Keywords
Neuropsychiatric disorders comorbid with HIV; Human Immunodeficiency Virus (HIV)
Introduction
Substance abuse is significantly associated with earlier progression to Acquired Immune Deficiency Syndrome (AIDS) in Human Immunodeficiency Virus (HIV)-infected people [1,2], and the use of recreational drugs have been found to induce CNS impairment independent of the effects caused by HIV infection [3]. Consequently, people living with HIV/AIDS face an additional burden of psychiatric comorbidity, and approximately 40% of patients with HIV infection will develop CNS involvement during the disease [4]. Although HIV may remain dormant in the CNS for many years, its mere presence might lead to subtle deficits in cognitive functioning. However, these deficits are not found in all patients, which has led some authors to suggest that peripheral triggers might be involved [5].
Psychopathology can occur: (1) as a risk factor for HIV infection; (2) as a psychological response to HIV infection and its complications, because of direct effect of HIV on the brain; (3) because of HIV-related opportunistic diseases; or (4) as side effects of HIV-related treatments. Despite the impressive reduction of HIV-related morbidity and mortality where antiretroviral therapy (ART) is available, psychiatric and neuropsychiatric repercussions of HIV disease are expected to become more relevant in the coming years [6], especially with the recent outbreak of communicable diseases associated with opioid use disorder [7].
Interestingly, African Americans have been disproportionately affected by HIV/AIDS since the epidemic began in the United States in 1981, and that disparity has deepened over time [8]. Although African Americans make up only 12.3 percent of the U.S. population, they have accounted for about 40 percent of the nearly 930,000 AIDS cases diagnosed in the country so far, and that proportion appears to be growing. In 2015, 17,670 African Americans were diagnosed with HIV infection in the United States and 48 percent (8,702) of those diagnosed with AIDS in the United States were African Americans [8].
In addition, drug abuse and addiction are also prevalent in District of Columbia (DC) and has traditionally been linked to HIV/AIDS because of the likelihood of opportunistic infections. In DC alone, three percent of the population have tested positive to HIV infection, which is the highest in the nation [9] and exceeds the World Health Organization’s (WHO) definition of a severe epidemic [10]. Consequently, although African Americans account for 48 percent of DC residents, they constitute 75 percent of all HIV cases, have the highest incidence of HIV infections, and account for the newest AIDS cases and AIDS-related deaths in the District [11].
Currently, no literature is available regarding the demographics and neurological manifestations in African American subsets with HIV infection. Because substances of abuse are psychoactive, and known to alter neurological function, we analyzed the relationship between various psychiatric diagnoses and specific drugs of abuse in HIVinfected African-American patients in the Washington DC metropolitan area, to determine if specific drugs of abuse or specific mental health conditions can be predictors of CD4 and CD8 count trends and if they confer vulnerabilities to HIV.
Methodhology
Study setting
This cross-sectional retrospective study was conducted using medical records of a convenient sample of African- American patients admitted into Howard University Hospital from January to December of 2010. The Institutional Review Board reviewed and approved this study protocol.
Site selection
Howard University hospital was selected because it is the most comprehensive health care facility in the Washington DC metro area, servicing mainly minority populations, a large percentage of which are African American.
Data sources
Data was collected from medical records of 282 consecutive patients with an ICD-9 coded diagnosis of HIV and concurrent neurological diagnosis for demographic variables (i.e., age and sex), antiretroviral therapy, drug use history, laboratory results and neurologic diagnosis. Subsequently, neurologic diagnoses were categorized as opportunistic infections; dementia; peripheral neuropathy, seizure and epilepsy; TIA and stroke; neuropsychiatric disorders. We confirmed the accuracy of ICD-9 case acquisition by extensive manual review of medical records by investigators. Additionally, we re-categorized neurological diagnosis as necessary by this manual review. We also included individuals with multiple neurological manifestations in each of the categories. Individuals admitted more than one time during the study interval were counted only once. However, data on substance use and abuse was based on self-report.
The anonymized data were retrospectively reviewed, and laboratory and clinical variables were pooled and analyzed centrallyfor frequencies of comorbidities of various neurological disorders, history of substance abuse, their relation to CD4 and CD8 counts, and in relation to HAART therapy. Any patient who reported using nicotine, alcohol, cocaine, marijuana, PCP, or heroin were considered drug users.
Data collection
Data for each patient was retrospectively recorded on a spreadsheet. Data abstracted onto a spreadsheet included age, gender, race, history of HIV related illnesses, comorbid medical or neurological conditions including current substance abuse or psychiatric illness, CD4 and CD8 cell counts, and antiretroviral medications.
Analyses
The data collected was subjected to extensive statistical analysis using the Pearson chi-squared and T tests within the SPSS 19 statistical software. Each drug (nicotine, alcohol, cocaine, marijuana, PCP and heroin) was analyzed for prevalence within a neuropsychiatric group (depression, schizophrenia, or bipolar disorder). CD4 and CD8 counts were also analyzed for each psychiatric group.
