Keywords
Liposomal formulation, bilayered vesicles, percent drug encapsulation, cytotoxic.
Introduction
Advances in combinatorial chemistry have led to the discovery of a wide number of new chemical entities (NCE) that have a potential therapeutic action on the biological systems. But most of the NCEs being discovered provide a challenge to the formulation scientist because of their physicochemical properties like poor solubility and permeability. Even though, above problems could be addressed, but most of the molecules fail to show their desired therapeutic action in vivo, which leads to lack of in vitro – in vivo correlation [1,2].
A majority of anti-neoplastic agents, which are highly cytotoxic to tumor cells in vitro, affect the normal cells also. This is due to their low therapeutic index (TI), i.e., the dose required to produce anti-tumor effect is toxic to normal cells. Such drugs have to be targeted to a specific site (diseased site) in order to reduce their toxic effects to normal tissues [3]. Hence, an efficient drug delivery system is required to present the maximum fraction of administered dose at the target site. Various carriers like nanoparticles, microparticles, polysaccharides, lectins and liposomes can be used to target the drug to a specific site [4-9].
Liposomal drug delivery is gaining interest due to its contribution to varied areas like drug delivery, cosmetics, and structure of biological membrane [10]. Liposomes can act as a carrier for a variety of drugs, having a potential therapeutic action. Liposomes are colloidal carriers, having a size range of 0.01 – 5.0 μm in diameter. Indeed these are bilayered vesicles that are formed when phospholipids are hydrated in excess of aqueous medium [11,12]. Liposomes have got a potential advantage of encapsulating hydrophilic as well as hydrophobic drugs and targeting them to the required diseased site in the body [10]. Fig. 1 depicts the structure of a liposome (bilayered vesicle) and phospholipid.
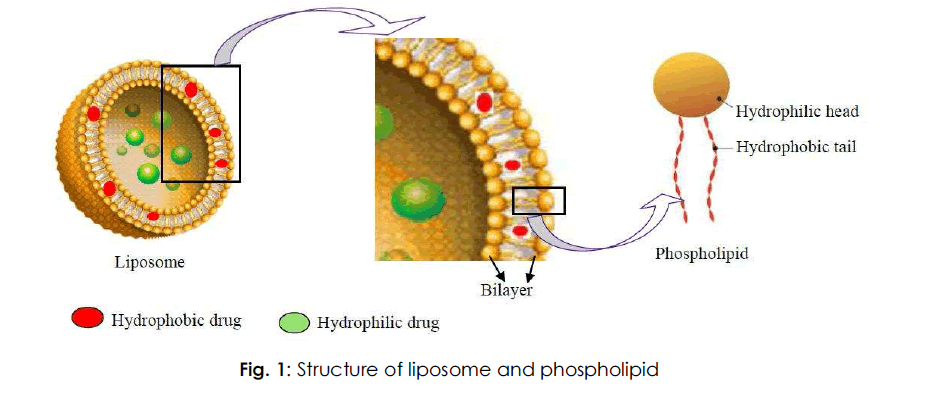
Fig. 1: Structure of liposome and phospholipid
Various therapeutic agents like anticancer drugs, vaccines, antimicrobials, genetic materials, proteins and macromolecules can be encapsulated within the bilayered vesicles [13]. Liposomal technology was used for the successful encapsulation of various drug molecules like paclitaxel [14], acyclovir [15], tropicamaide [16], arteether [17], chloroquine diphosphate [18], cyclosporine [19] and dithranol [20]. Table 1 indicates the list of few liposomal products that have been approved for human use [3].
| Drug |
Product |
Indication |
| Ambisome™ |
Amphoteracin B |
Fungal infection |
| DaounoXome™ |
Daunorubicin |
Kaposi's sarcoma |
| Doxil™ |
Doxorubicin |
Refractory Kaposi's sarcoma, recurrent breast cancer and ovarian cancer |
| Visudyne® |
Verteporfin |
Age-related macular degeneration, pathologic myopia and ocular
histoplasmosis |
| Myocet® |
Doxorubicin |
Recurrent breast cancer |
| DepoCyt® |
Cytarabine |
Neoplastic meningitis and lymphomatous meningitis |
| Lipoplatin® |
Cisplatin |
Epithelial malignancies |
| DepoDur® |
Morphine |
sulfate
Postoperative pain following major surgery |
Table 1: List of liposomal products approved for commercial use.
Mechanism Of Liposome Formation
The basic part of liposome is formed by phospholipids, which are amphiphilic molecules (having a hydrophilic head and hydrophobic tail). The hydrophilic part is mainly phosphoric acid bound to a water soluble molecule, whereas, the hydrophobic part consists of two fatty acid chains with 10 – 24 carbon atoms and 0 – 6 double bonds in each chain [21].
When these phospholipids are dispersed in aqueous medium, they form lamellar sheets by organizing in such a way that, the polar head group faces outwards to the aqueous region while the fatty acid groups face each other and finally form spherical/ vesicle like structures called as liposomes. The polar portion remains in contact with aqueous region along with shielding of the non-polar part (which is oriented at an angle to the membrane surface) [22].
