Keywords
Antioxidant potential; Acacia nilotica; Ocimum sanctum; Alpinia nigra; DPPH assay; Reducing power; Total phenolic; Total flavonoid
Introduction
The inequity between the manifestation of Reactive Oxygen Species (ROS) inside a biological system and detoxification capability of injurious intermediates is known as Oxidative stress [1]. The chain reaction initiated by a ROS can severely harm an individual cell and its surroundings. Research shows that a chain reaction evolves in the presence of different free radicals like superoxide, Peroxide, etc. [2].
In these recent years, concern on oxidative stress has raised to a higher level after numerous fundamental research were found to be finalizing significant relations between oxidative stress and aging or different chronic diseases including cancer [3]. Antioxidants have been playing a role against oxidative stress to ensure the safety of biomolecules and prevent oxidative deterioration. Many studies have shown that polyphenolic compounds are effective as antioxidants and act against lipid oxidation in phospholipids’ bilayers [4].
However a growing number of synthetic antioxidants have become another issue, scientists needed to consider carefully. At this situation, it is widely suggested that the use of natural antioxidants can be a very good alternative solution instead of using synthetic butylated hydroxyanisole, butylated hydroxytoluene, propyl gallate etc. where a few of these agents are believed to induce cancer and liver dysfunction [5,6]. On the contrary, it is very likely to assume that the natural antioxidants would be safer than most other synthetic materials serving as oxidative stress fighter.
A. nilotica is traditionally used as astringent, anti-oxidant, natriuretic, antispasmodial, diuretic, intestinal pains and diarrhea, coughs, leucorrhea and sclerosis [7]. O. sanctum Linn, a small herb seen throughout Bangladesh, have been recommended for the treatment of bronchitis, bronchial asthma, diarrhea, dysentery, skin diseases, chronic fever, insect bite, etc. [8]. The ethnopharmacological uses of A. nigra include treatment of jaundice, gastric ulcers, parasitic infections, inflammation, etc. [9,10].
As our continuous approaches [11-13] to justify traditional claims of different medicinal plants of Bangladesh, the antioxidant potentials of A. nilotica, O. sanctum, A. nigra were investigated.
Materials and Method
Plant material
Roots A. nilotica, leaves of O. sanctum and rhizomes of A. nigra from were collected from Noakhali district of Bangladesh and identified by officials of Bangladesh National Herbarium, Dhaka where voucher specimens (No. 38212, 38234, 38358, respectively) have also been deposited. Materials dried at room temperature and coarsely ground before extraction. Samples (250 g each) were extracted separately at room temperature by percolation method using 750 ml of 80% methanol for each sample. The resulting extract was concentrated over a rotary vacuum until a crude solid extract was obtained, which was then subject to solvent fractionation by n-hexane and little amount of water. The obtained n-hexane fraction was separately concentrated under room temperature and used directly for the study.
Chemicals
Methanol, n-hexane, Folin-Ciocalteu reagent, sodium carbonate, gallic acid, crystalline aluminum chloride, crystalline sodium acetate, quercetin, 1’-1’ diphenyl picryl-hydrazyl (DPPH), potassium ferricyanide, trichloroacetic acid, ferric chloride, butylated hydroxy toluene (BHT), ascorbic acid. All reagents were of analytical grade and purest quality available in a university lab.
Total phenolic content determination
Total phenolic compound contents were determined by the Folin-Ciocalteau method [14]. Extract solution of 250 μg/ml for each extract were prepared in water. To 0.5 ml of extract solution, 2.5 ml of Folin-Ciocalteu reagent (1:10 v/v diluted with distilled water) and 2.0 ml of Na2CO3 (7.5% w/v) solution were added. The mixture was incubated for 20 minutes at room temperature and then the absorbance was measured at 760 nm by UV-spectrophotometer and using the standard curve prepared from Gallic acid solution with different concentration (0.78125 μg/ml to 100 μg/ml), the total phenolic contents of the sample was measured and it was expressed as μg of GAE (Gallic acid equivalent)/mg of the extract [15].
Total flavonoid content determination
The flavonoids content of the n-hexane extracts of A. nilotica, O. sanctum, A. nigra were determined following Aluminium chloride colorimetric method [16]. Extracts were taken in methanol in different beakers to make the concentration of 500 μg/ml for each plant. Then 5 ml of an analyzed solution of three different extracts were taken in different test tubes and 2.5 ml of AlCl3 reagent (133 mg crystalline aluminum chloride and 400 mg crystalline sodium acetate were dissolved in 100 ml of methanol) was added individually in all taken test tubes and absorbance recorded at 430 nm. Various dilutions of quercetin (125 μg/ml to 6.25 μg/ml) were prepared in methanol and a standard curve was plotted. The amount of flavonoids was calculated as quercetin equivalent from the calibration curve of quercetin.
