Keywords
Computer tomography; Abdominal malignancy; Pain relief
Introduction
Patients with cancer and peritoneal carcinomatosis suffer greatly from severe pain [1,2]. The pain could be treated by either prescription medications or surgery. The medications include Patient-Controlled Analgesia (PCA), transdermal or transoral opiods including fentanyl, morphine, and hydromorphone [3-8]. The surgical approaches include celiac ganglion plexus neurolysis, celiac ganglionectomy, intrathecal pain pump, and spinal cord stimulation [1,2,9-14]. The visceral pain is carried by nociceptive fibers, which originate from abdominal viscera and organs, and pass through celiac ganglion plexus before entering the spinal cord. Hence, through blocking the celiac ganglion plexus, we could effectively relieve pain from these organs. After the pain is managed, we could thereby decrease the use of opioids and avoid adverse side effects of opioids, such as nausea, vomiting, or suppression of respiration.
The goals of this study are to introduce the practicality of intraoperative CT-guided percutaneous celiac plexus neurolysis (iCT-PCPN) and, subsequently, demonstrate the effectiveness of decreasing Numeric Rating Scale (NRS) and post-operational opioids use.
Materials and Methods
From January 2013 to December 2017, we completed iCT-PCPN for 14 patients with peritoneal carcinomatosis at Chia-Yi Chang Gung Memorial Hospital. This was a single center retrospective study. The patients were selected through consultation from internal medical departments. The patients had intractable pain even with prescribed pain medications such as steroids, NSAIDs, opioids, and PCA. The followings were the exclusion criteria: non-neoplasm related abdominal pain from herpes or chronic pancreatitis, coagulopathy, local or systemic infections, chemotherapy or radiotherapy within 4 weeks, or patient’s refusal.
All operations were conducted by the same experienced neurosurgeon. The distribution of patients is shown in Table 1. There were 18 patients enrolled in this study, but excluded 4 of them due to non-neoplasm related abdominal pain from chronic pancreatitis or herpes zoster infection. Fourteen patients were selected and iCT-PCPN was performed to manage pain stemmed from carcinomatosis.
| Case |
Gender |
Age |
Diagnosis (Origin) |
Approach |
Injected medicine |
Injected volume |
OP time (min) |
OP to death time (Day) |
| 1 |
M |
82 |
Pancreatic cancer |
Left postcrural |
66% alcohol |
30 |
28 |
0 |
| 2 |
F |
78 |
Rectal cancer |
Left postcrural |
66% alcohol |
30 |
15 |
0 |
| 3 |
F |
65 |
Pancreatic cancer |
Bilateral postcrural |
66% alcohol |
40 |
35 |
0 |
| 4 |
F |
51 |
Ureter tumor |
Bilateral postcrural |
66% alcohol |
22 |
6 |
0 |
| 5 |
M |
61 |
Cholangiocarcinoma |
Left postcrural |
70% alcohol |
15 |
19 |
0 |
| 6 |
M |
41 |
Ampular vater cancer |
Bilateral postcrural |
5% phenol |
40 |
30 |
0 |
| 7 |
M |
53 |
Pancreatic cancer |
Left postcrural |
5% phenol |
40 |
15 |
0 |
| 8 |
F |
56 |
Pancreatic cancer |
Bilateral postcrural |
5% phenol |
40 |
31 |
0 |
| 8' |
F |
56 |
Pancreatic cancer |
Bilateral postcrural |
5% phenol |
40 |
22 |
0 |
| 9 |
M |
60 |
Periampular cancer |
Bilateral postcrural |
5% phenol |
30 |
23 |
0 |
| 10 |
M |
53 |
Esophageal cancer |
Bilateral postcrural |
5% phenol |
40 |
35 |
0 |
| 11 |
M |
48 |
Esophageal cancer |
Left postcrural |
5% phenol |
30 |
22 |
0 |
| 12 |
M |
61 |
Lung small cell carcinoma |
Bilateral postcrural |
5% phenol |
40 |
27 |
0 |
| 13 |
M |
64 |
Pancreatic cancer |
Bilateral postcrural |
5% phenol |
40 |
20 |
N/A |
| 14 |
F |
67 |
Pancreatic cancer |
Bilateral postcrural |
5% phenol |
36 |
35 |
0 |
| Average |
- |
59.73 |
- |
- |
- |
- |
24.2 |
0 |
| Q1 |
- |
53 |
- |
- |
- |
- |
19.5 |
0 |
| Median |
- |
60 |
- |
- |
- |
- |
23 |
0 |
| Q3 |
- |
64.5 |
- |
- |
- |
- |
30.5 |
0 |
Table 1: Distribution of the 14 patients with the median age of 60 years old. Five out of the 14 patients were female and 9 were male. The origin of peritoneal carcinomatosis were pancreatic cancer (9 cases), esophageal cancer (2 cases), lung small cell carcinoma (1 case), cholangiocarcinoma (1 case), rectal cancer (1 case), and ureter tumor (1 case). Case 8 has had operation twice. The median of operation time is 23 minutes. The median of operation day to death day is 48.5 days. Q1: 25th percentile, Q3: 75th percentile.