When phospholipids are hydrated in water, along with the input of energy like sonication, shaking, heating, homogenization, etc. it is the hydrophilic/ hydrophobic interactions between lipid – lipid, lipid – water molecules that lead to the formation of bilayered vesicles in order to achieve a thermodynamic equilibrium in the aqueous phase [23]. The reasons for bilayered formation include:
• The unfavorable interactions created between hydrophilic and hydrophobic phase can be minimized by folding into closed concentric vesicles.
• Large bilayered vesicle formation promotes the reduction of large free energy difference present between the hydrophilic and hydrophobic environment.
• Maximum stability to supramolecular self assembled structure can be attained by forming into vesicles.
Classification Of Liposome
Various classes of liposomes have been reported in literature. They are classified based on their size, number of bilayers, composition and method of preparation. Based on the size and number of bilayers, liposomes are classified as multilamellar vesicles (MLV), large unilamellar vesicles (LUV) and small unilamellar vesicles (SUV) as depicted in Fig. 2. Based on composition, they are classified as conventional liposomes (CL), pH-sensitive liposomes, cationic liposomes, long circulating liposomes (LCL) and immuno-liposomes. Based on the method of preparation, they are classified as reverse phase evaporation vesicles (REV), French press vesicles (FPV) and ether injection vesicles (EIV). In this context, the classification based on size and number of bilayers is discussed below.
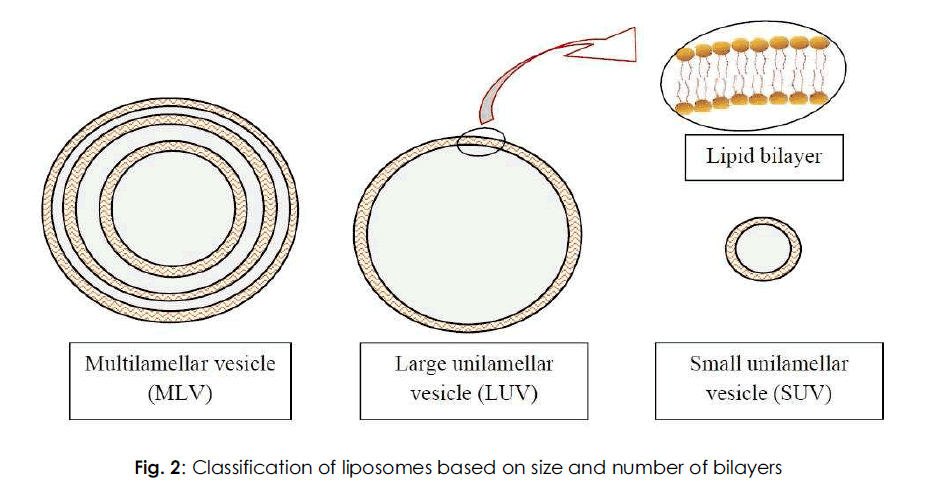
Fig. 2: Classification of liposomes based on size and number of bilayers.
Multilamellar vesicles (MLV)
MLV have a size greater than 0.1 μm and consist of two or more bilayers. Their method of preparation is simple, which includes thin – film hydration method or hydration of lipids in excess of organic solvent. They are mechanically stable on long storage. Due to the large size, they are cleared rapidly by the reticulo-endithelial system (RES) cells and hence can be useful for targeting the organs of RES [3]. MLV have a moderate trapped volume, i.e., amount of aqueous volume to lipid ratio. The drug entrapment into the vesicles can be enhanced by slower rate of hydration and gentle mixing [24]. Hydrating thin films of dry lipids can also enhance encapsulation efficiency [25]. Subsequent lyophilization and rehydration after mixing with the aqueous phase (containing the drug) can yield MLV with 40% encapsulation efficiency [26,27].
Large unilamellar vesicles (LUV)
This class of liposomes consists of a single bilayer and has a size greater than 0.1 μm. They have higher encapsulation efficiency, since they can hold a large volume of solution in their cavity [28]. They have high trapped volume and can be useful for encapsulating hydrophilic drugs. Advantage of LUV is that less amount of lipid is required for encapsulating large quantity of drug. Similar to MLV, they are rapidly cleared by RES cells, due to their larger size [3,8]. LUV can be prepared by various methods like ether injection, detergent dialysis and reverse phase evaporation techniques. Apart from these methods, freezethawing of liposomes [29,30], dehydration/ rehydration of SUV [31] and slow swelling of lipids in non-electrolyte solution [32] can also be used to prepare LUV.
Small unilamellar vesicles (SUV)
SUV are smaller in size (less than 0.1 μm) when compared to MLV and LUV, and have a single bilayer. They have a low entrapped aqueous volume to lipid ratio and characterized by having long circulation half life. SUV can be prepared by using solvent injection method (ethanol or ether injection methods) [33] or alternatively by reducing the size of MLV or LUV using sonication or extrusion process under an inert atmosphere like nitrogen or Argon. The sonication can be performed using either a bath or probe type sonicator. SUV can also be achieved by passing MLV through a narrow orifice under high pressure. These SUV are susceptible to aggregation and fusion at lower or negligible/ no charge [34].