DPPH radical-scavenging activity
The antioxidant activities of the plants extracts on the stable radical 1,1-diphenyl-2-picrylhydrazyl (DPPH) were estimated by the method of Brand-Williams [17]. Briefly, 3 mL of DPPH solution (20 μg/ml) was added to 2 ml each of the test solutions (500-0.977 μg/ml, total 10 different conc.), and was incubated in the dark at room temperature for 30 min. The absorbance values were read at 517 nm, and converted into percentage antioxidant activity, using the formula below. This procedure was the same for all three different plants extracts.
% inhibition=[1-(Absorbance of Sample/Absorbance of control)] × 100
Reducing power determination
The reducing power of three selected plants’ extracts were determined according to the method previously described [18]. 1 ml of test samples of different concentrations (500 μg/ml to 3.906 μg/mL) was mixed with 2.5 ml phosphate buffer (pH 6.6) and 2.5 ml of (1% w/v) potassium ferricyanide. The mixture was incubated at 50°C for 20 min. Trichloroacetic acid of 2.5 ml was added to the mixture and it was then centrifuged at 3000 rpm for 10 min. The supernatant solution of 2.5 ml was taken in another test tube and 2.5 ml of distilled water, 0.5 ml of ferric chloride (0.1% w/v) were added to the tube and mixed. The absorbance was measured against a blank at 700 nm. Increased absorbance of the reaction mixture indicated the increased reducing power. This procedure was the same for all three different plants extracts. Butylated hydroxytoluene (BHT) was used as a positive control in DPPH radical scavenging assay and ascorbic acid (AA) as a positive control in ferric reducing power assay.
Results and Discussion
Antioxidants are used in the food industry, to stabilize foods that by their composition would in the presence of oxygen and other reactive oxygen species undergo significant loss in quality such as the development of rancidity from the oxidation of unsaturated fats resulting in off-odors, off-flavors, and discoloration by decomposition of components of the food.
In this study, we examined total phenolics, total flavonoids and evaluated the antioxidant effects of n-hexane extracts of roots of A. nilotica, leaves of O. sanctum and rhizome of A. nigra.
Total phenolic content and total flavonoid content
Recent years have seen an exponential increase in research to identify and characterize of phenolic and flavonoid contents from medicinal plants. Phenols show antioxidant activity by destroying lipid free radicals or preventing the conversion of hydroperoxides into free radicals, that is why it is responsible for varying the antioxidant capacity of plants [19].
Total phenol compounds, as determined by Folin Ciocalteu method, was reported as μg gallic acid equivalents/mg of extract powder, by reference to a standard curve (y=0.018x+0.043, R²= 0.999). The total flavonoid contents were reported as μg quercetin equivalent/mg of extract powder, by reference to a standard curve (y=0.009x+0.005, R²=0.988) (Table 1).
| n-hexane extract of |
Total phenol contents gallic acid equivalent (μg/mg) |
Flavonoid contents Quercetin Equivalent (μg/mg) |
| A. nilotica |
74.89 |
46.67 |
| O. sanctum |
13.55 |
258.66 |
| A. nigra |
7.77 |
69 |
Table 1: Total phenol and flavonoids contents of tested plants extracts.
A. nilotica n-hexane root extracts showed the highest amount of phenolic content and O. sanctum leaves extract showed the highest amount of flavonoid content among three investigated plants ’ parts. Whereas the lowest amount of phenolic and flavonoid content were found in A. nigra and A. nilotica, respectively. The order of magnitude of total phenolic content among these plants is A. nilotica>O. sanctum>A. nigra and for total flavonoid is O. sanctum>A. nigra>A. nilotica. Phenolic acids are found as antioxidants in nature, e.g. caffeic acid, ferulic acid, vanillic acid, and rosmarinic acid are widely distributed in the plant kingdom [20] where the last one was found as potent active substance against HIV [21].
DPPH scavenging assay
The DPPH assay constitutes a quick and low cost sensitive method where the decolorization of the radical solution from purple to light yellow is evaluated in the presence of the plant extract at 517 nm using UV spectroscopy [22]. The IC50 value is defined as the concentration of plant extract that causes 50% loss of the DPPH activity and was calculated by linear regression plots of the percentage of antiradical activity against the concentration of the tested extract. IC50 value found from this study for A. nilotica, O. sanctum, A. nigra is 39.62 μg/ml, 48.81 μg/ml, 70.85 μg/ml, respectively.
