The soft compliant electrodes from the natural rubber latex as the matrix phase and the ionic filler solution as the dispersed phase by UV curing system have many advantages i.e. softness, flexibility, good appearance, compatibility, and good physical, electrical and mechanical properties. The electrical conductivity, Youngs modulus, tensile stress, critical strain, and curing time of soft compliant electrode added 0.5% V/V ZnCl2 ionic filler solution are 1.97 10-2 S/cm, 5.43 Â 103 Pa, 6.97 104 Pa, 28.8%, 7 min. respectively. The electrical conductivity, Youngs modulus, tensile stress, critical strain, and curing time of soft compliant electrode added 0.75% V/V CuCl2 ionic filler solution are 3.26 10-2 S/cm, 3.03 103 Pa, 5.15 Â 104 Pa, 6.84%, 7 min respectively. While the electrical conductivity, Youngs modulus, tensile stress, critical strain, and curing time of soft compliant electrode added 5.0% V/V CaCl2 ionic filler solution made from eggshell dissolved in HCl are 0.13 S/cm, 1.16 Â 106 Pa, 7.92 Â 105 Pa, 41.18%, 19 min. respectively. The electrical conductivity values of soft compliant electrode added with ZnCl2, CuCl2, and CaCl2 made from eggshell ionic filler solution are higher than that of cured natural rubber latex films without ionic filler solution with the magnitudes of 102 to 104. The eggshell is a calcium source used as the CaCl2 ionic filler solution which is potential for the soft compliant electrode and non-toxicity samples preparation. The soft compliant electrodes can be applied for various applications such as drug delivery, artificial muscle, robot, micro-pump, micro-valve, disk drive, flat panel speaker, linear motor in MRI, and etc.
Keywords
Soft compliant electrode; Electrical conductivity; Ionic filler solution; Eggshell
Introduction
Soft compliant electrode is a soft conductive material called the dielectric elastomer actuator which is an electro-active polymer having large strain and shape conformability [1]. Dielectric elastomers are a rubber-like. Dielectric material coated on polymer can acted as a compliant electrode having elastic, incompressible, and the ability to operate at large strain [2-4]. In general, dielectric elastomers are made from various materials such as silicone, polyacrylates, thermoplastic, polyurethane, and natural rubber [3,5]. Natural rubber has good mechanical properties such as elasticity and flexibility and the possibility of incorporation in bulk to make composite materials on electrical-thermal-mechanical properties [5-10]. Furthermore, the natural rubber is an interesting material to make an actuator because it is lightweight, easily fabricated in various shapes, and low cost. The dielectric elastomers can be applied for several actuator applications such as cardiac membrane pump, artificial bicep for orthotic and prosthetic technology or artificial muscle like that of natural muscle, a hopping robot or mobile robot, micro-pump, micro-valve, disk drive, flat panel speaker, intelligent endoscope, a six-legged robot, a linear motor (e.g. non-magnetic actuators for MRI applications), and used for rehabilitation purposes [2,11-14].
Eggs are one of the most complete foods as they contain protein, lipid, and carbohydrates which are essential for a good diet; they also contain vitamins and mineral elements that are necessary for the development of young and elderly people. Egg and its derivative are one of important raw materials to make food, drug, bakery, and cosmetic industries i.e. for manufacturing bread, cakes, crackers, ice creams, food additive agents. The eggshell obtained from by products provides approximately 11 wt% of the total weight (65 g to 70 g per egg); the main composition composed of more than 96 wt% calcium carbonate (CaCO3), 1 wt% magnesium carbonate (MgCO3), 1 wt% calcium phosphate (CaPO4), and 2 wt% of the other organic matters. In order to maximize the recycling opportunities for eggshells, reduce eggshell wastes, conserve the environment without pre-treatment, and increase agricultural evaluation, it is estimated that eggshells waste amounts to some million tons per day from hen, duck, and bird eggs. Thus, an efficient eggshell recycle or disposal method is required. Eggshells are porous ceramic materials as developing embryos require gases, oxygen and carbon dioxide, to breath in and out by diffusion through the pores. Mostly, the structure of eggshells is composed of micro-pores acting as catalysts or adsorbents [15-19].