Methods Of Preparation
The conventional methods for preparing liposomes include solubilizing the lipids in organic solvent, drying down the lipids from organic solution, dispersion of lipids in aqueous media, purification of resultant liposomes and analysis of the final product [35].
Of all the methods used for preparing liposomes, thin-film hydration method is the most simple and widely used one. MLV are produced by this method within a size range of 1 – 5 μm. If the drug is hydrophilic it is included in the aqueous buffer and if the drug is hydrophobic, it can be included in the lipid film. But the drawback of this method is poor encapsulation efficiency (5 – 15% only) for hydrophobic drugs. By hydrating the lipids in presence of organic solvent, the encapsulation efficiency of the MLV can be increased [36,37]. LUV can be prepared by solvent injection, detergent dialysis, calcium induced fusion and revese phase evaporation techniques. SUV can be prepared by the extrusion or sonication of MLV or LUV.
All these preparation methods involve the usage of organic solvents or detergents whose presence even in minute quantities can lead to toxicity. In order to avoid this, other methods like polyol dilution [38], bubble method [39] and heating method [40] have been developed without using any organic solvents or detergents. Detailed procedures for liposome preparation, can be obtained from literature [21,35].
Charaterization Of Liposomes
Liposomes produced by different methods have varying physicochemical characteristics, which leads to differences in their in vitro (sterilization and shelf life) and in vivo (disposition) performances [41-43]. Rapid, precise and reproducible quality control tests are required for characterizing the liposomes after their formulation and upon storage for a predictable in vitro and in vivo behavior of the liposomal drug product [44,45]. A liposomal drug product can be characterized for some of the parameters that are discussed below.
Size and size distribution
When liposomes are intended for inhalation or parenteral administration, the size distribution is of primary consideration, since it influences the in vivo fate of liposomes along with the encapsulated drug molecules [46-50]. Various techniques of determing the size of the vesicles include microscopy (optical microscopy [51], negative stain transmission electron microscopy [42], cryo-transmission electron microscopy [52], freeze fracture electron microscopy and scanning electron microscopy [45]), diffraction and scattering techniques (laser light scattering and photon correlation spectroscopy) [45] and hydrodynamic techniques (field flow fractionation [53], gel permeation [54] and ultracentrifugation).
Percent drug encapsulation
The amount of drug encapsulated/ entrapped in liposome vesicle is given by percent drug encapsulation. Column chromatography can be used to estimate the percent drug encapsulation of liposomes [55]. The formulation consists of both free (unencapsulated) and encapsulated drug. So as to know the exact amount of drug encapsulated, the free drug is separated from the encapsulated one. Then the fraction of liposomes containing the encapsulated drug is treated with a detergent, so as to attain lysis, which leads to the discharge of the drug from the vesicles into the surrounding medium. This exposed drug is assayed by a suitable technique which gives the percent drug encapsulated from which encapsulation efficiency can be calculated [56-59].
Trapped volume per lipid weight can also give the percent drug encapsulated in a liposome vesicle. It is generally expressed as aqueous volume entrapped per unit quantity of lipid, μl/μmol or μg/mg of total lipid [41,43]. Inorder to determine the trapped volume, various materials like radioactive markers, fluorescent markers and spectroscopically inert fluid [60] can be used. Radioactive method is mostly used for determining trapped volume [41]. It is determined by dispersing lipid in an aqueous medium containing a non-permeable radioactive solute like [22Na] or [14C] inulin [61]. Alternatively, water soluble markers like 6-carboxyfluorescein, 14C or 3H-glucose or sucrose can be used to determine the trapped volume [45]. A novel method of determining intravesicular volume by salt entrapment was also reported in literature [62].
Surface charge
Since the charge on the liposome surface plays a key role in the in vivo disposition, it is essential to know the surface charge on the vesicle surface. Two methods namely, free-flow electrophoresis and zeta potential measurement can be used to estimate the surface charge of the vesicle. The surface charge can be calculated by estimating the mobility of the liposomal dispersion in a suitable buffer (determined using Helmholtz– Smolochowski equation) [63].
Vesicle shape and lamellarity
Various electron microscopic techniques can be used to assess the shape of the vesicles. The number of bilayers present in the liposome, i.e., lamellarity can be determined using freezefracture electron microscopy [41] and 31P-Nuclear magnetic resonance analysis64. Apart from knowing the shape and lamellarity, the surface morphology of liposomes can be assessed using freeze-fracture and freeze-etch electron microscopy [64].
Phospholipid identification and assay
The chemical components of liposomes must be analyzed prior to and after the preparation [45]. Barlett assay [65], Stewart assay [66] and thin layer chromatography [67] can be used to estimate the phospholipid concentration in the liposomal formulation. A spectrophotometric method to quantify total phosphorous in a sample was given in literature, which measure the intensity of blue color developed at 825 nm against water [68]. Cholesterol oxidase assay or ferric perchlorate method [69] and Gas liquid chromatography techniques can be used to determine the cholesterol concentration [70].