In DPPH scavenging assay, A. nilotica extract exerts far better antioxidant activity than the others. This antioxidant test establishes the potency of A. nilotica as an oxidative stress fighter where O. sanctum and A. nigra shows relatively less potentiality (Table 2).
| Concentration (µg/ml) |
% inhibition |
| A. nilotica |
O. sanctum |
A. nigra |
BHT |
| 500 |
82.82 |
76.8 |
67.66 |
94.1 |
| 250 |
80.36 |
69.61 |
65.26 |
93.67 |
| 125 |
74.53 |
62.43 |
58.68 |
90.37 |
| 62.5 |
69.01 |
58.56 |
50.89 |
79.81 |
| 31.25 |
46.01 |
49.17 |
43.71 |
57.22 |
| 15.62 |
26.99 |
39.23 |
32.93 |
38.85 |
| 7.81 |
11.96 |
19.34 |
24.55 |
26.5 |
| 3.9 |
10.12 |
11.05 |
14.37 |
16.56 |
| 1.95 |
5.52 |
8.29 |
5.38 |
15.96 |
| 0.97 |
3.37 |
3.87 |
1.79 |
14.45 |
| IC50 (µg/ml) |
39.62 |
48.81 |
70.85 |
18.56 |
Table 2: DPPH free radical scavenging capacity (% inhibition) of selected plants extracts and reference standard.
This result also determines an approximately proportional relationship between phenolic content and free radical scavenging power that is similar to other studies [14,23]. The order of magnitude of antioxidant activity among these three plants is A. nilotica>O. sanctum>A. nigra.
Ferric reducing power assay
The reduction of ferric ion (Fe3+) to ferrous ion (Fe2+) is measured by the intensity of the resultant Perl’s Prussian blue solution from yellow which absorbs at 700 nm. Greater the intensity of the colour, greater will be the absorption; consequently, greater will be the antioxidant activity [24]. Many studies reported that reducing power was associated with the antioxidant activity and this relationship of phenolic compound has been well established in several plant sources [25,26].
The reducing power of selected plants’ extracts and positive reference standards is shown in Table 3.
| Conc. (µg/ml) |
Absorption at 700 nm |
| A. nilotica |
O. sanctum |
A. nigra |
AA |
| 500 |
2.245 |
2.589 |
0.429 |
3.035 |
| 250 |
1.493 |
1.338 |
0.322 |
1.995 |
| 125 |
0.88 |
0.719 |
0.292 |
1.234 |
| 62.5 |
0.463 |
0.402 |
0.26 |
0.762 |
| 31.25 |
0.32 |
0.339 |
0.244 |
0.516 |
| 15.62 |
0.29 |
0.28 |
0.235 |
0.369 |
| 7.81 |
0.211 |
0.273 |
0.224 |
0.297 |
| 3.9 |
0.197 |
0.229 |
0.218 |
0.232 |
Table 3: Reducing the potential of plants extracts and ascorbic acid.
The highest absorption (2.589) was found for O. sanctum and lowest (0.429) for A. nigra among the three extracts. A. nilotica and O. sanctum showed fairly good antioxidant activity close to positive standard ascorbic acid. The reducing power of extracts increased steadily with increasing concentrations (Figure 1) and varied significantly with different concentrations. The found order of magnitude of ferric reducing power in this study is ascorbic acid>O. sanctum>A. nilotica>A. nigra.
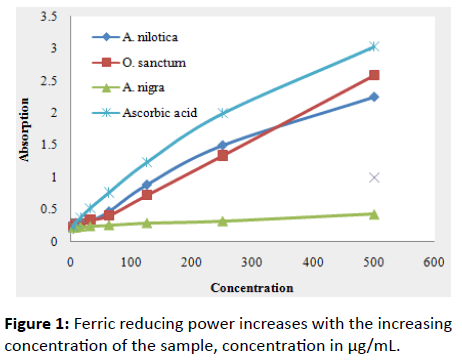
Figure 1: Ferric reducing power increases with the increasing concentration of the sample, concentration in μg/mL.
Conclusion
A. nilotica and O. sanctum showed good antioxidant properties and A. nigra showed comparatively less antioxidant properties. It is however, worthwhile to further investigate the in vivo potentials of this plant and also isolate the active components which could ultimately lead to their application in the food industry as an antioxidant flavour or in pharmaceutical formulations.
Acknowledgement
This research received financial support from the Ministry of Science and Technology, Bangladesh under the grant number NST/Fellow/2012-13/Life Sciences and Medical Sciences/168.
24409
References
- Fu W, Chen J, Cai Y, Lei Y, Chen L, et al. (2010) Antioxidant, free radical scavenging, anti-inflammatory and hepatoprotective potential of the extract from Parathelypteris nipponica (Franch. et Sav.) Ching. J Ethnopharmacol 130: 521-528.
- Halliwell B (2007) Oxidative stress and cancer: have we moved forward? Biochem J 401: 1-11.
