdt S, Orfanos CE [1999] Other functions, other genes: alternative activation of antigen-presenting cells. Immunity, 10: 137-142.
- Namangala B, Noel W, De Baetselier P, Brys L, Beschin A [2001] Relative contribution of interferon-gamma and interleukin-10 to resistance to murine African trypanosomiasis. J Infect Dis 183: 1794-1800.
- de Waal, Malafyt, R. and Moore, K.W. [1998].Interleukin-10. In: Thompson AW., Ed. The cytokine handbook. 3rd ed. San Diego: Academic Press. pp. 333-364.
- Scharton-Kersten, MT, Sher A [2002] Roles of natural killer cells in innate resistance to protozoan infections. CurrOpinImmunol 9[1]: 44-51.
- Ahmed A, Saha B, Patwardhan A, Shivprasad S, Nandi D [2009] The major players in adaptive immunity.1. Humoral immunity. Resonance 14[5]: 455-471.
- Mills CD, Kincaid K, Alt JM, HeilmanMJ, Hill AM [2000] M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol 164:6166-6173.