Stability Of Liposomes
During the development of liposomal drug products, the stability of the developed formulation is of major consideration. The therapeutic activity of the drug is governed by the stability of the liposomes right from the manufacturing steps to storage to delivery. A stable dosage forms is the one which maintains the physical stability and chemical integrity of the active molecule during its developmental procedure and storage. A well designed stability study includes the evaluation of its physical, chemical and microbial parameters along with the assurance of product’s integrity throughout its storage period. Hence a stability protocol is essential to study the physical and chemical integrity of the drug product in its storage.
Physical stability
Liposomes are bilayered vesicles that are formed when phospholipids are hydrated in water. The vesicles obtained during this process are of different sizes. During its storage, the vesicles tend to aggregate and increase in size to attain thermodynamically favorable state. During storage, drug leakage from the vesicles can occur due to fusion and breaking of vesicles, which deteriorates the physical stability of the liposomal drug product. Hence morphology, size and size distribution of the vesicles are important parameters to assess the physical stability [28]. In order to monitor this, a variety of techniques like light scattering and electron microscopy [71] can be used to estimate the visual appearance (morphology) and size of the vesicles.
Chemical stability
Phospholipids are chemically unsaturated fatty acids that are prone to oxidation and hydrolysis, which may alter the stability of the drug product. Along with this, pH, ionic strength, solvent system and buffered species also play a major role in maintaining a liposomal formulation. Indeed chemical reaction can be induced even by light, oxygen, temperature and heavy metal ions.
Oxidation deterioration involves the formation of cyclic peroxides and hydroxyperoxidases due to the result of free radical generation in the oxidation process. Liposomes can be prevented from oxidative degradation by protecting them from light, by adding anti-oxidants such as alpha – tocopherol or butylated hydroxyl toluene (BHT), producing the product in an inert environment (presence of nitrogen or Argon) or by adding EDTA to remove trace heavy metals [21,28].
Hydrolysis of the ester bond at carbon position of the glycerol moiety of phospholipids leads to the formation of lyso-phosphatidylcholine (lysoPC), which enhances the permeability of the liposomal contents. Hence, it becomes necessary to control the limit of lysoPC within the liposomal drug product. This can be achieved by formulating liposomes with phosphatidylcholine free from lysoPC [21].
In Vivo Behavior Of Liposomes
During the optimization of liposomal formulation, various physico-chemical parameters are altered in order to achieve a desired bio-distribution and cellular uptake of drugs. Those parameters which affect the in vivo (biological) performance of liposomes are described below [72].
Liposome size
The size of the vesicle governs the in vivo fate of liposomes, because it determines the fraction cleared by RES [73]. The rate of uptake of liposome by RES increases with the vesicle size. Liposomes larger than 0.1 μm are taken up (opsonized) more rapidly by RES, when compared to liposomes smaller than 0.1 μm.
The size of the vesicle also determines the extravasation of liposomes. Tumor capillaries are more permeable than normal capillaries. Due to such leaky vasculature, fluids along with small sized liposomes can pass through the gaps leading to increased accumulation of drug loaded liposomes in the tumor tissue. The difference between intravascular hydrostatic and interstitial pressure acts as a driving force for the extrvasation of small sized liposomes [74].
Surface charge
The lipid – cell interaction can be governed by the nature and density of charge on the liposome surface. Charging the lipid composition can alter the nature and charge on the liposome. Lack of charge in the SUV liposomes can lead to their aggregation and thereby reducing the stability of the liposome; whereas, the interaction of neutrally charged liposome with the cell is almost negligible [75,76]. High electrostatic surface charge on the liposome may provide useful results in promoting lipid – cell interaction. Negatively charged density influences the extent of lipid – cell interactions and increase the intracellular uptake of liposomes by target cells [77]. But positively charged liposomes are cleared more rapidly after systemic administration. Unlike negatively charged liposomes, cationic liposomes deliver the contents to cells by fusion with cell membrane [78].
Surface hydration
Liposomes with hydrophilic surface coatings are less prone to opsonization, hence reducing its uptake by RES cells. This can be attributed to the hydrophilic surface coating, which reduces the interaction of liposomes with cell and blood components [79-81]. These sterically stabilized liposomes are more stable in the biological environment and exhibit high circulation half lives, when compared to liposomes coated with hydrophobic coatings. Monogangliosides, hydrogenated phosphotidyl inositol, polyethylene glycol are some of the hydrophilic groups responsible for steric stabilization of liposomes [82,83].
Bilayer fluidity
Lipid exists in different physical states above and below the phase transition temperature (Tc). They are rigid and well ordered below Tc but are in fluid like liquid – crystalline state above Tc. Table 2 inidcates the phase transition temperatures of various phospholipids [3,21]. Liposomes with low Tc (less than 37°C) are fluid like and are prone to leakage of the drug content at physiological temperature. But, the liposomes with high Tc (greater than 37°C) are rigid and less leaky at physiological temperature.