There are many kinds of conductive fillers or ionic fillers composed of ionic crystals, solid electrolytes, and liquid electrolytes acted as a dispersed phase in the soft polymeric matrix consisting of oil, gel, or elastomer such as mainly carbon black, graphite, carbon nanotube, graphene, piezoelectric materials i.e. PZT, PLZT, dielectric ceramic i.e. inorganic liquid electrolyte in terms of ionic salt solution CaCl2, AlCl3, MgCl2, and AuCl3 or oxide ceramic i.e. Fe2O3, CuO, AgO, and PdO. The polymeric matrix provides the mechanical properties to the composites while the conductive fillers or ionic fillers provide the electrical conductivity by using a melt rheometer in the tension mode to determine mechanical properties and electrical conductivity. However, one of the major challenges in the development of dielectric elastomer actuators is the high electric field requirement to actuate these elastomer films [20-23]. In addition, compliant electrodes can deform with the substrate without generating an opposing stress or losing conductivity, are the key to the development of electro-active polymer actuator technology including the softest possible electrodes and the amount of conductive filler or ionic fillers as low as possible in order to maintain the elastic modulus of the composite low. The amount of ionic fillers or dielectric materials or conductive fillers depends on the shape of the filler particles and on how well dispersed the ionic fillers are in the matrices. Ionic filler particles with high respect ratios and shape different from spherical, or which form highly structured agglomerates, are all also display percolation thresholds far below the limiting 16% volume of spherical particles [24]. As a mentioned shape of ionic fillers, therefore, in this research the ionic filler is prepared in terms of liquid or solution in order to easily compatibility and a low percolation threshold in the natural rubber latex compounds to obtain high electrical conductivity and good flexibility.
In this work, the objective is to comparison study effect of adding three kinds of ionic electrolyte filler solution among ZnCl2, CuCl2, and CaCl2 made from eggshells in order to increase physical, electrical, and mechanical properties of the compliant electrode samples. Furthermore, the physical properties (smoothness of rubber latex film surface, color, and thickness), mechanical properties (elastic modulus, tensile stress, Young’s modulus, and tensile strain), and electrical properties (electrical conductivity, deformation of samples in the electrical field) of soft compliant electrode samples also reported here.
Experimental
Materials
Chicken eggshell was used as a CaCO3 source collected from the local cafeteria to prepare calcium chloride solution (CaCl2) via the chemical reaction process. Hydrochloric acid (HCl; analytical reagent grade-AR) was purchased from Lab-Scan Co. Ltd., Thailand. Natural rubber latex concentrated 60.69 wt% was supplied by Thai rubber latex group Co. Ltd., Thailand. Photoinitiator (C15H21NO2S) namely 2-methyl-4´- (methylthio)-2-morpholinopropiophenone (Sigma-Aldrich) was supplied by Chemical Express Co. Ltd., Thailand. Crosslink agent (C15H26O6S3) trimethylopropane tris (3- mercaptopropionate) (Sigma-Aldrich) was purchased from Chemical Express Co. Ltd., Thailand. Surfactant Tween 80: Polysorbate 80 (Sigma-Aldrich) was supplied by Chemical Express Co. Ltd., Thailand. Commercial zinc chloride (ZnCl2) is high purity more than 99.0 wt% (Ajak Australia) supplied by Italmar Co., Ltd. Thailand. Commercial copper chloride (CuCl2) is high purity more than 99.0 wt% (Ajak Australia) supplied by Italmar Co. Ltd. Thailand.
Instrument
X-ray fluorescence (XRF) was used to determine the chemical compositions: Philips, model PW2400 was used with the tube current of 1000 mA and an acquisition lifetime of 30s. Universal testing machine was measured mechanical properties (Yield strength, Young’s modulus, and tensile stress) of compliant electrode samples with loading 50 kN by Hounsfield, H50 KS. The tensile testing was measured according to the ASTM D412-98 a (Standard test methods for vulcanized rubber and thermoplastic elastomers tension). Rapid mill model RM 1105 with speed 500 rpm was supplied by from Compound Clay Co., Ltd., Thailand. The rapid mill composed of a porcelain pot and porcelain balls ground by electric motor for grinding the eggshell in order to prepare the calcium chloride ionic filler solution. UV oven model UVA F400 A1, power 400 W was supplied by A to S Bangkok supply Co. Ltd., Thailand. The UVA oven was used for curing the natural rubber latex compound. Fourier transform infrared spectra (FTIR) were recorded with a spectrometer (PerkinElmer, Spectrum One) at a spectral resolution of 4 cm-1. The raw material and cured natural rubber film samples were measured the chemical functional groups in the range of 500 cm-1 to 4000 cm-1. Melt rheometer model rheometric scientific model (ARES) was used to measure the electrical conductivity of the natural rubber latex film samples in the tension mode which able to determine electrical conductivity and mechanical deformation at electrical field 10 V.