- Lin SD, Liu EH, Mau JL (2008) Effect of different brewing methods on antioxidant properties of steaming green tea. LWT-Food Sci Technol 41: 1616-1623.
- Botterweck AA, Verhagen H, Goldbohm RA, Kleinjans J, Van den Brandt PA (2000) Intake of butylated hydroxyanisole and butylated hydroxytoluene and stomach cancer risk: results from analyses in the Netherlands cohort study. Food and Chemical Toxicology 38: 599-605.
- Imaida K, Fukushima S, Shirai T, Ohtani M, Nakanishi K, et al. (1983) Promoting activities of butylated hydroxyanisole and butylated hydroxytoluene on 2-stage urinary bladder carcinogenesis and inhibition of γ-glutamyl transpeptidase-positive foci development in the liver of rats. Carcinogenesis 4: 895-899.
- Saini ML, Saini R, Roy S, Kumar A (2008) Comparative pharmacognostical and antimicrobial studies of acacia species (Mimosaceae). J Med Plants Res 2: 378-386.
- Prakash P, Gupta N (2005) Therapeutic uses of Ocimum sanctum Linn (Tulsi) with a note on eugenol and its pharmacological actions: a short review. Indian J Physiol Pharmacol 49: 125-131.
- Roy B, Swargiary A, Giri BR (2012) Alpinia Nigra (Family Zingiberaceae): An anthelmintic medicinal plant of North-East India. Adv Life Sci 2: 39-51.
- Rahman MA, Uddin S, Wilcock C (2007) Medicinal plants used by Chakma tribe in Hill Tracts districts of Bangladesh. Indian J Traditional Knowledge 6: 508-517.
- Sikder M, Rashid R, Islam F, Hossian A, Siddique A, et al. (2013) Screening of ten medicinal plants of Bangladesh for analgesic activity on Swiss-albino mice. Orient Pharm Exp Med 13: 327-332.
- Sikder MAA, Kaisar MA, Rahman MS, Hasan CM, Al-Rehaily AJ, et al. (2012) Secondary metabolites from seed extracts of Syzygium Cumini (L.). J Physical Sci 23: 83-87.
- Chowdhury SR, Tasdique FI, Quadery M, Shihan MH, Rashid MA (2012) In vitro antioxidant, total phenolic content and preliminary toxicity studies of Gmelina philippensis chem. Afr J Pharm Pharmacol 6: 855-859.
- Ebrahimzadeh MA, Pourmorad F, Hafezi S (2008) Antioxidant activities of Iranian corn silk. Turk J Biol 32: 43-49.
- Rekha C, Poornima G, Manasa M, Abhipsa V, Devi JP, et al. (2012) Ascorbic acid, total phenol content and antioxidant activity of fresh juices of four ripe and unripe citrus fruits. Chem Sci Trans 1: 303-310.
- Chang CC, Yang MH, Wen HM, Chern JC (2002) Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J Food Drug Anal 10: 178-182.
- Brand-Williams W, Cuvelier M, Berset C (1995) Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci Technol 28: 25-30.
- Oyaizu M (1986) Studies on product of browning reaction prepared from glucose amine. Jpn J Nutr 44: 307-315.
- Cai Y, Luo Q, Sun M, Corke H (2004) Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci 74: 2157-2184.
- Larson RA (1988) The antioxidants of higher plants. Phytochemistry 27: 969-978.
- Mazumder A, Neamati N, Sunder S, Schulz J, Pertz H, et al. (1997) Curcumin analogs with altered potencies against HIV-1 integrase as probes for biochemical mechanisms of drug action. J Med Chem 40: 3057-3063.
- Molyneux P (2004) The use of the stable free radical diphenylpicrylhydrazyl (DPPH) for estimating antioxidant activity. Songklanakarin J Sci Technol 26: 211-219.
- Nahak G, Sahu RK. (2010) In vitro antioxidative acitivity of Azadirachta indica and Melia azedarach leaves by DPPH scavenging assay. Nat Sci 8: 22-28.
- Zou Y, Lu Y, Wei D (2004) Antioxidant activity of a flavonoid rich extract of Hypericum perforatum L. in vitro. J Agric Food Chem 52: 5032-5039.
- Siddhuraju P, Becker K (2003) Antioxidant properties of various solvent extracts of total phenolic constituents from three different agroclimatic origins of drumstick tree (Moringa oleifera Lam.) leaves. J Agric Food Chem 51: 2144-2155.
- Amarowicz R, Pegg R, Rahimi-Moghaddam P, Barl B, Weil J (2004) Free-radical scavenging capacity and antioxidant activity of selected plant species from the Canadian prairies. Food Chem 84: 551-562.