The phase transition temperature also governs the liposomal cell interaction. Liposomes with low Tc lipids have high extent of uptake by RES when compared to those with high Tc lipids [80]. Incorporation of cholesterol in the bilayer can decrease the membrane fluidity at a temperature greater than phase transition temperature, which gives stability to liposomes.
| Name of the phospholipid |
Molecular weight |
Phase transition temperature (°C) |
| Dimyristoyl phosphatidylcholine (DMPC) |
677.94 |
23 |
| Dioleoyl PC (DOPC) |
786.12 |
-22 |
| Distearoyl PC (DSPC) |
790.15 |
55 |
| Dipalmitoyl phosphatidylethanolamine (DPPE) |
691.97 |
67 |
| Dipalmitoyl PC (DPPC) |
734.05 |
41 |
| Dipalmitoyl phosphatidylglycerol (DPPG) |
744.96 |
41 |
Table 2: Phase transition temperatures of various phospholipids.
Therapeutic Applications Of Liposomes
When a conventional dosage form fails to provide a desired therapeutic effect, then new drug delivery systems are developed. Liposomes are among such systems which provide a superior therapeutic efficacy and safety in comparison to existing formulations. Some of the major therapeutic applications of liposomes in drug delivery include:
Site-avoidance delivery
The cytotoxicity of anti-cancer drugs to normal tissues can be attributed to their narrow therapeutic index (TI). Under such circumstances, the TI can be improved by minimizing the delivery of drug to normal cells by encapsulating in liposomes. Free doxorubicin has a severe side effect of cardiac toxicity, but when formulated as liposomes, the toxicity was reduced without any change in the therapeutic activity [3,84].
Site specific targeting
Delivery of larger fraction of drug to the desired (diseased) site, by reducing the drug’s exposure to normal tissues can be achieved by site specific targeting. Encapsulating the drug in liposomes can be used for both active and passive targeting of drugs in order to achieve a safer and efficacious therapy [3]. On systemic administration, long circulating immunoliposomes are able to recognize and bind to target cells with greater specificity [85,86]. In patients with recurrent osteosarcoma, there was an enhanced tumoricidal activity of monocytes, when muramyl peptide derivatives were formulated as liposomes and administered systemically [87].
Intracellular drug delivery
Increased delivery of potent drugs to the cytosol (in which drug’s receptors are present), can be accomplished using liposomal drug delivery system [3]. N-(phosphonacetyl)-L-aspartate (PALA) is normally poorly taken up into cells. Such drugs when encapsulated within liposomes, showed greater activity against ovarian tumor cell lines in comparison to free drug [76].
Sustained release drug delivery
Liposomes can be used to provide a sustained release of drugs, which require a prolonged plasma concentration at therapeutic levels to achieve the optimum therapeutic efficacy [3]. Drugs like cytosine Arabinoside can be encapsulated in liposomes for sustained release and optimized drug release rate in vivo [88].
IntraperitoneaI administration
Tumors that develop in the intra-peritoneal (i.p.) cavity can be treated by administering the drug to i.p. cavity. But the rapid clearance of the drugs from the i.p. cavity results in minimized concentration of drug at the diseased site. However, liposomal encapsulated drugs have lower clearance rate, when compared to free drug and can provide maximum fraction of drug in a prolonged manner to the target site [89,90].
Immunological adjuvants in vaccines
Immune response can be enhanced by delivering antigens encapsulated within liposomes. Depending on the lipophilicity of antigens, the liposome can accommodate antigens in the aqueous cavity or incorporate within the bilayers [3]. In order to enhance the immune response to diphtheria toxoid, liposomes were first used as immunological adjuvants [91].
Conclusion
A number of drug candidates which are highly potent and have low therapeutic indication can be targeted to the required diseased site using the liposomal drug delivery system. Drugs encapsulated in liposomes can have a significantly altered pharmacokinetics. The efficacy of the liposomal formulation depends on its ability to deliver the drug molecule to the targeted site over a prolonged period of time, simultaneously reducing its (drug’s) toxic effects. The drugs are encapsulated within the phospholipid bilayers and are expected to diffuse out from the bilayer slowly. Various factors like drug concentration, drug to lipid ratio, encapsulation efficiency and in vivo drug release must be considered during the formulation of liposomal drug delivery systems. The development of deformable liposomes and ethosomes along with the administration of drug loaded liposomes through inhalation and ocular route are some of the advances in the technology. Thus liposomal approach can be successfully utilized to improve the pharmacokinetics and therapeutic efficacy, simultaneously reducing the toxicity of various highly potent drugs.
5745
References
- Amidon, G.L., Lennernas, H., Shah, V.P., Crison, J.R. A theoretical basis for a biopharmaceutic drug classification: the correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm Res. 1995; 12: 413-420.
- Sunil, P., Maru, O., Chan, M. Novel lipid-based formulations enhancing the in vitro dissolution and permeability characteristics of a poorly water-soluble model drug, piroxicam. Int J Pharm. 2005; 301(1-2): 209-216.
- Sharma, A., Sharma, U.S. Liposomes in drug delivery: progress and limitations. Int J Pharm. 1997; 154: 123-140.
- Gregoriadis, G. Liposomes, In Gregoriadis, G., (Ed.), Drug Carriers in Biology and Medicine. Academic Press, New York. 1979; Ch. 14. Pp 287- 341.