Results
Of the 757 admissions between January 2010 to December 2010 with a diagnosis of HIV, 282 (37.3%) had a concurrent diagnosis after excluding readmissions. The demographics of the 282 HIV-infected individuals used in the study are listed in Table 1. Patient age ranges were between 16 and 83, with a median age of 47 years. 54 percent of the patients were male, and 46 percent were female. 99 percent had their HIV diagnosis pre-admission while 1 percent had their diagnosis at the hospital. Forty-two percent (42%) met WHO CD 4 criteria for AIDS diagnosis. Of the 282 patients, 276 had record of their antiretroviral therapy status. 62 percent of these patients were observed to be on antiretroviral therapy while 39 percent were not. Also, 275 records showed drug abuse history, with 86 percent indicating positive for drug abuse (Table 1).
| Characteristics |
Number |
Percentage |
| Gender (n=282) |
| Male |
152 |
54 |
| Female |
130 |
46 |
| Age |
16-83 |
47(Mean) |
| HIV Diagnosis (n=282) |
| Pre-admission |
279 |
99 |
| At Hospital |
3 |
1 |
| Antiretroviral therapy (n=276) |
| Yes |
170 |
62 |
| No |
106 |
39 |
| Drug abuse history (n=275) |
| Yes |
236 |
86 |
| No |
39 |
14 |
| Signs and symptoms of neuropsychiatric disorders |
| Depression |
113 |
50 |
| Bipolar disorder |
70 |
31 |
| Schizophrenia |
43 |
19 |
Table 1: Demographic characteristic of the patients.
Figure 1 shows at least a prevalence of neurological disorders (inclusive of neuropsychiatric diseases, seizure/ epilepsy, transient ischemic attack/cerebrovascular accident (TIA/CVA), peripheral neuropathy, and opportunistic infections) in patients aged less than 50. As expected, dementia was twice as prevalent in patients aged more than 50 years, and twice as prevalent in females compared to males.
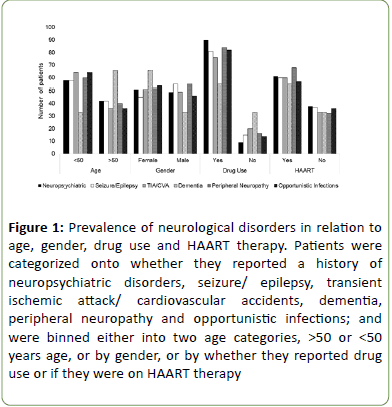
Figure 1: Prevalence of neurological disorders in relation to age, gender, drug use and HAART therapy. Patients were categorized onto whether they reported a history of neuropsychiatric disorders, seizure/ epilepsy, transient ischemic attack/ cardiovascular accidents, dementia, peripheral neuropathy and opportunistic infections; and were binned either into two age categories, >50 or <50 years age, or by gender, or by whether they reported drug use or if they were on HAART therapy
There was however no gender-specific difference in the prevalence of the other neurological disorders. The number of patients who reported drug use was significantly greater when categorized by each neurological disease, ranging from 10-fold greater association for those with a neuropsychiatric disease compared to only 0.67-fold increase in drug use among patients with dementia. In addition, two thirds of all patients were undergoing HAART therapy whereas one third was not (Figure 1).
Frequency of neuropsychiatric disease in HIV patients with neurological complications
When the frequency of psychiatric disorders in HIV-infected African American patients was analyzed, depression (MDD) was highest, with a 16 percent greater frequency than bipolar disorder (BD), 26 percent greater than Schizophrenia (SCZ) patients and 18 percent more than patients with a history of seizures (Figure 2A). It was also observed that some of the patients were diagnosed with more than one psychiatric disorder: 29 had both MDD and BD, 11 had both MDD and SCZ, 12 had both BD and SCZ while 10 were diagnosed with all three of the disorders (Figure 2B). Therefore, patients reported in the BD cohort in the current study, may exhibit bipolar disorder only or have co-morbid SCZ and MDD symptoms.
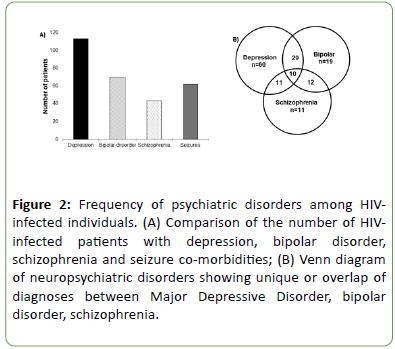
Figure 2: Frequency of psychiatric disorders among HIVinfected individuals. (A) Comparison of the number of HIVinfected patients with depression, bipolar disorder, schizophrenia and seizure co-morbidities; (B) Venn diagram of neuropsychiatric disorders showing unique or overlap of diagnoses between Major Depressive Disorder, bipolar disorder, schizophrenia.
Relationship of drugs of abuse with neuropsychiatric disease in HIV patients
To test the possibility that a specific drug class may contribute to or exacerbate mental health disease, we evaluated MDD, BP, or SCZ, in correlation with nicotine, alcohol, cocaine, cannabis, heroin, or PCP use. Interestingly, overall, 86 percent of the patient cohort reported a history of drug use, and thus the prevalence of specific drugs of abuse in the patients was further investigated. Nicotine, alcohol, marijuana and heroin use were not significantly correlated with a specific neuropsychiatric disorder.
Compared to the other drugs evaluated, cocaine use was significantly associated with patients with an MDD diagnosis compared to the rest of the population (57.5% vs. 38.5%; p<0.01) (Figure 3A). BD was statistically linked to a history of drug use, in general, compared to non-bipolar patients (p<0.05) and cocaine use, in specific, was most prevalent in BD patients (61.2% vs. 38.9%; p<0.01) (Figure 3B). Finally, PCP use was significantly associated with patients diagnosed with SCZ compared to non-SCZ patients (14% vs. 3.6%; p<0.05) (Figure 3C).