Soft compliant electrode preparation
The soft compliant electrode samples were prepared totally nine formula according to the Table 1. Formula 1 contains only natural rubber latex 20 ml without adding any chemical substances. Formula 2 is the sample added only 3 V/V% crosslink agent and 1 V/V% photo-initiator without ionic filler solution adding.
| Samples |
Formula 1 |
Formula2 |
Formula 3 |
Formula 4 |
Formula5 |
Formula6 |
Formula
7 |
Formula
8 |
Formulag9 |
| Conc. Natural rubber latex (ml) |
20 |
20 |
20 |
20 |
20 |
20 |
20 |
20 |
20 |
| Crosslink agenta (g) |
- |
0.112 |
0.112 |
0.112 |
0.112 |
0.112 |
0.112 |
0.112 |
0.112 |
| Photoinitiatorb (ml) |
- |
0.06 |
0.06 |
0.06 |
0.06 |
0.06 |
0.06 |
0.06 |
0.06 |
Surfactantc
(ml) |
- |
1.0 |
1.0 |
1.0 |
1.0 |
1.0 |
1.0 |
1.0 |
2.0 |
| ZnCl2 solutiond |
- |
- |
0.0896 g
0.5 V/V% |
0.1342 g
0.75V/V% |
0.1790 g 1.0 V/V% |
- |
- |
- |
- |
| CuCl2 solutione (g) |
- |
- |
- |
- |
- |
0.1270 g
0.5 V/V% |
0.1906 g 0.75V/V% |
0.2540 g
1.0 V/V% |
- |
| CaCl2 solutionf (g) |
- |
- |
- |
- |
- |
- |
- |
- |
12.8 g
5.0 V/V% |
| Water (ml) |
- |
- |
10 |
10 |
10 |
10 |
10 |
10 |
10 |
aCrosslink agent is trimethylopropane tris (3-mercaptopropionate) (C15H26O6S3) having true density 1.21 g/cm3.bPhotoinitiator is 2-methyl-4-(methylthio)-2-morpholino propiophenone (C15H21NO2S) having true density1.1932 g/cm3.cSurfactant is tween 80 (Polysorbate 80) having true density 1.07 g/cm3 composed of polyethyleneglycol sorbitanmonooleate, polyoxyethy lenesorbitan monooleate, and polysorbate 80.dZnCl2solution composed of ZnCl2 having true density 1.79 g/cm3.eCuCl2 solution composed of CuCl2.2H2O having true density 2.54 g/cm3.fCaCl2 solution prepared from eggshell powder react with hydrochloric acid bydissolving the eggshell powder in hydrochloric acid (HCl). The true density of eggshell powder is 2.2 g/cm3.gFormula 9 added 5.0 V/V% CaCl2 made from eggshell powder needs 2.0 ml surfactant in order tohomogeneous mixture.
Table 1: Formula of the soft compliant electrode prepared by UV curing system
Formula 3, 4, and 5 are the cured natural rubber film added 3 V/V% crosslink agent, 1 V/V% photo-initiator, 1 V/V% surfactant, and 0.5, 0.75, and 1.0 V/V% ZnCl2 as an ionic filler solution, respectively. Formula 6, 7, and 8 are the cured natural rubber latex films added 3 V/V% crosslink agent, 1 V/V % photo-initiator, 1 V/V% surfactant, and 0.5, 0.75, and 1.0 V/V% CuCl2 as an ionic filler solution.
Formula 9 is the cured natural rubber latex film added 3 V/V % crosslink agent, 1 V/V% photo-initiator, 2 V/V% surfactant, and 5 V/V% CaCl2 made from eggshell react with HCl according to the equation 1. All samples were prepared by mixing 3 V/V% crosslink agent and 1 V/V% photo-initiator with the magnetic stirrer for 3 min, then pour the obtain mixture into the natural rubber latex and stir for 3 min. After that, the surfactant (tween 80) was added into the natural rubber latex compound and stirred for 3 min.