- Albertsson, A.C., Donaruma, L.G., Vogl, O. Synthetic polymers as drugs, In Tirrell, D.A., Donaruma, L.G., Turek, A.B., (Eds.), Macromolecules as drugs and drug as carriers for biologically active material. Ann NY Acad Sci. 1985; 446: 105-115.
- Donaruma, L.G., Warner, R.J. Some biologically active (thiosemicarbazides). In Tirrell, D.A., Donaruma, L.G. and Turek, A.B. (Eds.), Macromolecules as drugs and drug as carriers for biologically active materials. Ann NY Acad Sci. 1985; 446: 116-133.
- Abra, R.M., Hunt, C.A. Liposome disposition in vivo. III. Dose and vesicle size effects, Biochim Biophys Acta. 1981; 666: 493-503.
- Tirrell, D.A., Heath, T.D., Colley, C.M., Ryman, B.E. New aspects of liposomes, Biochim Biophys Acta. 1976; 457: 259.
- Tirrell, D.A., Takigawa, D.Y., Seki, K. pH sensitization of phospholipid vesicles via complexation with synthetic poly (carboxylic acids). In Tirrell, D.A., Donaruma, L.G., Turek, A.B. (Eds.), Macromolecules as drugs and drug as carriers for biologically active materials. Ann NY Acad Sci. 1985; 446: 237- 248.
- Mozafari, M.R. Liposomes: an overview of manufacturing techniques. Cell Mol Biol Lett. 2005; 10: 711-719.
- Bangham, A.D., Horne, R.W. Negative Staining of Phospholipids and Their Structural Modification by Surface-Active Agents As Observed in the Electron Microscope. J Mol Biol.1964; 8: 660-668.
- Bangham, A.D., Hill, M.W., Miller, N.G.A. Preparation and use of liposomes as models of biological membranes, In Korn, E.D. (Ed.), Methods in Membrane Biology. Vol. 1. Plenum Press, New York. 1974; pp. l-68.
- Gregoriadis, G., Florence, A.T. Liposomes in drug delivery: Clinical, diagnostic and ophthalmic potential. Drugs, 1993; 45: 15-28.
- Wu. J., Liu. Q., Lee, R.J. A folate receptor- targeted liposomal formulation for paclitaxel. Int J Pharm. 2006; 316(1-2): 148-153.
- Pavelic, Z., Skalko-Basnet, N., Filippvic-Grcic, J., Martinac, A., Jalsenjak, I. Development and in vitro evaluation of a liposomal vaginal delivery system for acyclovir. J Control Release. 2005; 106: 34-43.
- Nagarsenker, M.S., Londhe, V.Y., Nadkarni, G.D. Preparation and evaluation of liposomal formulations of tropicamide for ocular delivery. Int J Pharm. 1999; 190(1): 63-71.
- Al-Angary, A.A., Al-Meshal, M.A., Bayomi, M.A., Khidr, S.H. Evaluation of liposomal formulations containing the anti-malarial agent arteether. Int J Pharm. 1996; 128(1-2): 163-168.
- Qiu, L., Jing, N., Jin, Y. Preparation and in vitro evaluation of liposomal chloroquine diphosphate loaded by a transmembrane pH- gradient method. Int J Pharm. 2008; 361(1-2): 56- 63.
- Al-Meshal, M.A., Khidr, S.H., Bayomi, M.A., Al- Angary, A.A. Oral administration of liposomes containing cyclosporine: a pharmacokinetic study. Int J Pharm. 1998; 168(2): 163-168.
- Agarwal, R., Katare, O.P., Vyas, S.P. Preparation and in vitro evaluation of liposomal/ niosomal delivery systems for anti-psoriatic drug dithranol. Int J Pharm. 2001; 228(1-2): 43-52.
- Vyas, S.P., Khar, R.K. In Vyas, S.P., Khar, R.K. (Eds.), Targeted and controlled drug delivery: Novel carrier systems. CBS publishers. 2002; 173- 248.
- Lasic, D.D. The mechanism of vesicle formation. Biochem J. 1988; 256: 1-11.
- Lasic, D.D., Joannic, R., Keller, B.C., Frederik, P.M., Auvray, L. Spontaneous vesiculation. Adv Colloid Interfac Sci. 2001; 89-90: 337-349.
- Olson, F., Hunt, T., Szoka, F., Vail, W.J., Papahadjopoulos, D. Preparation of liposomes of defined size distribution by extrusion through polycarbonate membranes. Biochim Biophys Acta. 1979; 557: 9-23.
- Barenholz, Y., Gibbes, D., Litman, B.J., Gall, J., Thompson, T.E., Carlson, R.D. A simple method for the preparation of homogeneous phospholipid vesicles. Biochemistry. 1977; 16: 2806-2810.
- Ohsawa, T., Miura, H., Harada, K. A novel method for preparing liposome with a high capacity to encapsulate proteinous drugs: freeze-drying method. Chem Pharm Bull. 1984; 32: 2442-2445.
- Kirby, C.J., Gregoriadis, G. A simple procedure for preparing liposomes capable of high encapsulation efficiency under mild conditions, In Liposome Technology, Vol. 1. CRC Press, Boca Raton, FL .1984, pp 19-27.