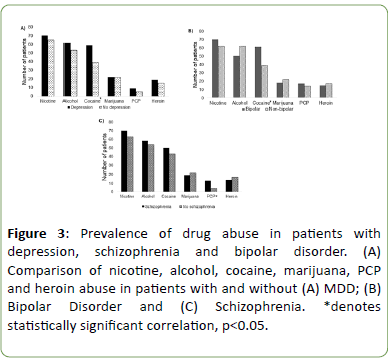
Figure 3: Prevalence of drug abuse in patients with depression, schizophrenia and bipolar disorder. (A) Comparison of nicotine, alcohol, cocaine, marijuana, PCP and heroin abuse in patients with and without (A) MDD; (B) Bipolar Disorder and (C) Schizophrenia. *denotes statistically significant correlation, p<0.05.
CD4 and CD8 counts as a function of neurological/neuropsychiatric disease in HIV patients
The CD4 clinical readout is used to measure CD4+ helper T lymphocytes in the circulation, whereas the CD8 count measures the number of cytotoxic T lymphocytes which is normally low in a non-infected, non-cancerous state. A normal CD4 count is from 500 to 1500 cells per cubic millimeter of blood. CD4+ T lymphocytes are primarily targeted for destruction by HIV viral cells and play an important role in host immune defenses against opportunistic infection [12]. With progressive HIV disease, CD4 counts drop to low levels, typically <200, resulting in AIDS defining illness and increasing mortality. The normal CD8 range in healthy individuals is from 150 to 1000, but as HIV infection progresses, there is gradual loss of CD4 T-cells and elevation of CD8 cells. An elevated CD8 count may be one of the few indicators of future virologic failure. Of the 282 medical records, only 210 had a record of their CD4/CD8 counts. 58 percent of these patients presented had CD4 counts >200 and 42 percent presented with CD4 counts <200.
The most common neurological diagnosis was neuropsychiatric in nature, and within this subgroup, 12 percent more patients with CD4 count >200 than those with CD4 counts <200; In specific, patients with bipolar and schizophrenia comorbidities had a mean CD4 count that was non-statistically distinguishable from those without these neuropsychiatric comorbidities (Figure 4A), however for MDD, there was a significant increase in mean CD4 counts associated with the diagnosis (362.6 ± 38.6 vs. 272 ± 13.1, p<0.05). The mean CD8 counts did not seem affected by neuropsychiatric status (Figure 4B). We then analyzed and compared CD4 and CD8 counts in non-neuropsychiatric neurological manifestations. Interestingly, compared to the neuropsychiatric and peripheral neuropathy categories, the seizure/epilepsy category had a significant number of patients (61%) with low CD4 counts, like those with opportunistic infections that show 88 percent of patients having CD4 counts <200 (Figure 5A). When a within group assessment of patients with MDD, BD, and SCZ was conducted as in Figure 5B, the data indicate a respective 61%, 63%, and 77% of patients with CD4 counts >200 (Figure 5).
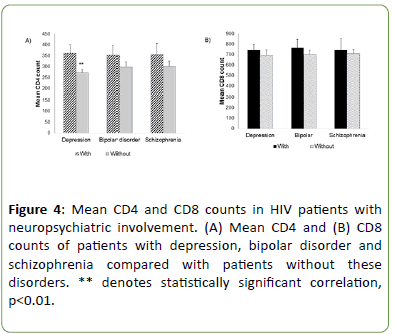
Figure 4: Mean CD4 and CD8 counts in HIV patients with neuropsychiatric involvement. (A) Mean CD4 and (B) CD8 counts of patients with depression, bipolar disorder and schizophrenia compared with patients without these disorders. ** denotes statistically significant correlation, p<0.01.
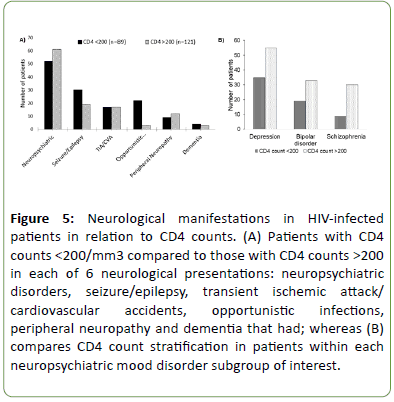
Figure 5: Neurological manifestations in HIV-infected patients in relation to CD4 counts. (A) Patients with CD4 counts <200/mm3 compared to those with CD4 counts >200 in each of 6 neurological presentations: neuropsychiatric disorders, seizure/epilepsy, transient ischemic attack/ cardiovascular accidents, opportunistic infections, peripheral neuropathy and dementia that had; whereas (B) compares CD4 count stratification in patients within each neuropsychiatric mood disorder subgroup of interest.
Next, patients were stratified into two groups of CD8 counts <1200 or >1200. All neurological disease, including neuropsychiatric, were associated with multi-fold (5-50) greater number of patients with CD8 >1200 except Dementia (Figure 6A). Furthermore, 75% of MDD, 90% BD, and 88% of SCZ exhibited a mean CD8 count >1200 (Figure 6B).
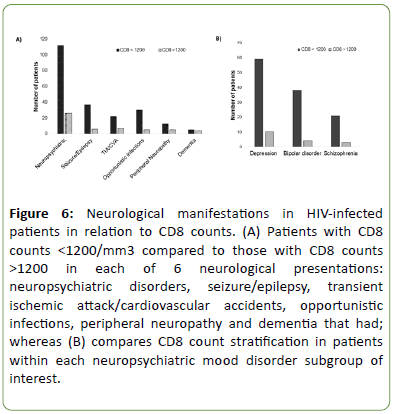
Figure 6: Neurological manifestations in HIV-infected patients in relation to CD8 counts. (A) Patients with CD8 counts <1200/mm3 compared to those with CD8 counts >1200 in each of 6 neurological presentations: neuropsychiatric disorders, seizure/epilepsy, transient ischemic attack/cardiovascular accidents, opportunistic infections, peripheral neuropathy and dementia that had; whereas (B) compares CD8 count stratification in patients within each neuropsychiatric mood disorder subgroup of interest.