Finally, the ionic filler solution was added into the natural rubber latex compound and stirred with the glass rod in order to prevent the bubble formation. The natural rubber latex compound was poured into the petri-dish and cured in the UVA oven until the natural rubber latex compound was dried to be the natural rubber latex films. The optimum amount of CaCl2 as an ionic filler solution added into the natural rubber latex compound is 5.0 V/V% and still compatibility and homogeneous suspension.
Results and Discussion
The chemical compositions of hen eggshell measured by XRF are tabulated in Table 2. The main composition of eggshell powder is CaCO3 97.04 wt%, and other oxide compounds 2.96 wt%. The calcium carbonate (CaCO3) can be dissolved in concentrated 37% hydrochloric acid (HCl) as following the equation (1) to completed form the CaCl2 ionic filler solution added in the natural rubber latex compound to prepare the soft compliant electrode samples.
| Composition |
Raw hen eggshells |
| Na2O |
<0.01 |
| MgO |
0.10 |
| SiO2 |
0.01 |
| P2O5 |
0.80 |
| SO3 |
1.79 |
| K2O |
0.21 |
| CaO |
- |
| CaCO3 |
97.04 |
| SrO |
0.02 |
| ZrO2 |
0.02 |
Table 2: Chemical composition of hen eggshells measured by wavelength dispersive XRF
Physical-mechanical-thermal properties of cured natural rubber latex films are data tabulated in Table 3. Formula 1 is the pure natural rubber latex films without adding crosslink agent and other chemical substances. Therefore, formula 1 takes long time for natural rubber latex films formation for 70 mins whereas the formula 2 is the natural rubber latex film sample added both crosslink agent and photo-initiator to cure the natural rubber latex films. Therefore, the curing time of formula 2 is only 5 mins. Formulae 3-5, 6-8, and 9 are the cured natural rubber latex films added crosslink agent, photoinitiator, surfactant, and ionic filler solution of commercial ZnCl2 and CuCl2, and CaCl2 made from eggshell dissolved in HCl, respectively.
| Samples |
Thickness
(mm) |
Film characteristics |
Tensile stress
(Pa) |
Young’s modulus (Pa) |
Electrical conductivity (s/cm) |
Critical strain
(%) |
| Formula 1 |
0.812 ± 0.062 |
Smooth film surface
Light yellow-brown |
N/A |
N/A |
N/A |
N/A |
| Formula 2 |
0.916 ± 0.156 |
Smooth film surface, white, and translucence |
1.61105 |
6.78 Â 103 |
1.96 Â 10-4 |
12.00 |
| Formula 3 |
0.553 ± 0.028 |
Smooth film surface, pale yellow, and translucence |
6.97 Â 104 |
5.43 103 |
1.97 Â 10-2 |
28.80 |
| Formula 4 |
0.400 ± 0.004 |
Smooth film surface, light yellow, and translucence |
1.47 Â 105 |
6.47 Â 103 |
2.04 10-4 |
8.76 |
| Formula 5 |
0.879 ± 0.033 |
Smooth film surface, light yellow, and translucence |
1.92 Â 105 |
8.23 103 |
3.29 10-4 |
7.21 |
| Formula 6 |
0.633 ± 0.057 |
Smooth film surface, yellow, and translucence |
2.66 104 |
3.55 103 |
2.78 Â10-2 |
44.30 |
| Formula 7 |
0.548 ± 0.003 |
Smooth film surface, yellow, and translucence |
5.15 104 |
3.03 103 |
3.26 10-2 |
6.84 |
| Formula 8 |
0.500 ± 0.067 |
Smooth film surface, yellow, and translucence |
5.35 104 |
3.50 Â 103 |
7.85 10-3 |
5.34 |
| Formula 9 |
0.543 ± 0.003 |
Smooth film surface, homogeneous, light yellow, and translucency |
1.39 104 |
1.98 Â 106 |
1.30 10-1 |
41.18 |
Table 3: Physical-mechanical-electrical properties of the soft compliant electrode prepared by UV curing system.