Patients had diagnoses of pancreatic cancer (8 patients), esophageal cancer (2 patients), rectal cancer (1 patient), cholangiocarcinoma (1 patient), ureter cancer (1 patient), and lung small cell carcinoma (1 patient). Nine patients were male, and 5 patients were female. Five patients were treated with 66% or 70% alcohol, and 9 patients were treated with 5% phenol. This was due to the unavailability of phenol at our institution in the recent years. Five patients were treated with left post-crural approach, while 9 patients were treated with bilateral post-crural approach.
The operation procedures are shown in Table 2 and performed under local anesthesia. Patients were placed at prone position, draped and applied antiseptic. A pre-operative CT with combination and navigation system of BrainLab® was completed. 22-gauge block needles were used unilaterally or bilaterally under guidance of iCT navigation. Several repeats of CT of abdomen were performed to check the needle tip placement toward the space of celiac ganglion plexus at the level of T12-L1 from posterior approach, in which the celiac ganglion lays. Contrast enhancement was also performed to check the celiac ganglion plexus. Intra-abdominal organs, including aorta, were noted and caution was taken to avoid injury to them. Injections of alcohol or phenol were performed for celiac plexus block. CT images of iCT-PCPN and post-operative needle puncture wounds are shown in Figure 1. After the whole procedure was completed, we then removed the needles and care for the wounds closely. We sent the patients to Post-Operative Room (POR) for careful observation. After each operation, we checked patients’ pain status using NRS. The NRS feedback was recorded pre-operatively, and on day 1, day 3, day 7, day 14, day 21, and day 28 postoperatively. We also documented the use of opioid for pain relieve.
| Operation procedures |
| 1. Obtain informed consent |
| 2. Patient was placed at prone position |
| 3. Draped and antiseptic |
| 4. CT of abdomen was performed before operation and integrated with navigation system of BrainLab® |
| 5. Celiac trunk level was checked by navigation |
| 6. Local anesthesia |
| 7. A 22-gauge block needle was placed via post-crural approach at level of T12-L1 disc space bilaterally or unilaterally |
| 8. Injection of contrast was performed |
| 9. Intraoperative CT was performed and celiac ganglion and periaortic space was checked |
| 10. Injection of 5% phenol or 66% alcohol |
| 11. Remove the needle |
| 12. Wound care and send the patient to POR |
Table 2: The operation procedures of iCT-PCPN. The patient is placed with prone position and draped and antiseptic. The CT of abdomen is performed with navigation system of Brain Lab®. The celiac trunk level is checked at T12-L1 disc space. Under local anesthesia, the celiac plexus neurolysis is performed by injection of 5% phenol or 66% alcohol to the space of celiac plexus through post-crural approach with 22-gauge block needle bilaterally or unilaterally. After the whole procedure, needles are removed and we send the patient to Post-Operative Room (POR), observe the vital signs and give wound care and pain control.
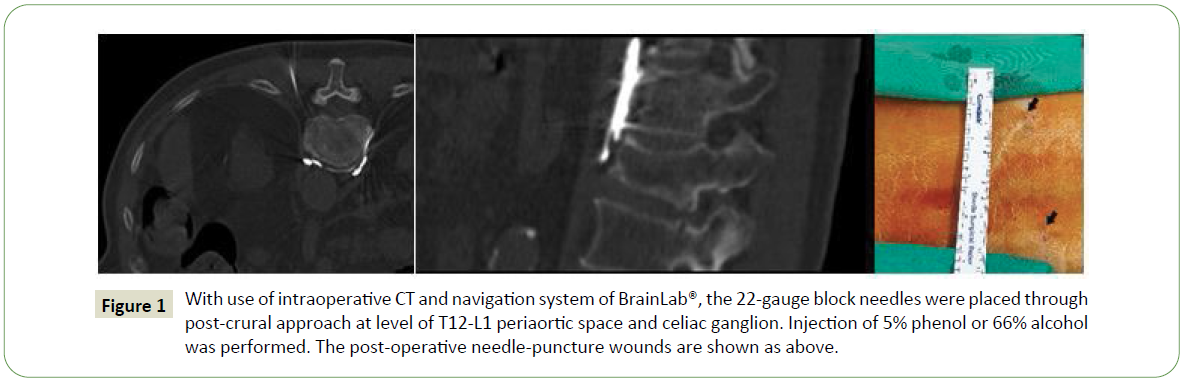
Figure 1: With use of intraoperative CT and navigation system of BrainLab®, the 22-gauge block needles were placed through post-crural approach at level of T12-L1 periaortic space and celiac ganglion. Injection of 5% phenol or 66% alcohol was performed. The post-operative needle-puncture wounds are shown as above.
The data was analyzed with IBM SPSS Statistics Version 25 (IBM SPSS Statistics for Windows, IBM, Armonk, NY, USA). Kruskal– Wallis and Wilcoxon rank-sum tests were used for statistical analyses where appropriate, with significance thresholds set at p< 0.05.