Discussion
Even as efforts have been successful in reducing the rate of HIV infection around the world and in the District of Columbia, the rate remains at epidemic levels of 2.7 percent, nearly 10 times the nationwide values. Our results are the first to evaluate the incidence of substance abuse in HIV-infection, comorbid with neuropsychiatric and neurological disease in an urban Washington D.C. population. Whereas much data has been published for Caucasian Americans with HIV, where psychiatric or mental disorders are common co-morbidities amongst those at risk for or infected by HIV [2,13], this unique study focuses on an African-American urban population. Here, we report a high co-morbidity of neuropsychiatric disease and drug use in HIV patients, a specific correlation of certain drug classes with MDD, BD, or SCZ, and a surprising partial protection of CD4 degradation associated with neuropsychiatric disease, and no other neurological diagnoses, even as CD8 levels maintain their elevated levels.
Our study shows a prevalence of neurological manifestations in HIV-infected patients younger than 50, except dementia which occurred in patients older than 50. Studies have shown that different brain regions that interact to promote cognitive functions show less-coordinated activation with ageing, suggesting a vast loss of integrative function, which is related, in part, to disruption of neuronal connections in different cortical regions [14,15]. There was a very high percentage of drug users in our cohort. 6 percent of HIV/AIDS cases in the United states are attributed to drug use [8], but in this cohort, there may be a higher number. These data do not allow an interpretation of the cause of HIV infection, however because of the 86% incidence of drug use in the subjects, it is likely a major contributing factor. It is well established that drugs of abuse alter judgment, induces loss-of-inhibition, and plays a massive role in the spread of HIV by leading to engagement in impulsive, unsafe behaviors [16].
The use of potent antiretroviral combinations, like the highly active antiretroviral therapy (HAART), has provided unprecedented opportunities for effectively treating HIV disease and led to a dramatic decline in HIV mortality by profoundly inhibiting viral replication and delaying disease progression [17,18]. This, however, would require adherence to the treatment regimen as non-adherence may result in the selection of drug-resistant HIV strains [19,20], and though our results indicate that over 60% of patients were on HAART therapy, the records did not account for adherence and thus disease treatment could be severely impacted in this population. Various demographic, psychosocial, and diagnostic factors affect adherence to HAART [21], and neuropsychiatric disorders often correlate with increased substance use behaviors and can interfere with cognitive functioning [22-24] which also negatively affects HAART adherence.
Our results show that MDD was 2-3 times more prevalent than other neuropsychiatric disorders comorbid in our African- American HIV-infected cohort (Figure 2). This is consistent with reports from numerous studies in which syndromes ranging from MDD to apathetic and irritable mood have been observed to be frequently present among HIV-infected individual [25-27]. MDD is the most prevalent psychiatric disorder among HIV-infected persons [28,29], two to four times higher than those found in general populations [2,28-30]. The depressive symptoms may be either a primary consequence of the central nervous system (CNS) effects of HIV infection, or the frustrations, and stigmatization sometimes associated with living with HIV/AIDS [31]. It is well recognized that lesions in specific neuroanatomic structures due to disease or insult, such as those resulting from a stroke or brain tumor, may contribute to the development of secondary depression. In HIV-positive people with MDD, morphological alterations have been identified in various neuroanatomical structures, including cortical and subcortical regions [26].
Drug use, especially cocaine and PCP use, was observed to be higher in the HIV-infected patients with MDD, BD and SCZ respectively, compared to patients without these comorbidities (Figure 3). These provocative data suggest a possible relationship between certain psychostimulant drugs and psychiatric disorder presentation in HIV-infected individuals. The neurocognitive effects of cocaine, PCP or other drugs of abuse may influence the development and/or sustainment of psychiatric disease in immunocompromised individuals. Additionally, the medications used to treat HIV infection could have additive or synergistic effects on the incidence of psychiatric disease presentation in HIV patients when combined with drugs of abuse. Substance abuse disorders are three to five times more prevalent in patients with SCZ compared to healthy controls [32,33]. The core pathophysiologic feature of SCZ involves hypofunction of Nmethyl- D-aspartate receptors (NMDARs) [34-37], implicated in the aberrant regulation of synaptic plasticity which may promote substance abuse and addiction. Specifically, modulation of the mesocorticolimbic dopamine system by glutamatergic inputs from cortical and subcortical regions appears to regulate aspects of maladaptive drug seeking [38].