The curing time of natural rubber latex films added ZnCl2 and CuCl2 solution is approximately 7 mins to obtain the smooth film surface, translucency, and pale to light yellow due to amount and effect of ionic filler ions. While the formula 9 is the natural rubber latex film added CaCl2 made from eggshell used the curing time 19 mins. The different curing time depends on many factors i.e. types and amount of crosslink agent, photo-initiator, surfactant, especially type and amount ionic filler. Although Zn2+, Cu2+, and Ca2+ have the same divalent cations, but the different ionic radius of Zn2+, Cu2+, and Ca2+ are 0.60, 0.63, and 0.71 nm, respectively. The different ionic radius affects to intercalate and exfoliate of cations into the polymer chain. Therefore, the different ionic radius of ionic filler adding affects to the curing time of natural rubber latex films. The average film thickness of all samples is in the range of 0.5 mm to 1.0 mm due to the volume limitation of natural rubber latex compounds added in the petri-dishes before curing by the UVA.
The mechanical properties (tensile stress and Young’s modulus) of cured natural rubber latex films with and without ionic filler solution measured by universal testing at room temperature 27°C are tabulated in Table 3. Formula 1 cannot measure the mechanical properties due to without UV curing. Formula 2 is the cured natural rubber latex films without adding ionic filler solution having good tensile stress 1.61 × 105 Pa. The comparison study on mechanical properties of the cured natural rubber latex films added ionic filler solution among ZnCl2, CuCl2, and CaCl2 show high tensile stress and Young’s modulus especially formula 3,7, and 9. The mechanical properties of the cured natural rubber latex films added CaCl2 ionic filler solution made from eggshell are better than the cured natural rubber latex films added ZnCl2 and CuCl2 ionic filler solution although Zn2+, Cu2+, and Ca2+ having the same divalent ions. The different ionic radius of Zn2+, Cu2+, and Ca2+ are 0.60, 0.63, and 0.71 nm, respectively. The different ionic radius affects to intercalate and exfoliate of cations into the polymer chain related to the elasticity of polymer chains. Therefore, the optimum percentage volume of ionic filler solution adding into the natural rubber latex is different.
The electrical conductivity data measured by ARES or melt rheometer as tabulated in Table 3 and shown in Figures 1-3.Formula 3 (0.5 V/V% ZnCl2), formula 7 (0.75 V/V% CuCl2), and formula 9 (5 V/V% CaCl2) are the best soft compliant electrode samples which give the highest electrical conductivity, good tensile stress, and good Young’s modulus as shown in Figures 1-3, respectively. The highest electrical conductivity data of formula 3,7, and 9 on the top line are 0.0197, 0.0326, and 0.1300 S/cm, respectively. The obtained electrical conductivity values of natural rubber latex films are consistent with the ionic conduction from the hand book chemistry and physics data: Zn2+ 52.8 × 10-4 m2 S/mol-1, Cu2+ 53.6 × 10-4 m2 S/mol-1, and Ca2+ 59.47 × 10-4 m2 S/mol-1 [25]. The order of ionic radius from high to low values is Ca2+ > Cu2+> Zn2+. The ionic conductivity values orientation from high to low is also Ca2+ > Cu2+ > Zn2+.
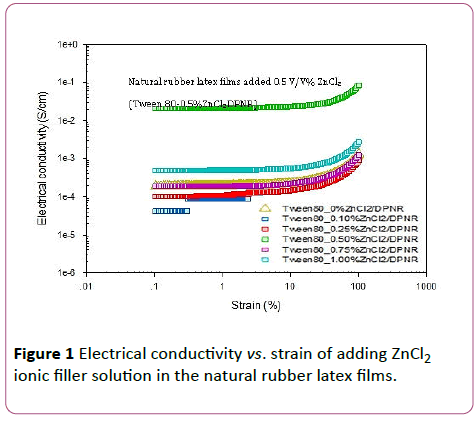
Figure 1: Electrical conductivity vs. strain of adding ZnCl2 ionic filler solution in the natural rubber latex films.
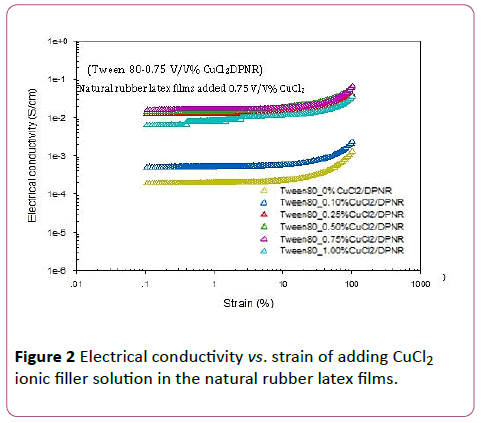
Figure 2: Electrical conductivity vs. strain of adding CuCl2 ionic filler solution in the natural rubber latex films.
