Assem Aweimer1*, Thorsten Brechmann2, Leif I. Bösche1, Michael Gotzmann1 and Andreas Mügge1
1Department of Cardiology and Angiology, BG-University Hospital Bergmannsheil, Ruhr-University of Bochum, Germany
2Department of Gastroenterology and Hepatology, BG-University Hospital Bergmannsheil, Ruhr-University of Bochum, Germany
Corresponding Author:
Assem Aweimer
Department of Cardiology and Angiology, BG-University Hospital Bergmannsheil Ruhr-University of Bochum
Buerkle-de-la-Camp-Platz 1, D-44789 Bochum Germany
Tel: 00049-234-3020
Fax: 0049-234-302-6051
E-mail: Assem.Aweimer@rub.de
Case Report
Intrahepatic cholestatic hepatitis is a rare but therapy-limiting side effect of antiarrhythmic drugs, in literature only few reports are related to the subscription of ajmaline [1-3]. As potential causes for development of intrahepatic cholestatic hepatitis drug-triggered autoimmune reactions as well as direct toxic effects are recently discussed. However, the mechanism of this severe hepatic side effect remains unclear.
Here we present a 53-year-old male who was admitted to our emergency department with sudden-onset palpitation and dizziness without angina or dyspnoea. The initial ECG and the vital parameters revealed a hemodynamic stable ventricular tachycardia (VT) with 210 bpm. Tachycardia showed a right bundle branch block and left anterior hemiblock with a superior axis. The laboratory testing showed a slightly elevated troponin I (0,41 μg/l [ref.<0,04 μg/l]) and mild cholestasis (γGT 202 U/l [ref. < 56 U/l]) without hepatitis. A pre-hopsital i.v. application of 300 mg amiodarone and 5 mg metoprolole by the emergency physician was effectless, whereas an application of 30 mg ajmaline i.v. induced a conversion to normofrequent sinus rhythm.
At the second day after admission the patient was referred to coronary angiography and right ventricular electrophysiological examination. A coronary heart disease could be excluded; no tachycardia could be induced during electrophysiological testing. The echocardiography showed a mild to moderate impaired left ventricular function with a global hypokinesis (LV-EF 40-45%). At the third day after admission a VT-storm occurred, which was successfully terminated several times (13 times) with i.v. bolus application of 30 mg ajmaline for each VT. Therefore we conducted an emergency left ventricle electrophysiological examination, which revealed a flecainide-sensitive idiopathic focal ventricular tachycardia located in the inferolateral wall (Figure 1). Subsequently we performed a successful catheterassisted endocardial ablation by use of 3D-mapping. After the procedure we started oral flecainide (300 mg/d) for long-termtherapy as relapse prevention, as the patient’s hemodynamics was impaired due to VT-storm. Hereafter no recurrence of ventricular tachycardia was observed. A post-procedural magnetic resonance tomography of the heart showed a marked late-enhancement in projection to the ablation region with a LV ejection fraction of 42%. After all, as a secondary prophylactic step and a safety procedure concerning a relapse of VT we implanted an ICDsystem (day 14 after admission).
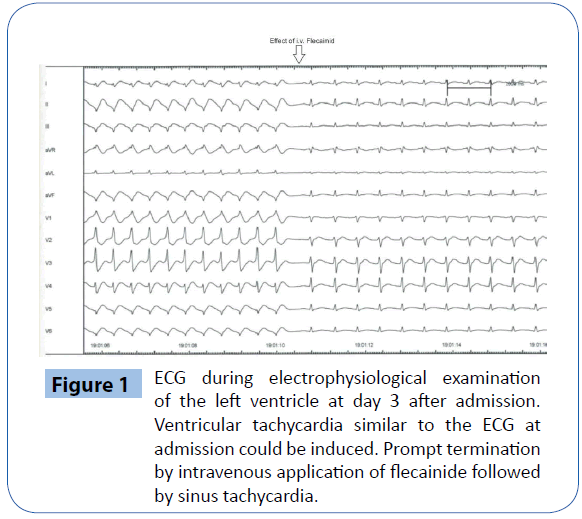
Figure 1: ECG during electrophysiological examination of the left ventricle at day 3 after admission. Ventricular tachycardia similar to the ECG at admission could be induced. Prompt termination by intravenous application of flecainide followed by sinus tachycardia.