The finding that mean CD4 counts in HIV-positive patients with depression comorbidity was statistically higher compared to non-MDD patients (Figure 4A) but that CD8 counts were not statistically more or less elevated, implicates a possible interaction with MDD-mediated protective mechanisms against degradation of CD4+ helper T lymphocytes. (Figure 4B). Indeed, among patients with CD4>200, the category of neuropsychiatric disorders was prominent (Figure 5). This could indicate a paradigm shift of neurological diseases from opportunistic infections to neuropsychiatric manifestations. CD4 count decline is linked to HIV disease progression, and previous work has shown that depression can significantly increase plasma viral load, accelerating the decline of CD4 cell counts. In addition, MDD in HIV negatively affects the rate of decline of cognitive symptoms, and is associated with higher deficits in comprehension, attention, and memory [31,39]. Manic symptoms in the early stages of HIV and a CD4 count greater than 200 is indicative of BD [40,41]. New-onset psychosis in patients with CD4 counts <200 has been described as a symptom of late-stage HIV/AIDS, and may be due to CNS infection, tumors, HIV invasion into the brain, and cognitive impairment [42]. In our study, the CD counts were not specifically correlated with stage of disease or delay-to-onset of comorbidities. Nonetheless, untreated neuropsychiatric manifestations in HIV-infected patients may lead to further spread of the disease, due to the prevalence of risk-taking behavior [43,44]. Along with MDD, other conditions including dementia, anxiety, psychosis, substance abuse, are all psychiatric factors associated with non-adherence to HIV treatment [45-48]. CD8 cells have an important role in fighting untreated HIV infection. However, an overstimulation of CD8 response and an elevated CD8 cell count has been associated with accelerated HIV disease progression. Elevated CD8 cell count has also been associated with greater risk of future antiretroviral treatment failure. Our data concerning CD8 cell count (Figure 6) supports previous studies’ conclusion that drug use is not a major predictor of disease progression as CD8 values were not altered [26].
We propose a model (Figure 7) based on the findings from our study of an HIV+ urban cohort. Comorbidity of HIV with MDD and BD was correlated with a statistically significant increase in cocaine use, and increased CD4 and CD8 cell counts, which may result in increased rate of HIV disease progression. A statistically significant increase in PCP use was observed in correlation with SCZ, but CD4 and CD8 counts were not further elevated or diminished. This implies that HIV comorbidity with SCZ has no immunological consequence on HIV progression, which correlates to findings by Helleberg et al. [49]. Zhang et al. [50] reported a decrease, while Muller et al. [51] reported increased CD4 cell numbers. Despite the varying reports of immune alteration in SCZ, there is a lack of clinical support for specific immune dysfunction in SCZ. Besides, the stage of disease when the sample was collected may likely influence findings. This, however, could not be determined from the medical records.
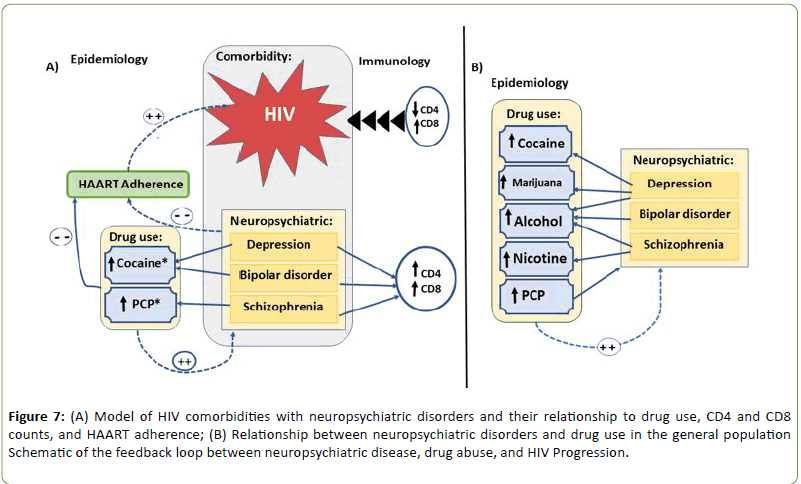
Figure 7: (A) Model of HIV comorbidities with neuropsychiatric disorders and their relationship to drug use, CD4 and CD8 counts, and HAART adherence; (B) Relationship between neuropsychiatric disorders and drug use in the general population Schematic of the feedback loop between neuropsychiatric disease, drug abuse, and HIV Progression.
Comorbidity of HIV with depression and bipolar disorder was correlated with increase in cocaine use and increased CD4 and CD8 cell counts. Increase in PCP use was observed with schizophrenia, including increased CD4 count and CD8 counts.
In literature, low CD4 counts and high CD8 counts are correlated with rapid HIV disease progression. Drug use is negatively correlated with HAART adherence, which also impacts HIV disease progression.
In the general, non-HIV-infected population, drug abuse is also correlated with neuropsychiatric disorders: depression is prevalent in cocaine, marijuana and alcohol abusers. Bipolar disorder is significantly associated with alcohol abuse; while nicotine and alcohol dependence are more common with schizophrenic patients than with the general population. PCP use is correlated with most of the neuropsychiatric disorders.
Adherence to HIV treatment regimens is sometimes markedly compromised when a person has serious mental illness [2]. Drug abuse and addiction can also worsen the progression of HIV and its consequences, especially in the brain. HIV patients with drug dependence have been reported to have a greater neurocognitive impairment and poorer HAART adherence compared to non-drug users [3,27,52,53]. Increased life expectancy in HIV patients may add cerebrovascular or degenerative encephalitis to the clinical presentation of HIV neurocognitive disorders [54]. Although it is suggested that neurocognitive complications are usually mild, and survival is not compromised [55,56], they may negatively affect quality of life [56], independence in daily activities, or treatment adherence [48,57-61]. This may result in antiviral drug resistance and poor HIV outcomes. HAART slows down the progression of HIV to AIDS, and so incomplete adherence can lead to immunosuppression and drug resistant HIV [47], while non-adherence increases HIV-related morbidity and mortality [62].