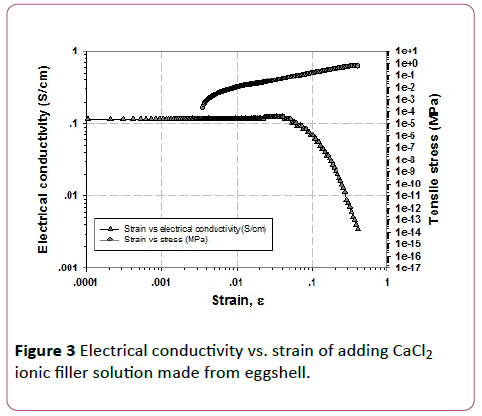
Figure 3: Electrical conductivity vs. strain of adding CaCl2 ionic filler solution made from eggshell.
FTIR spectra of raw materials: pure natural rubber latex, crosslink agent, photo-initiator, surfactant, and ionic filler for natural rubber latex films preparation are shown in Figure 4. The wave numbers at 504.02 cm-1 and 567.87 cm-1 belongs to strong ? (Zn-O), ? (Cu-O), and ν (Ca-O), respectively. The FTIR spectra at 1084 cm-1 to 1128 cm-1 show (C-O) st, (S=O) st, and(=C-N) stretching. The high peak intensity at 1376 cm-1 is S=O bond stretching. At the wave number in the range of 1390 cm-1 to 1800 cm-1 show C-O, CH3 asymmetric deformation, and C=C bonds. Furthermore, the Ca-O bond can occur at 1465 cm-1. In addition, at the wave number of 1609 cm-1, 1617.14 cm-1, and 1800 cm-1 show ?(Zn-O), ?(Cu-O), ?(Ca-O), respectively. At 1930 cm-1 shows strong ? (C=C), at 2927 cm-1 and 2961 cm-1 show the small peak of ? (C=H). The chemical functional group of ? (O-H) appears at the wave number of 3427 cm-1, 3519 cm-1, and 3583 cm-1. In addition, the C-H and O-H bonds also show the FTIR spectra at 3090 cm-1 and 3389 cm-1, respectively.
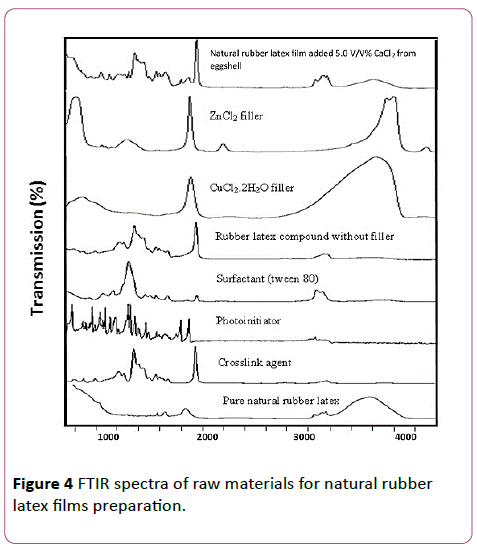
Figure 4: FTIR spectra of raw materials for natural rubber latex films preparation.
The FTIR spectra comparison of natural rubber latex films with/without adding ionic filler solution ZnCl2, CuCl2, and CaCl2 made from eggshell powder in the range of wave number 500 cm-1 to 4000 cm-1 are shown in Figure 5.
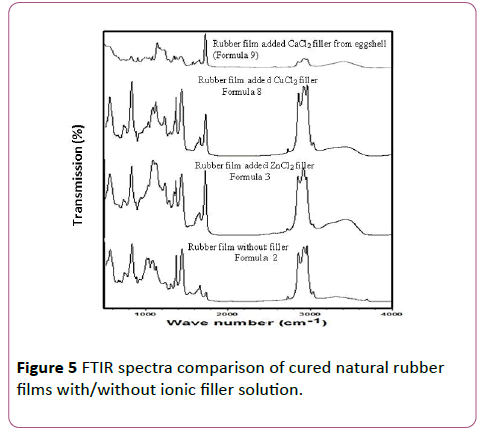
Figure 5: FTIR spectra comparison of cured natural rubber films with/without ionic filler solution.