Seven days after discharge the patient complained of pruritus, jaundice, fatigue and pain in the right upper abdomen for the duration of three days. Laboratory testing revealed the constellation of cholestatic hepatitis with strikingly elevated cholestatic enzymes ( γ-glutamyl transferase (γGT) 2037 U/l [ref<56 U/l], Alkaline phosphatase (AP) 394 U/l [ref.<127 U/l]) and bilirubinaemia (3,7 mg/dl [ref.<1,2 mg/dl], accompanied by a significant eosinophilia (0,7/nl l [ref.<0,45/nl],. Infective (viral hepatitis A, B, C) and autoimmune (IgM, IgG, ANA, AMA-M2) causes of hepatitis were excluded. Ultrasound examination of the abdomen showed slightly hyperechoic parenchyma of the liver, whereas intra- and extrahepatic bile ducts were normal.
In consideration of the predominantly elevated cholestatic enzymes, the eosinophilia and the patients’ history we suspected drug-induced cholestatic hepatitis due to ajmaline or flecainide. Consistent with this diagnosis, evaluation of CIOMS/RUCAMScale [4] (9 points) classified our case as “highly probable” for an adverse drug reaction. We reduced the dosage of oral flecainide medication (150 mg/d) and observed the patient in our outpatient clinic. Over the course of time the liver enzymes decreased continuously and normalized within 2 months (Figure 2).
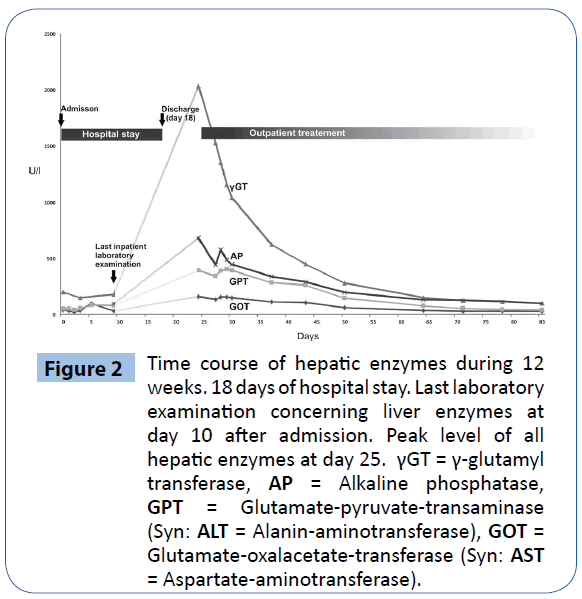
Figure 2: Time course of hepatic enzymes during 12 weeks. 18 days of hospital stay. Last laboratory examination concerning liver enzymes at day 10 after admission. Peak level of all hepatic enzymes at day 25. γGT = γ-glutamyl transferase, AP = Alkaline phosphatase, GPT = Glutamate-pyruvate-transaminase (Syn: ALT = Alanin-aminotransferase), GOT = Glutamate-oxalacetate-transferase (Syn: AST = Aspartate-aminotransferase).
Pharmacogenetic genotyping of Cytochrome P450 2D6 (CYP2D6) revealed a heterozygous genotype with a defective CYP2D6 allele (CYP2D6*5) resulting in a decreased enzyme activity accordingly to an “intermediate metabolizer”.
Ajmaline and flecainide are well-known antiarrhythmic drugs and indicated for emergency treatment of supraventricular and ventricular tachycardia. These antiarrhythmic drugs are metabolized in the liver via the cytochrome P450 enzyme CYP2D6 [5,6]. The polymorphism of this enzyme results in poor, intermediate, efficient or ultrarapid metabolizers of CYP2D6 drugs. About 5-10% of the European Caucasian population are poor metabolizers and nearly 10-17% are intermediate metabolizers [7].