In the general, non-HIV-infected population, drug abuse is also correlated with neuropsychiatric disorders (Figure 7B). MDD is prevalent in cocaine [63-65], marijuana [66] and alcohol abusers [67-70], BD is significantly associated with alcohol abuse [24,71,72], and nicotine and alcohol dependence is more common with SCZ patients than with the general population [73-76]. PCP use is correlated with most neuropsychiatric disorders, especially in individuals with genetic predisposition to SCZ.
Conclusion
In summary, it is important to study the variation of disease progression in different populations for a more comprehensive understanding of the pathology associated with various comorbidities and behaviors. We have presented data from a mostly African-American urban population set, and although we found many similarities to other studies of HIV, neurological disease, and drugs of abuse already reported in the literature, we also found a high prevalence of drug abuse and neuropsychiatric complications, with specific correlations with certain drugs of abuse. We focused on mood disorders/ Neuropsychiatric Disease and found a relative protection of CD4 T lymphocyte degradation, which did not seem to influence progression of disease; however, illicit drugs may have a direct correlation with neuropsychiatric disease incidence. In the future, it will be important to study length of time with HIV Status, adherence to HAART therapy, and extent of drug use on HIV pathology.
Acknowledgement
This work was supported by grants from the National Institute of Health through the 1SC2NS065385-01.
22206
References
- Webber MP, Schoenbaum EE, Gourevitch MN, Buono D, Kleina RS (1999) Prospective study of HIV disease progression in female and male drug users. AIDS 13: 257-262.
- Blank MB, Himelhoch S, Walkup J, Eisenberg MM (2013) Treatment considerations for HIV-infected individuals with severe mental illness. Curr HIV/AIDS Rep 10: 371-379.
- Sanchez AB, Kaul M (2017) Neuronal stress and injury caused by HIV-1, CART and drug abuse: Converging contributions to hand. Brain Sci 7: 25.
- Singh R, Kaur M, Arora D (2011) Neurological complications in late-stage hospitalized patients with HIV disease. Ann Indian Acad Neurol 14: 172-177.
- Gartner S (2000) AIDS: HIV Infection and dementia. Science 287: 602-604.
- Gallego L, Barreiro P, López-Ibor JJ (2011) Diagnosis and clinical features of major neuropsychiatric disorders in HIV infection. AIDS Rev 13: 171-179.
- Williams AR, Bisaga A (2016) From AIDS to opioids - How to combat an epidemic. N Engl J Med 375: 813-815.
- Tanne JH (2009) HIV prevalence in US capital is at epidemic level. BMJ 338: b1205.
- https://doh.dc.gov/sites/default/files/dc/sites/doh/publication/attachments/HAHSTA%20Annual%20Report%202013_Final.pdf.
- Huynh-Nhu Le, Hipolito MMS (2016) Culturally sensitive approaches to identification and treatment of depression among HIV infected african american adults: A qualitative study of primary care providers' perspectives. J Depress Anxiety 5.
- Saag MS (1997) Use of HIV viral load in clinical practice: back to the future. Ann Intern Med 126: 983-985.
- Wainberg ML, McKinnon K, Cournos F (2014) Epidemiology of psychopathology in HIV. HIV and Psychiatry 1-60.
- Anand A, Yu Li, Yang W, Jingwei W, Sujuan G, et al. (2005) Activity and connectivity of brain mood regulating circuit in depression: a functional magnetic resonance study. Biol Psychiatry 57: 1079-1088.
- Eggers C, Arendt G, Hahn K, Husstedt IW, Maschkeet M, et al. (2017) HIV-1-associated neurocognitive disorder: epidemiology, pathogenesis, diagnosis, and treatment. J Neurol 264: 1715-1727.
- King KM, Nguyen HV, Kosterman R, Bailey JA, Hawkins JD (2012) Co-occurrence of sexual risk behaviors and substance use across emerging adulthood: evidence for state- and trait-level associations. Addiction 107: 1288-1296.
- Hogg RS (1998) Improved survival among HIV-infected individuals following initiation of antiretroviral therapy. JAMA 279: 450-454.
- Palella FJ, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, et al. (1998) Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med 338: 853-860.
- Montaner JSG, Reiss P, Cooper D, Vella S, Harris M, et al. (1998) A randomized, double-blind trial comparing combinations of nevirapine, didanosine, and zidovudine for HIV-infected patients: the INCAS Trial. Italy, The Netherlands, Canada and Australia Study JAMA 279: 930-937.
- Vanhove GF (1996) Patient compliance and drug failure in protease inhibitor monotherapy. JAMA 276: 1955-1956.
- Ickovics JR, Meade CS (2002) Adherence to HAART among patients with HIV: Breakthroughs and barriers. AIDS Care 14: 309-318.
- Porter RJ, Gallagher P, Thompson JM, Young AH (2003) Neurocognitive impairment in drug-free patients with major depressive disorder. Br J Psychiatry 182: 214-220.
- Swendsen JD (2000) The comorbidity of depression and substance use disorders. Clin Psychol Rev 20: 173-189.
- Grant BF, Stinson FS, Hasin DS, Dawson DA, Chou SP, et al. (2005) Prevalence, correlates, and comorbidity of bipolar I disorder and axis I and II disorders: Results from the National Epidemiologic Survey on Alcohol and Related Conditions. J Clin Psychiatry 66:1205-1215.
- Dube B (2005) Neuropsychiatric manifestations of HIV infection and AIDS. J Psychiatry Neurosci 30: 237-246.
- Arseniou S, Arvaniti A, Samakouri M (2013) HIV infection and depression. Psychiatry Clin Neurosci 68: 96-109.