- Vemuri, S., Rhodes, C.T. Preparation and characterization of liposomes as therapeutic delivery systems: a review. Pharmaceutica Acta Helvetiae. 1995; 70: 95-111.
- Pick, U.I. Liposomes with a large trapping capacity prepared by freezing and thawing of sonicated phospholipid mixtures. Arch Biochem Biophys. 1981; 212: 186-194.
- Kasahara, M., Hinkle, P.C. Reconstitution and purification of the o-glucose transporter from human erythrocytes. J Biol Chem. 1977; 252: 7384-7390.
- Shew, R.L., Deamer, D. A novel method for encapsulation of macromolecules in liposomes. Biochim Biophys Acta. 1985; 816: 1-8.
- Reeves, J.P., Dowben, R.M. Formation and properties of thin-walled phospholipid vesicles. J Cell Physiol. 1969; 734: 49-60.
- Deamer, D.W., Bangham, A.D. Large volume liposomes by an ether vaporization method. Biochim Biophys Acta. 1976; 443: 629-634.
- Hamilton, R.L., Goerke, J., Guo, L. Unilamellar liposomes made with the French pressure cell: A simple preparative and semi-quantitative technique. J Lipid Res. 1980; 21: 981-992.
- New, R.R.C. Preparation of liposomes, In: New, R.R.C. (Ed.), Lipsomes: a practical approach. IRL Press, Oxford, 1990; pp. 33-104.
- Papahadjopoulos, D., Watkins, J.C. Phospholipid model membranes. II. Permeability properties of hydrated liquid crystals. Biochim Biophys Acta. 1967; 135: 639-652.
- Gruner, S.M., Lenk, R.P., Janoff, A.S., Ostro, M.J. Novel multilayered lipid vesicles: comparison of physical characteristics of multi-lamellar liposomes and stable pluri-lamellar vesicles. Biochemistry. 1985; 24: 2833-2842.
- Kikuchi, H. Yamauchi, H., Hirota, S. A polyol dilution method for mass production of liposomes. J Liposome Res. 1994; 4: 71-91.
- Talsma, H., Van Steenbergen, M.J., Borchert, J.C.H., Crommelin, D.J.A. A novel technique for the one-step preparation of liposomes and nonionic surfactant vesicles without the use of organic solvents. Liposome formation in a continuous gas stream: The bubble method. J Pharm Sci. 1994; 83: 276-280.
- Mozafari, M.R., Reed, C.J., Rostron, C., Kocum, C., Piskin, E. Construction of stable anionic liposome-plasmid particles using the heating method: A preliminary investigation. Cell Mol Biol Lett. 2002; 7: 923- 927.
- Ostro, M.J. In: Liposomes: from Biophysics to therapeutics. Marcel Dekker, New York, 1987, pp 383.
- New, R.R.C. In: New, R.R.C. (Ed.), Lipsomes: a practical approach. OIRL Press, Oxford, London, 1989; 1.
- Weiner, N., Martin, F., Riaz, M. Liposomes as drug delivery systems. Drug Dev Ind Pharm. 1989; 18: 1523-1554.
- Talsma, H., Crommelin, D.J.A. Liposomes as drug delivery systems, part II: Characterization. Pharmaceutical Technology. 1992b; 16: 52-58.
- Barenholz, Y., Cromellin, D.J.A. In: Encyclopedia of pharmaceutical technology. Swabrick, J. (Ed.), Marcel Dekker, New York, 1994; 1-39.
- Vemuri, S., Yu, T., De Groot, J., Roosdrop, N. In- vitro interaction of sized and unsized liposome vesicles with high density lipoproteins. Drug Dev Ind Pharm. 1990; 16: 1579-1584.
- Ellens, H., Mayhew, E., Rustum, Y.M. Reversible depression of the reticulo-endothelial system by liposomes. Biochim Biophys Acta. 1982; 714: 479- 485.
- Kao, Y.J., Juliano, R.L. Interaction of liposomes with the reticulo-endothelial system. Biochim Biophys Acta. 1981; 677: 453- 461.
- Juliano, R.L., Stamp, D. Effect of particle size and charge on the clearance rate of liposomes and liposome encapsulated drugs. Biochem Biophys Res Commun. 1975; 63: 651.
- Guiot, P., Baudhuin, P., Gotfredsen, C. Morphological characterization of liposome suspensions by stereological analysis of freeze- fracture replicas from spray-frozen samples. J Microsc. 1980; 120: 159-174.
- Katare, O.P., Vyas, S.P. Proliposomes of Indomethacin for oral administration. J Microencap, 1991; 8: 1-7.
- Schmidtgen, M.C., Drechsler, M., Lasch, J., Schubert, R. Energy-filtered cryotransmission electron microscopy of liposomes prepared from human stratum corneum lipids. J Microsc. 1998; 191: 177-186.
- Moon, M.H., Giddings, J.C. Size distribution of liposomes by flow field-flow fractionation. J Pharm Biomed Anal. 1993; 11: 911-920.