Results
Shown in Table 1, iCT-PCPN has the advantages of precision and short operation time. The average operation time of this study is 24.2 minutes (Q1: 19.5, median: 23, Q3: 30.5). The average time between the operation and patients’ death is 62.54 days (Q1: 27, median: 48.5, Q3: 60.5).
As depicted in Table 3, we assessed the pre-operative pain status by NRS and NRS post-operatively on day 1, day 3, day 7, day 14, day 21, andday 28. There is a statically significant decrease in the reported NRS. The median of NRS pre-operatively is 10 (Q1: 9, Q3: 10). The median of NRS at day 1 post-operatively is 3 (Q1: 2, Q3: 5), p<0.005. The median of NRS at day 3 is 2 (Q1:0, Q3: 4.5), p<0.0005. The median of NRS at day 7 is 2 (Q1:0, Q3: 2.5), p<0.0005. The median of NRS at day 14 is 0 (Q1:0, Q3: 4), p<0.0005. We noted the median of NRS hit bottom at day 14. The median of NRS at day 21 is 4 (Q1:0, Q3: 5.5), p<0.005. The median of NRS at day 28 is 5 (Q1:4, Q3: 6), p<0.005.
| NRS assessment time |
Score in NRS (Median) |
p-value (statistical significance<0.05, the Kruskal-Wallis test, reference: Pre-op) |
| Pre-op |
10(Q1: 9, Q3: 10) |
- |
| 1 day |
3(Q1: 2, Q3: 5) |
P<0.005 |
| 3 days |
2(Q1: 0, Q3: 4.5) |
P<0.0005 |
| 7 days |
2(Q1: 0, Q3: 2.5) |
P<0.0005 |
| 14 days |
0(Q1: 0, Q3: 4) |
P<0.0005 |
| 21 days |
4(Q1: 0, Q3: 5.5) |
P<0.005 |
| 28 days |
5(Q1: 4, Q3: 6) |
P<0.005 |
Table 3: The median of NRS decreased from 10 to 3(Q1:2, Q3:5), p<0.005, on post-operative day 1 with statistical significance compared to preoperative NRS. The NRS reached 0 on the 14th day (Q1:0, Q3:4), p<0.0005. The NRS decrease remained at 5 (Q1:4, Q3:6), p<0.005 with statistical significance until the 28th day. Q1: 25th percentile, Q3: 75th percentile.
Additionally, the distribution of the reported NRS by the 14 patients is shown in Figure 2. p<0.05 at post-operative on day 1, day 3, day 7, day 14, day 21, and day 28 is compared to preoperative pain status. The null hypothesis that there is no difference between pre-operative NRS and post-operative NRS is rejected. Therefore, the patients had reported improvement of NRS with statistical significance. Figure 2 also discloses the fact that p>0.05 is noted between each post-operative time point, for example, on day 1 and day 3. This means there was no statistical significance difference of NRS between each time point post-operatively. As aforementioned, p<0.05 is noted comparing the pre-operative NRS to each time point. Therefore, the iCTPCPN remains effective for patients with pain from peritoneal carcinomatosis for at least 28 days.