- Watkins CC, Treisman G (2015) Cognitive impairment in patients with AIDS - Prevalence and severity. HIV AIDS (Auckl) 7: 35-47.
- Starace F (2002) Depression is a risk factor for suboptimal adherence to highly active antiretroviral therapy. J Acquir Immune Defic Syndr 31: S136-S139.
- Ciesla JA, Roberts JE (2001) Meta-analysis of the relationship between HIV infection and risk for depressive disorders. Am J Psychiatry 158: 725-730.
- Morrison MF, Petitto JM, Have TT, Gettes DR, Chiappini MS, et al. (2002) Depressive and anxiety disorders in women with HIV infection. Am J Psychiatry 159: 789-796.
- Hinkin CH, Castellon SA, Atkinson JH, Goodkin K (2001) Neuropsychiatric aspects of HIV infection among older adults. J Clin Epidemiol 54: S44-S52.
- Kavanagh DJ, John McG, John BS, Dore G, Clark D (2002) Substance misuse in patients with schizophrenia: epidemiology and management. Drugs 63: 743–755.
- Coyle JT (2006) Substance use disorders and Schizophrenia: A question of shared glutamatergic mechanisms. Neurotox Res 10: 221-233.
- Javitt DC, Zukin SR (1991) Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry 148: 1301-1308.
- Coyle JT (1996) The glutamatergic dysfunction hypothesis for schizophrenia. Harv Rev Psychiatry 3: 241-253.
- Coyle JT, Tsai G, Goff D (2002) Ionotropic glutamate receptors as therapeutic targets in schizophrenia. Curr Drug Targets CNS Neurol Disord 1: 183-189.
- Daniel CJ (2007) Glutamate and schizophrenia: Phencyclidine, N-methyl-D-aspartate receptors, and dopamine-glutamate interactions. Int Rev Neurobiology 69-108.
- Carlezon WA, Thomas MJ (2009) Biological substrates of reward and aversion: A nucleus accumbens activity hypothesis. Neuropharmacology 56: 122-132.
- Fellows RP, Byrd DA, Morgello S (2013) Major depressive disorder, cognitive symptoms, and neuropsychological performance among ethnically diverse HIV+ men and women. J Int Neuropsychol Soc 19: 216-225.
- Mpungu EN, Musisi S, Mpungu SK, Katabira E (2009) Clinical presentation of bipolar mania in HIV-positive patients in Uganda. Psychosomatics 50: 325-330.
- Matinella A, Lanzafame M, Bonometti MA, Gajofatto A, Concia, E, et al. (2015) Neurological complications of HIV infection in pre-HAART and HAART era: a retrospective study. J Neurol 262: 1317-1327.
- Dolder CR, Patterson TL, Jeste DV (2004) HIV, psychosis and aging: past, present and future. AIDS 18: S35-S42.
- Kopnisky KL, Stoff DM, Rausch DM (2004) Workshop report: The effects of psychological variables on the progression of HIV-1 disease. Brain Behav Immun 18: 246-261.
- Pappas MK, Halkitis PN (2011) Sexual risk taking and club drug use across three age cohorts of HIV-positive gay and bisexual men in New York City. AIDS Care 23: 1410-1416.
- Mellins CA, Havens JF, McDonnell C, Lichtenstein C, Uldall K, et al. (2009) Adherence to antiretroviral medications and medical care in HIV-infected adults diagnosed with mental and substance abuse disorders. AIDS Care 21: 168-177.
- Ettenhofer ML, Hinkin CH, Castellon SA, Durvasula R, Ullman J, et al. (2009) Aging, neurocognition, and medication adherence in HIV infection. Am J Geriatr Psychiatry 17: 281-290.
- Bangsberg DR (2004) Paradoxes of adherence and drug resistance to HIV antiretroviral therapy. J Antimicrob Chemother 53: 696-699.
- Gonzalez JS, Batchelder AW, Psaros C, Safren SA (2011) Depression and HIV/AIDS treatment nonadherence: a review and meta-analysis. J Acquir Immune Defic Syndr 58: 181-187.
- Helleberg M, Pedersen MG, Pedersen CB, Mortensen PB, Obel N (2015) Associations between HIV and schizophrenia and their effect on HIV treatment outcomes: a nationwide population-based cohort study in Denmark. Lancet HIV 2: 344-350.
- Zhang XY, Zhou DF, Cao LY, Zhang PY, Wu GY (2002) Decreased production of interleukin-2 (IL-2), IL-2 secreting cells and CD4+ cells in medication-free patients with schizophrenia. J Psychiatr Res 36: 331-336.
- Norbert M, Ackenheil M, Elisabeth H, Mempel W, Eckstein R (1991) Cellular immunity in schizophrenic patients before and during neuroleptic treatment. Psychiatry Res 37: 147-160.
- Meade CS, Fitzmaurice GM, Sanchez AK, Griffin ML, McDonald LJ, et al. (2011) The relationship of manic episodes and drug abuse to sexual risk behavior in patients with co-occurring bipolar and substance use disorders: A 15-month prospective analysis. AIDS Behav 15: 1829-1833.
- Volkow ND, Montaner J (2011) The urgency of providing comprehensive and integrated treatment for substance abusers with HIV. Health Aff (Millwood) 30: 1411-1419.
- Sevigny JJ, Albert SM, McDermott MP, Schifitto G, McArthuret JC, et al. (2007) An evaluation of neurocognitive status and markers of immune activation as predictors of time to death in advanced HIV infection. Arch Neurol 64: 97-102.