The obtained FTIR spectra are consistent with the types of ionic filler solution adding ZnCl2, CuCl2, and CaCl2 consistent with the FTIR spectra in Figure 4. However, the FTIR spectra of soft compliant electrode added only photo initiator and crosslink agent (Formula 2) show the chemical functional group at the wave number 1090 cm-1 to 1100 cm-1, 1376 cm-1, 1390 cm-1 to 1800 cm-1, 1646 cm-1 belonging to st, (S=O) st, (=C-N) st, (C-O) st, (C=O), and (C=S) st. In addition, at the wave numbers 3090 cm-1, and 3389 cm-1 are (-C-H) and (=C-H) stretching, respectively. Furthermore, at the wave number 504.02 and 567.87 belongs to strong ? (Zn-O), ? (Cu-O), and ν (Ca-O), respectively consistent with the FTIR spectra of ionic filler in Figure 4.
Conclusion
The soft compliant electrodes are prepared as the composite materials with the natural rubber latex compound as the matrix phase and the ionic filler as the dispersed phase by UV curing system. The advantages of using the natural rubber as the matrix phase are light weight, flexibility, low cost, easy to formation. The matrix provides the mechanical and physical properties whereas the ionic fillers or dielectric materials provide electrical and thermal conductivities.
Since the compliant electrode samples usually require the softest and flexibility possible electrodes, the amount of ionic fillers should be as low as possible to maintain the elastic modulus of the composites low. The amount of ionic fillers added in the natural rubber latex compound depends on the shape and amount of ionic filler also, effect to a percolation threshold and compatibility in the natural rubber matrix. Therefore, adding the ionic fillers solution of both commercial ZnCl2 and CuCl2, and CaCl2 prepared from eggshell in the natural rubber latex compound to prepare the soft compliant electrode has many advantages i.e. to increase compatibility, to increase electrical conductivity, to maintain flexibility and mechanical property of the elastomer matrix, to obtain good physical properties such as smoothness on film surface, translucency, short curing time, and good appearance. The electrical conductivity, tensile stress, critical strain, and curing time of soft compliant electrode added 0.5 V/V% ZnCl2 ionic filler solution are 0.0197 S/cm, 6.97 × 104 Pa, 28.80%, 7 min, respectively. The electrical conductivity, tensile stress, critical strain, and curing time of soft compliant electrode added 0.75 V/V% CuCl2 ionic filler solution are 0.0326 S/cm, 5.15 × 104 Pa, 6.84%, 7 min, respectively. While the electrical conductivity, tensile stress, critical strain, and curing time of soft compliant electrode added CaCl2 ionic filler solution prepared from eggshell dissolved in HCl are 0.13 S/cm, 132.71 ± 15.17%, 1.28 × 106 Pa, 41.18%, 19 min, respectively. Furthermore, the eggshell as a calcium source to prepare the CaCl2 ionic filler solution is potential for the soft compliant electrode samples preparation as well and still has potential to reduce the extractable protein on the natural rubber film surface. In addition, the other important reason of using eggshell is to reduce eggshell waste in the world and the environmental conservation.
Acknowledgment
The authors would like to thank the Petroleum and Petrochemical College and the Scientific and Technological Research Equipment Centre, at Chulalongkorn University and the Department of Materials Engineering, at Kasetsart University for the use of their analytical equipment. We are also grateful for grant support from the Kasetsart University Research and Development, Conductive and Electroactive Polymers Research Unit of Chulalongkorn University, Thailand Research Fund (TRF-RTA), the Royal Thai Government, and the Center for Advanced Studies in Industrial Technology, Kasetsart University.
17207
References
- Zhenyi M, Scheinbeim JI, Lee JW, Newman BA (1994) High field electrostrictive response of polymers. J Polym Sci B: Polym. Phys 32: 2721-2731.
- Fox JW, Goulbourne NC (2008) On the dynamic electromechanical loading of dielectric elastomer membranes. J Mechan Phys 56: 2669-2686.
- Niklaus M, Rosset S, Dubois S, Shea H (2009) Ion-implanted compliant electrodes used in dielectric electroactive polymer actuators with large displacement. Procedia Chem 1: 702-705.
- Pelrine R, Kornbluh R, Joseph J, Heydt R, Pei Q, et al. (2000) High-field deformation of elastomeric dielectrics for actuators. Mater Sci Eng C 11: 89-100.