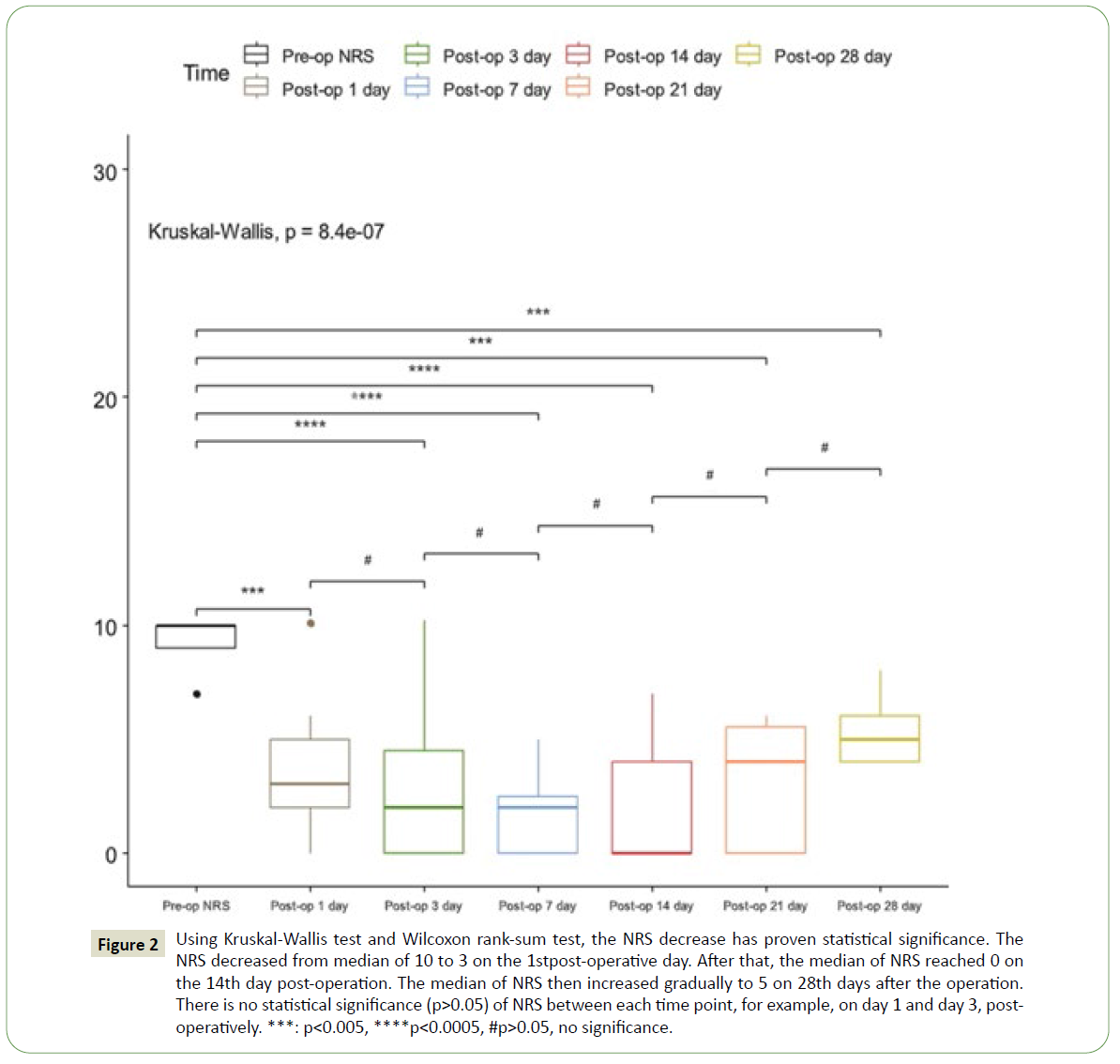
Figure 2: Using Kruskal-Wallis test and Wilcoxon rank-sum test, the NRS decrease has proven statistical significance. The NRS decreased from median of 10 to 3 on the 1st post-operative day. After that, the median of NRS reached 0 on the 14th day post-operation. The median of NRS then increased gradually to 5 on 28th days after the operation. There is no statistical significance (p>0.05) of NRS between each time point, for example, on day 1 and day 3, postoperatively. ***: p<0.005, ****p<0.0005, #p>0.05, no significance.
We compared the opioid dosage pre-operatively, postoperative day 7, and day 21. Figure 3 shows the distribution of morphine dosage and illustrated the median of morphine consumption preoperatively at 23.33 mg, which increased to 33.33 mg on the 7th day post-operatively and remains 33.33 mg on the 21st day. We conducted Kruskal-Wallis test and p>0.05 was noted. The null hypothesis that there is no difference in administered morphine dosage pre-operatively, on the 7th day, and on the 21st day is true. Therefore, there is an increasing trend of use of opioids post-operatively without statistical significance.
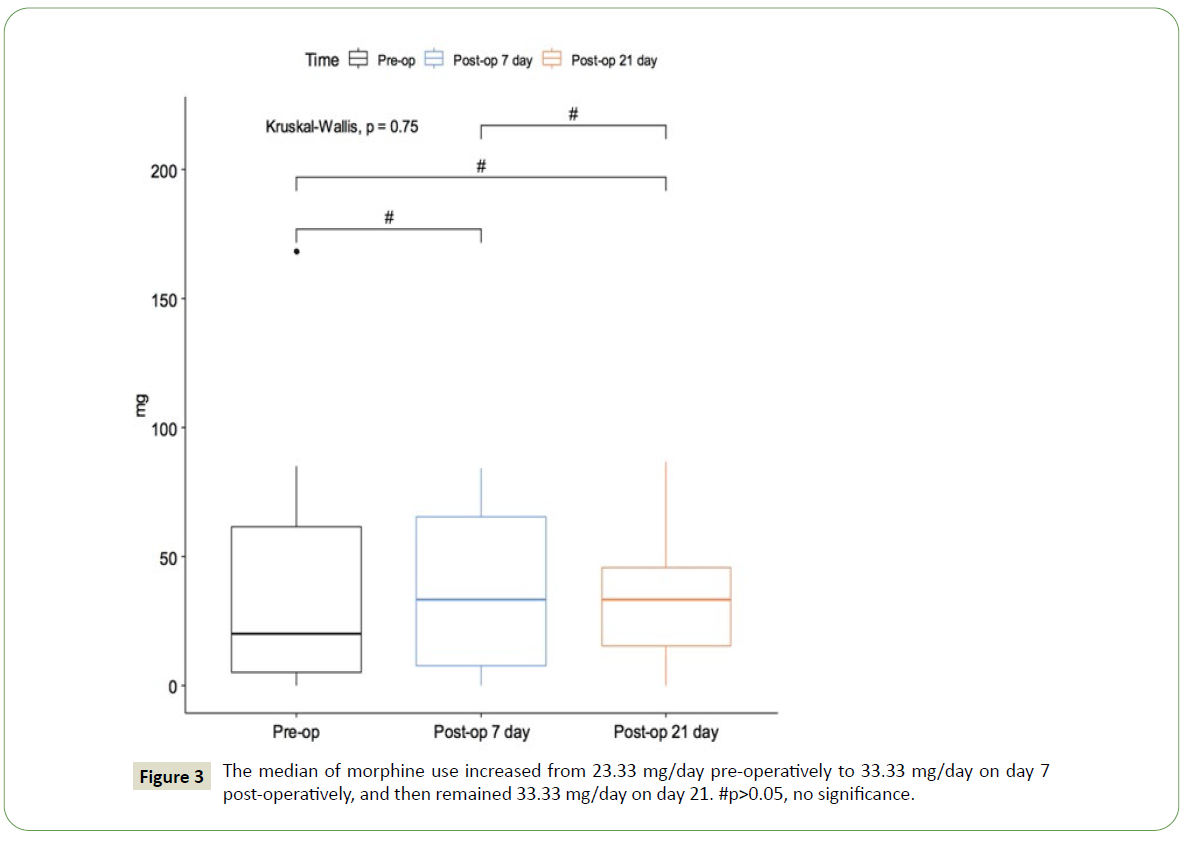
Figure 3: The median of morphine use increased from 23.33 mg/day pre-operatively to 33.33 mg/day on day 7 post-operatively, and then remained 33.33 mg/day on day 21. #p>0.05, no significance.
Table 4 also shows the detailed distribution of consumption of morphine dosage before and after iCT-PCPN. Especially, the Q3 value of morphine use on pre-operative day was 78.34 mg, at 7 days post-operation decreased to 65.20 mg, and that at 21 days post-operation decreased further to 45.83 mg. Hence, iCT-PCPN may help decrease the use of opioids for patients with peritoneal carcinomatosis and with heavier adhesion to opioids.
| Case |
Pre-op morphine use |
7 days post-op morphine use |
21 days post-op morphine use |
| 1 |
5.00 |
0.00 |
27.00 |
| 2 |
0.00 |
20.00 |
0.84 |
| 3 |
20.00 |
33.33 |
33.33 |
| 4 |
71.67 |
21.25 |
40.80 |