- Tozzi V, Balestra P, Bellagamba R, Corpolongo A, Salvatori MF, et al. (2007) Persistence of neuropsychologic deficits despite long-term highly active antiretroviral therapy in patients with HIV-related neurocognitive impairment: Prevalence and risk factors. J Acquir Immune Defic Syndr 45: 174-182.
- DiMatteo MR, Lepper HS, Croghan TW (2000) Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med 160: 2101-2107.
- Heaton RK, Marcotte TD, Mindt MR, Sadek J, Moore J, et al. (2004) The impact of HIV-associated neuropsychological impairment on everyday functioning. J Int Neuropsychol Soc 10: 317-331.
- Yun LWH, Maravi M, Kobayashi JS, Barton PL, Davidson AJ (2005) Antidepressant treatment improves adherence to antiretroviral therapy among depressed HIV-infected patients. J Acquir Immune Defic Syndr 38: 432-438.
- Weber R, Huber M, Rickenbach M, Furrer H, Elzi L, et al. (2009) Uptake of and virological response to antiretroviral therapy among HIV-infected former and current injecting drug users and persons in an opiate substitution treatment programme: The Swiss HIV Cohort Study. HIV Med 10: 407-416.
- Glass TR, Battegay M, Cavassini M, Geest SD, Furrer HJ, et al. (2010) Longitudinal analysis of patterns and predictors of changes in self-reported adherence to antiretroviral therapy: Swiss HIV Cohort Study. J Acquir Immune Defic Syndr 54: 197-203.
- Haddad P, Brain C, Scott J (2014) Nonadherence with antipsychotic medication in schizophrenia: Challenges and management strategies. Patient Relat Outcome Meas 5: 43-62.
- Kidorf M, Disney ER, King VL, Neufeld K, Beilenson PL, et al. (2004) Prevalence of psychiatric and substance use disorders in opioid abusers in a community syringe exchange program. Drug Alcohol Depend 74: 115-122.
- Wild TC, Guebaly NE, Fischer B, Brissette S, Brochu S, et al. (2005) Comorbid depression among untreated illicit opiate users: results from a multisite Canadian study. Can J Psychiatry 50: 512-518.
- Hammond ER, Crum RM, Treisman GJ, Mehta SH, Clifford DB, et al. (2016) Persistent CSF but not plasma HIV RNA is associated with increased risk of new-onset moderate-to-severe depressive symptoms: A prospective cohort study. J Neurovirol 22: 479-487.
- Wright NE, Scerpella D, Lisdahl LM (2016) Marijuana Use Is Associated with Behavioral Approach and Depressive Symptoms in Adolescents and Emerging Adults. PLoS One 11: e0166005.
- Bellis MD, Narasimhan A, Thatcher DL, Keshavan MS, Soloff P, et al. (2005) Prefrontal cortex, thalamus and cerebellar volumes in adolescents and young adults with adolescent-onset alcohol use disorders and comorbid mental disorders. Alcoholism: Clinical and experimental research 29:1590-1600.
- Bolton JM, Robinson J, Sareen J (2009) Self-medication of mood disorders with alcohol and drugs in the National Epidemiologic Survey on Alcohol and Related Conditions. J Affect Disord 115: 367-375.
- Mushquash AR, Stewart SH, Sherry SB, Sherry DL, Mushquash CJ, et al. (2013) Depressive symptoms are a vulnerability factor for heavy episodic drinking: a short-term, four-wave longitudinal study of undergraduate women. Addict Behav 38: 2180-2186.
- Zhang Y, Sloan FA, (2014) Depression, alcohol dependence and abuse, and drinking and driving behavior. J Behav Health 3: 212-219.
- Merikangas KR, Akiskal HS, Angst J, Greenberg PE, Hirschfeld RMA, et al. (2007) Lifetime and 12-month prevalence of bipolar spectrum disorder in the National Comorbidity Survey replication. Arch Gen Psychiatry 64: 543-552.
- Brown SA, McGue M, Maggs J, Schulenberg J, Hingson R, et al. (2008) A developmental perspective on alcohol and youths 16 to 20 years of age. Pediatrics 121: S290-S310.
- De Leon J, Diaz FJ (2005) A meta-analysis of worldwide studies demonstrates an association between schizophrenia and tobacco smoking behaviors. Schizophr Res 76: 135-157.
- Chaves L, Shirakawa I (2008) Nicotine use in patients with schizophrenia evaluated by the Fagerström Tolerance Questionnaire: a descriptive analysis from a Brazilian sample. Rev Bras Psiquiatr 30: 350-352.
- Wijesundera H, Hanwella R, De Silva VA (2014) Antipsychotic medication and tobacco use among outpatients with schizophrenia: a cross-sectional study. Ann Gen Psychiatry 13: 7.
- Yee A, Mohamed NNBN, Hashim AHB, Loh HS, Singh MKH, et al. (2015) The Effect of Nicotine Dependence on Psychopathology in Patients with Schizophrenia. BioMed Research International 2015: 1-6.
- Murray JB (2002) Phencyclidine (PCP): A dangerous drug, but useful in schizophrenia research. J Psychol 136: 319-327.
- Reynolds LM, Cochran SM, Morris BJ, Pratt JA, Reynolds GP (2005) Chronic phencyclidine administration induces schizophrenia-like changes in N-acetylaspartate and N-acetylaspartylglutamate in rat brain. Schizophr Res 73: 147-152.