Drug-induced cholestasis may occur under conditions of increased drug concentrations or genetic alterations such as CYP2D6 polymorphism. In accordance to the few reported cases of ajmaline-induced cholestatic hepatitis [1,2], our patient had a similar clinical course characterized by a latency of nearly 3 weeks until occurrence of symptoms and liver enzyme activation. Previously, Mellor et al. supposed that the mechanism of ajmaline-induced cholestatic hepatitis may relate to CYP2D6 polymorphism. Recently, to the best of our knowledge and in comparison to previous reports we demonstrate for the first time that a CYP2D6 polymorphism leads to intrahepatic cholestatic hepatitis due to antiarrhythmic medication, probably related to ajmaline.
Despite metabolization by CYP2D6, the long-term medication with flecainide was necessary to avoid a relapse of potential lifethreatening ventricular tachycardia, so that we did not interrupt this therapeutic strategy. Nevertheless we reduced the flecainidedose adjusted to the reduced enzyme-activity. Compared to ajmaline, flecainide is supposed to have minor clinical significance regarding CYP2D6 metabolism [5-8]. Consistent with this assumption a continuation of flecainide therapy did not hinder recovery of the cholestatic hepatitis, even though a prolongation cannot be excluded.
Our case demonstrates that a continuation of dosage-reduced flecainide is reasonable in an intermediate metabolizer of CYP2D6 while subsiding cholestatic hepatitis. As mentioned above our patient had a mild cholestasis at admission, which is associated with a higher risk for development of drug-induced cholestasis [9].
Furthermore, genotype testing of CYP2D6 is not routinely implemented in the clinical setting for risk stratification concerning the development of severe hepatic side effects following treatment by antiarrhythmic drugs. In consideration of the clinical situation the evaluation of CYP2D6 polymorphism in patients at higher risk for liver side effects and medication with antiarrhythmic agents, which are metabolized by this enzyme could be helpful for prevention of adverse drug reactions. This algorithm needs to be proven in further studies. Nevertheless a strict clinical and laboratory observation of these patients in an outpatient manner is necessary.
7714
References
- Larrey D, Pessayre D, Duhamel G, Casier A, Degott C, et al. (1986) Prolonged cholestasis after ajmaline-induced acute hepatitis. J Hepatol 2: 81-87.
- Mellor G, Fellows I, Williams I (2013) 'Intrahepatic cholestatic hepatitis following diagnostic ajmaline challenge'. Europace 15: 314.
- Padda MS, Sanchez M, Akhtar AJ, Boyer JL (2011) Drug-induced cholestasis. Hepatology 53: 1377-1387.
- Lucena MI, García-Cortés M, Cueto R, Lopez-Duran J, Andrade RJ (2008) Assessment of drug-induced liver injury in clinical practice. Fundam Clin Pharmacol 22: 141-158.
- Bertilsson L, Dahl ML, Dalén P, Al-Shurbaji A (2002) Molecular genetics of CYP2D6: clinical relevance with focus on psychotropic drugs. Br J Clin Pharmacol 53: 111-122.
- Anzenbacher P, Anzenbacherová E (2001) Cytochromes P450 and metabolism of xenobiotics. Cell Mol Life Sci 58: 737-747.
- Zhou SF (2009) Polymorphism of human cytochrome P450 2D6 and its clinical significance: Part I. Clin Pharmacokinet 48: 689-723.
- Eichelbaum M, Gross AS (1990) The genetic polymorphism of debrisoquine/sparteine metabolism--clinical aspects. Pharmacol Ther 46: 377-394.
- Andrade RJ, Camargo R, Lucena MI, González-Grande R (2004) Causality assessment in drug-induced hepatotoxicity. Expert Opin Drug Saf 3: 329-344.