| 5 |
4.58 |
3.33 |
45.83 |
| 6 |
6.67 |
70.00 |
EXPIRED |
| 7 |
85.00 |
1440.00 |
960.00 |
| 8 |
504.00 |
504.00 |
40.00 |
| 8' |
24.00 |
10.00 |
30.83 |
| 9 |
23.33 |
84.17 |
202.83 |
| 10 |
190.21 |
40.83 |
6.67 |
| 11 |
168.33 |
46.67 |
86.67 |
| 12 |
61.67 |
60.40 |
EXPIRED |
| 13 |
5.00 |
5.00 |
0.00 |
| 14 |
11.23 |
5.40 |
15.40 |
| Average |
78.71 |
156.29 |
114.63 |
| Q1 |
5.83 |
7.70 |
15.40 |
| Median |
23.33 |
33.33 |
33.33 |
| Q3 |
78.34 |
65.20 |
45.83 |
Table 4: The median of morphine is 23.33 mg/day pre-operatively and it increased to 33.33 mg on day 7 post-operatively, and remains 33.33 mg on day 21. The 75th percentile of opioids use decreased from 78.34 mg/day pre-operatively to 65.20 mg/day on day 7 post-operatively, and eventually 45.83 mg/day on day 21 post-operatively. Q1: 25th percentile, Q3: 75th percentile.
There were no complications like motor impairment, urinary incontinence, infections, bowel perforation, intra-abdominal bleeding due to aortic injury or injury to major vessels noted after each operation.
Discussion
Celiac ganglion block was firstly introduced to the medical world by Kappis et al. in 1914 [15]. The procedure was initially performed by identifying external bony landmarks. Subsequently, anterior percutaneous approach was reported by Wendling et al. [16]. Jones initiated the use of ethanol for celiac ganglion neurolysis [17]. During 1950s, fluoroscopy-guided celiac ganglion block was performed, but was limited due to inaccuracy of needle tip and retroperitoneal anatomic structures. In 1977, Hagga et al. described CT-guided celiac ganglion block [18]. Ultrasoundguided endoscopic approach for celiac ganglion block was documented in recent years [19-23]. iCT-PCPN with navigation for celiac ganglion block has not been published in English medical journals to date.
Intraoperative CT is featured by precision with combination of navigation system. iCT has been previously documented for radio ablation surgery for trigeminal neuralgia, dorsal root ganglion ablation for thoracic or lumbar compression fractures, and navigated screws insertions for spondylolisthesis [24-27]. With theuse of iCT and navigation system, the present study of iCTPCPN for block of celiac ganglion plexus also has the advantages of increased accuracy and shorter operation time.