- Barboza-Filho CG, Cabrera FC, Dos Santos RJ, De Saja Saez JA (2012) The influence of natural rubber/Au nanoparticle membranes on the physiology of Leishmania brasiliensis. Exp Parasitol 130: 152-158.
- Braga M, Costa CR, Leite CP, Galembeck F (2001) Scanning electric potential microscopy imaging of polymer latex films: detection of supramolecular domains with nonuniform electrical characteristics. J Physl Chem B 105: 3005-3011.
- Job AE, Oliveira FA, Alves N, Glacometti JA, Mattoso LC (2003) Conductive composites of natural rubber and carbon black for pressure sensors, Synth. Met 135: 99-100.
- Camillo EC, Constantino CI, Teruya MY, Alves N, Mattoso LC, et al. (2005) Dependence of the electrical conductivity and elastomeric properties on sample preparation of blends of polyaniline and natural rubber. J Appl Polym Sci 97: 1498-1503.
- Rippel MM, Leite CP, Lee LT, Galembeck F (2005) Direct imaging and elemental mapping of microgels in natural rubber particles. Colloid Polym Sci 283: 570-574.
- Oliveira FA, Alves N, Glacometti JA, Constantino CI, Mattoso LC, et al. (2007) Study of the thermomechanical and electrical properties of conducting composites containing natural rubber and carbon black. J Appl Polym Sci 106: 1001-1006.
- Vogan J, Wingert A, Plante JS, Dubowsky S, Hafez M, et al. (2004) Manipulation in MRI devices using electrostrictive polymer actuators: with an application to reconfigurable imaging coils. in Proc. IEEE International conference on Robotics and Automotion JCRA 3: 2498-2504.
- Carpi F, Mannini A, de Rossi D (2008) Elastomeric contractile actuators for hand rehabilitation splints. in Electroactive Polymer Actuators and Devices (EAPAD), Y.Bar-Cohen (Edn), SPIE 692705.
- Pelrine RE, Kornbluh RD, Joseph JP (1998) Electrostriction of polymer dielectrics with compliant electrodes as a means of actuation, Sens. Actuators 64: 77-85.
- Cottinet PJ, Guyomar D, Galineau J, Sebald G (2000) Electro-thermo-elastomers for artificial muscles, Sens. Actuators, A. 180 (2012), 105-112.
- Richardson JT, PengYD(2000) Remue, Properties of ceramic foam catalyst supports: pressure drop. Appl Catal A: General 204: 19-32.
- Tullett SG (1984) Eggshell Conductance and Other Functional Qualities of Ostrich Egg, Comp. Biochem. Physiol 5-13.
- Nys Y, Gautron J, Garcia-Ruiz JM, Hincke MT (2004) Avian eggshell mineralization: biochemical and functional characterization of matrix proteins. C.R.Palevol 3: 549-562.
- Tsai WT, Yang JM, Lai CW, Cheng YH, Lin CC, et al. (2006) Characterization and adsorption properties of eggshells and eggshell membrane, Bioresour. Technol 97: 488-493.
- Toro P, Quijada R, Yazdani-Pedram M, Arias JL (2007) Eggshell, a new bio-filler for polypropylene composite. Mater Lett 61: 4347-4350.
- Adeyeye EI (2009) Comparative study on the characteristics of egg shell of some bird spicies. Bull Chem Soc Ethiop 159-166.
- Ohalloran A, Omalley F, McHugh P (2008) A review on dielectric elastomer actuators technology, applications, and challenges. J Appl Phys 071101-071110.
- Capsal JF, Dantras E, Laffont L, Dandurand J, Lacabanne C (2010) Nanotexture influence of BaTiO3 particles on piezoelectric behavior of PA 11/BaTiO3 nanocomposites. J Non-Cryst Solids 629-634.
- Bauer F, Capsal JF, Larcher Q, Dos Santos FD (2011) Advances in Relaxor Ferroelectric Terpolymer, New Applications, IEEE, New York, 2011.
- Capsal JF, Dantras E, Dandurand J, Lacabanne C (2010) Dielectric relaxations and ferroelectric behavior of even-odd polyamide. Polymer 4606-4610.
- Klppel M, Schuster RH, Heinrich G (1997) Structure and properties of reinforcing fractal filler networks in elastomers. Rubber Chem Technol 70: 243-255.