In the present study, iCT-PCPN has shown an effective decrease of NRS. From the statistics, the NRS decreases from median of 10 to 3 at day 1 postoperatively. Consequently, the median NRS reached bottom at 0 on day 7. The median of NRS then increased gradually to 5 at day 28.
The abdominal visceral pain fibers gather at celiac ganglion plexus before ascending into the spinal cord. Therefore, we could treat the peritoneal carcinomatosis-related pain by blockade of celiac ganglion plexus. However, there are some literatures stated that somatic pain may not be effectively treated by celiac ganglion block [2].
The celiac ganglion block has long been adapted for treatments of abdominal-related pain from peritoneal carcinomatosis. The technique could be performed by identifying the external bony landmarks under fluoroscopy guidance, under CT guidance, or under ultrasound guidance, and endoscopy. The procedure of celiac ganglion block or neurolysis could be performed through these four different approaches, each of them with attempts of reaching the peri-aortic space at level of T12-L1, where the celiac plexus is located. Direct celiac ganglion neurolysis is more difficult to approach and requires visualization by either endoscopy or surgery, however, it is conceptionally more effective [9,13,22]. Anterior approach has also been reported but there are risks of bowel perforation or intra-abdominal bleeding. Posterior approach has been the mainstream approach for celiac ganglion neurolysis. There are different posterior approaches, such as, postcrural approach, transdiscal approach, and transaortic approach. There are literatures showing risks of bleeding from the transaortic approach. There are also literatures showing transient back pain by transdiscal approach. Transdiscal approach also belongs to posterior approaches, but compared to postcrural approach, it is less likely than transdiscal approach to injure the adjacent intra-abdominal organs while performing procedure of celiac ganglion block. The postcrural approach has the advantage of easier localization of trajectory from the lateral, but it is more likely to injure abdominal organs. In the present study, we use the postcrural approach and identify the T12-L1 level peri-aortic space by iCT and navigation. Therefore, we could identify and avoid injury to the abdominal organs while performing the postcrural approach.The celiac ganglion block is performed with the aid of image guidance since the 1950s [2,11,12,14]. Fluoroscopy could provide adequate localization of T12-L1 disc level by lateral view, but it is less precise compared to other approaches. At the same time, surgeons may also be exposed to harmful radiation. CT-guide PCPN could also ensure more accuracy of injection to celiac ganglion plexus and the identification of abdominal organs. However, compared to iCTPCPN, CT-guided PCPN lack navigation and, thus, may require more scans, which will lead to more radiation exposure to the patient. Ultrasound-guided endoscopy has become more popular recently with the preciseness of localizing and visualizing the celiac ganglion plexus from the anterior approach. On the other hand, it is a more invasive procedure compared to iCT-PCPN due to the insertion of endoscopy.
After reviewing prior English literatures, the current study of iCT-PCPN has not been documented. iCT-PCPN procedure is similar to CT-guided celiac ganglion neurolysis, but it is more accurate with the combination of CT and navigation. Our procedure is performed in an operation room with the use of physiological monitors and local anesthesia. Anesthetists are present throughout the procedure. 22-gauge needles are used for injection in contrast of phenol or alcohol. The average time of operation is 24.2 minutes. With the application of navigation, we can approach celiac ganglions with more exact guidance and proper modification for appropriate trajectory. Therefore, we can reach celiac ganglion plexus with more precision and less injury to adjacent intra-abdominal organs.
Luz and his colleague have depicted direct block of celiac ganglion as Celiac Ganglion Neurolysis (CGN), and indirect block of celiac ganglion by injection of alcohol or phenol to the adjacent space as Celiac Plexus Neurolysis (CPN). CGN is conceptionally more effective than CPN. Compared to Endoscopic Ultrasound Celiac Ganglion Neurolysis (EUS-CGN) or Endoscopic Ultrasound Celiac Plexus Neurolysis (EUS-CPN), our procedure is less invasive by only inserting 22-gauge needles and more veracity by iCT and navigation. Luz has also documented that EUS-CGN and EUS-CPN are effective methods with up to 70% accuracy of identifying celiac ganglia, namely, performing CGN, and a 94% of pain relief rate in patients with peritoneal carcinomatosis. Literatures have recorded better pain relief with direct block on celiac ganglion, but pain block with injection of alcohol at peri-ganglion space also provides pain relief [22]. Sheshadri et al. haveexecuted celiac ganglionectomy during Hyperthermic Intraperitoneal Chemotherapy (HIPEC) surgery and have proven effectiveness in pain relief from peritoneal carcinomatosis, but this is a far more invasive procedure compared to the this study [13].
Opioids have an important role in peritoneal carcinomatosisrelated pain control. The iCT-PCPN or other pain block procedures are aimed to decrease the use of opioids in pain control due to adverse side effects like respiratory inhibition, dizziness, nausea or vomiting, and other discomforts. In our study, the median of morphine dosage increased from 23.33 mg/day pre-operatively to 33.33 mg/day at day 7, and then remained at 33.33 mg/day at day 21. This result showed an increase of opioids dosage without a statistical significance. Interestingly, the 75th percentile of opioids dosage decreased from 78.34 mg/day pre-operatively to 65.20 mg/day at day 7, and eventually reached 45.83 mg/day at day 21. Therefore, from this study, the patients with initially higher attachment to opioids later showed a better improvement in decreased dosage of opioids.
In this study, we noted that case No.8 had been performed twice with iCT-PCPN. The patient was satisfied with the effectiveness of pain control each time. The morphine dosage did not decrease after each procedure. This may be attributed to the relatively late intervention by iCT-PCPN. From our study, we concluded that iCT-PCPN provides patients with effective pain control. The procedurecanbe repeated throughout the end-stage of oncologic diseases. Better quality of pain management may be achieved by early intervention of iCT-PCPN and repeated treatment of iCT-PCPN. Sousa Correia shared similar results in ganglion impar block (GIB) with fluoroscopy guidance for patients with pelvic carcinomatosis and recorded beneficial outcome of decreased opioids-use and a significant VAS decrease of median from 7 to 4 in the following three months [28].
Common complications from PCPN include hypotension, transient back pain or shoulder pain, infection, or intra-abdominal bleeding [29,30]. In this study, none of the 14 cases experienced the aforementioned complications.
Searching prior English literatures regarding pain control for carcinomatosis, we found pain management is mainly treated either surgically or with pain medication. The dosage of medication prescription for peritoneal carcinomatosis could escalate from 100 mg/day to 300 mg/day, causing severe adverse effects of respiratory inhibition or impaired consciousness [3- 9,13,31,32]. For surgical treatments, intrathecal morphine pump and spinal cord stimulation are alternative treatments for peritoneal carcinomatosis but the operation for either treatment is more invasive than PCPN itself [4,33-37].
Limitations
Limitations for our study include the following: small sample size, relatively late intervention in the natural course of peritoneal carcinomatosis, and retrospective study. Further prospective or randomized studies could be carried for patients with peritoneal carcinomatosis in the future.
Conclusion
iCT-PCPN is an effective procedure for pain management of peritoneal carcinomatosis. It has the advantages of precision with navigation, less invasive surgery, less radiation exposure to patient or surgeon, improved patient safety, and the possibility of repeating procedure on the same patient.
38738
References
- Filippiadis DK, Yevich S, Deschamps F, Jennings JW, Tutton S, et al. (2019) The role of ablation in cancer pain relief. Curr Oncol Rep 21: 1-7.
- Kambadakone A, Thabet A, Gervais DA, Mueller PR, Arellano RS (2011) CT-guided celiac plexus neurolysis: A review of anatomy, indications, technique, and tips for successful treatment. Radiographics 31: 1599-1621.
- Bandieri E, Romero M, Ripamonti CI, Artioli F, Sichetti D, et al. (2016) Randomized trial of low-dose morphine versus weak opioids in moderate cancer pain. J Clin Oncol 34: 436-442.
- Caraceni A, Hanks G, Kaasa S, Bennett MI, Brunelli C, et al. (2012) Use of opioid analgesics in the treatment of cancer pain: Evidence-based recommendations from the EAPC. Lancet Oncol 13: e58-e68.
- Coluzzi PH (1998) Cancer pain management: Newer perspectives on opioids and episodic pain. Am J Hosp Palliat Med 15: 13-22.
- Dale O, Moksnes K, Kaasa S (2011) European Palliative Care Research Collaborative pain guidelines: Opioid switching to improve analgesia or reduce side effects. A systematic review. Palliat Med 25: 494-503.
- Dowell D, Haegerich TM, Chou R (2016) CDC guideline for prescribing opioids for chronic pain-United States, 2016. JAMA 315: 1624-1645.
- Mercadante S, Bruera E (2016) Opioid switching in cancer pain: From the beginning to nowadays. Crit Rev Oncol Hematol 99: 241-248.
- Arakelian E, Gunningberg L, Larsson J, Norlén K, Mahteme H (2011) Factors influencing early postoperative recovery after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Eur J Surg Oncol 37: 897-903.
- Fares KM, Mohamed SA, Abdel-Ghaffar HS (2014) High dose intrathecal morphine for major abdominal cancer surgery: A prospective double-blind, dose-finding clinical study. Pain Physician 17: 255-264.
- Koizuka S, Nakajima K, Mieda R (2014) CT-guided nerve block: a review of the features of CT fluoroscopic guidance for nerve blocks. J Anesth 28: 94-101.
- Lee MJ, Mueller PR, VanSonnenberg E, Dawson SL, D'Agostino H, et al. (1993) CT-guided celiac ganglion block with alcohol. AJR Am J Roentgenol 161: 633-636.
- Sheshadri DB, Chakravarthy MR (2016) Anaesthetic considerations in the perioperative management of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Indian J Surg Oncol 7: 236-243.
- Vahedian J, Saraee A, Baghai Wadji M, Safari S, Chavoshi Khamneh A (2020) Pain Relieving Effect of Intraoperative Chemical Splanchnicectomy of Celiac Ganglions in Patients with Resectable Pancreatic or Gastric Masses: A Randomized Clinical Trial. Pain Res Manag 2020: 1-6.
- Munisi K (1914) Erfahrungen mit local anasthesie bie bauchoperationen. Vehr Dtsch Gesellsch Chir 43: 87-89.
- Wendling H (1917) Ausschaltung der Nervi splanchnici durch Leitungsanästhesie bei Magenoperationen und andern Eingriffen in der oberen Bauchhöhle: Ein Beitrag zur Kenntnis der Sensibilität der Bauchhöhle: Inaugural-Dissertation zur Erlangung der Doktorwürde an der hohen medizinischen Fakultät der Universität Basel (Doctoral dissertation, Druck von H. Laupp jr.). 1917.
- Jones RR (1957) A technic for injection of the splanchnic nerves with alcohol. Anesth Analg 36: 75-77.
- Haaga JR, Havrilla TR, Alfidi RJ (1977) Interventional CT scanning. Radiol Clin North Am 15: 449-456.
- Doi S, Yasuda I, Kawakami H, Hayashi T, Hisai H, et al. (2013) Endoscopic ultrasound-guided celiac ganglia neurolysis vs. celiac plexus neurolysis: a randomized multicenter trial. Endoscopy 45: 362-369.
- Kappelle WF, Bleys RL, van Wijck AJ, Siersema PD, Vleggaar FP (2017) EUS-guided celiac ganglia neurolysis: a clinical and human cadaver study (with video). Gastrointest Endosc 86: 655-663.
- Koulouris AI, Alexandre L, Hart AR, Clark A (2021) Endoscopic ultrasound-guided celiac plexus neurolysis (EUS-CPN) technique and analgesic efficacy in patients with pancreatic cancer: A systematic review and meta-analysis. Pancreatology 21: 434-442.
- Luz LP, Al-Haddad MA, DeWitt JA (2014) EUS-guided celiac plexus interventions in pancreatic cancer pain: An update and controversies for the endosonographer. Endosc Ultrasound 3: 213.
- Yasuda I, Wang HP (2017) Endoscopic ultrasoundÃÂâ?ÃÂÃÂguided celiac plexus block and neurolysis. Dig Endosc 29: 455-462.
- Huang WC, Lin MH, Lee MH, Chen KT, Cheng CY, et al. (2018) Percutaneous dorsal root ganglion block for treating lumbar compression fracture-related pain. Acta Neurochir 160: 1283-1289.
- Lee MH, Lin MH, Weng HH, Cheng WC, Tsai YH, et al. (2013) Feasibility of intraoperative computed tomography navigation system for pedicle screw insertion of the thoracolumbar spine. Clin Spine Surg 26: E183-E187.
- Lin CH, Lee MH, Lin MH, Wang TC, Cheng WC, et al. (2013) Percutaneous dorsal root ganglion lysis with phenol for the treatment of pain associated with thoracic compression fracture. Acta Neurochir 155: 2313-2320.
- Tsai PJ, Lee MH, Chen KT, Huang WC, Yang JT, et al. (2019) Foramen ovale cannulation guided by intraoperative computed tomography with magnetic resonance image fusion plays a role in improving the long-term outcome of percutaneous radiofrequency trigeminal rhizotomy. Acta Neurochir 161: 1427-1434.
- Correia JS, Silva M, Castro C, Miranda L, Agrelo A (2019) The efficacy of the ganglion impar block in perineal and pelvic cancer pain. Supportive Care Cancer 27: 4327-4330.
- Nitschke AM, Ray Jr CE (2013) Percutaneous neurolytic celiac plexus block. Thieme Med Publishers 30: 318.
- Wang PJ, Shang MY, Qian Z, Shao CW, Wang JH, et al. (2006) CT-guided percutaneous neurolytic celiac plexus block technique. Abdom Imaging 31: 710-718.
- World Health Organization (2018) WHO guidelines for the pharmacological and radiotherapeutic management of cancer pain in adults and adolescents. 2018.
- Suzuki M, Narita M, Hasegawa M, Furuta S, Kawamata T, et al. (2012) Sensation of abdominal pain induced by peritoneal carcinomatosis is accompanied by changes in the expression of substance P and μ-opioid receptors in the spinal cord of mice. J Am Soc Anesthesiol 117: 847-856.
- Bonica JJ. Role of Anæsthetist in Management of Intractable Pain. Proceedings of the Royal Society of Medicine 47: 1029-1032.
- Bridenbaugh LD, Moore DC, Campbell DD (1964) Management of upper abdominal cancer pain: treatment with celiac plexus block with alcohol. JAMA 190: 877-880.
- Babu V, Kura K, Gritsenko K (2018) Celiac Plexus Blocks and Splanchnic Nerve Blocks. InEssentials of Interventional Techniques in Managing Chronic Pain 2018: 595-607.
- Jeon YH (2012) Spinal cord stimulation in pain management: a review. Korean J Pain 25: 143.