- Andrieux, K., Lesieur, S., Ollivon, M., Grabielle- Madelmont, C. Methodology for vesicle permeability study by high-performance gel exclusion chromatography. J Chromatogr Biomed Sci Appl. 1998; 706(1): 141-147.
- Maddan, T.D., Harrigan, P.R., Tai, L.C.L., Bally, M.B., Mayer, L.D., Redelmeier, T.E., et al. The accumulation of drugs within large unilamellar vesicles exhibiting a proton gradient: a survey. Chem Phys Lipids. 1990; 53: 37.
- Vemuri, S., Rhodes, CT. Development and characterization of a liposome preparation by a pH gradient method. J Pharm Pharmacol. 1994a; 46: 778-783.
- Vemuri, S., Rhodes, CT. Separation of liposomes by a gel filtration chromatographic technique: a preliminary evaluation. Pharmaceutica Acta Helvetiae. 1994b; 69: 107-113.
- Vemuri, S., Rhodes, CT. Encapsulation of a water soluble drug in a liposome preparation: removal of free drug by washing. Drug Dev Ind Pharm. 1995; 21(11): 1329-1338.
- Vemuri, S., Rhodes, CT. Development and validation of a drug release rate method for a water soluble drug in a liposome preparation. Drug Dev Ind Pharm. 1995; 21(11): 1353-1364.
- Yoss, N.L., Propescu, O., Pop, V.I., Porutiu, D., Kummerow, F.A., Benga, G. Comparison of liposome entrapment parameters by optical and atomic absorption spectrophotometry. Biosci Rep. 1985; 5: 1-5.
- Hope, M.J., Bally, M.B., Webb, G., Cullis, P. Production of large unilamellar vesicles by rapid extrusion procedure: characterization of size distribution, trapped volume, and ability to maintain a membrane potential. Biochim Biophys Acta. 1985; 812: 55-65.
- Gruber, H.J., Wilmsen, H.U., Schurga, A., Pilger, A., Schindler, H. Measurement of intravesicular volumes by salt entrapment, Biochim Biophys Acta. 1995; 1240: 266-276.
- Adamson, A.W. In: Physical chemistry of surface, II Ed., Interscience, New York, 1967; 23.
- Mandal, T.K., Downing, D.T. Freeze-fracture electron microscopic and osmotic water permeability studies of epidermal lipid liposomes derived from stratum corneum lipids of porcine epidermis. Derm Venereol. 1993; 73: 12-17.
- Barlett, G.R. Phosphorus assay in column chromatography. J Biol Chem. 1959; 234: 466- 468.
- Stewart, J.C.M. Colorimetric determination of phospholipids with ammonium ferrothiocyanate. Anal Biochem. 1959; 104: 10-14.
- Terao, J., Asano, I., Matsushito, S. Preparation of hydroperoxy and hydroxy derivatives of rat liver phosphatidylcholine and phosphatidylethanolamine. Lipids. 1985; 20(5): 312-317.
- McClare, C.W.F. An accurate and convenient organic phosphorus assay. Anal Biochem. 1971; 38: 527-530.
- Wybenga, D.R., Pileggi, V.J., Dirstine, P.H., Di Giorgio, J. Direct manual determination of serum total cholesterol with single stable reagent. J Clin Chem. 1970; 16: 980-984.
- Brooks, C.J.W., MacLachlan, J., Cole, W.J., Lawrie, T.D.V. In: Proceedings of symposium on anlaysis of steroids, Szeged, Hungary, 1984; 349.
- Szoka, F., Papahadjopoulos, D. Comparative properties and methods of preparation of lipid vesicles (liposomes). Ann Rev Biophys Bioeng. 1980; 9: 467-508.
- Straubinger, R., Sharma, A., Murray, M., Mayhew, E. Novel taxol formulations: taxol containing liposomes. J Natl Cancer Inst Monograph. 1993; 15: 69-78.
- Harashima, H., Sakata, K., Funato, K., Kiwada, H. Enhanced hepatic uptake of liposomes through complement activation depending on the size of liposomes. Pharm Res. 1994; 11: 402-406.
- Yuan, F., Leunig, M., Huang, S.K., Berk, D.A., Papahadjopoulos, D., Jain, R.K. Microvascular permeability and interstitial penetration of sterically stabilized (stealth) liposomes in a human xenograft. Cancer Res. 1994; 54: 3352- 3356.
- Sharma, A., Straubinger, R.M. Novel taxol formulations: preparation and characterization of taxol-containing liposomes. Pharm Res. 1994; 11: 889-896.
- Sharma, A., Straubinger, N.L., Straubinger, R.M. Modulation of human ovarian tumor cell sensitivity to N-(phosphonacetyl)-L-aspartate (PALA) by liposome drug carriers. Pharm Res. 1993a; 10: 1434-1441.
- Gabizon, A., Price, D.C., Huberty, J., Bresalier, R.S., Papahadjopoulos,D. Effect of liposome composition and other factors on the targeting of liposomes to experimental tumors: biodistribution and imaging studies. Cancer Res. 1990; 50: 6371-6378.
- Felgner, J.H., Kumar, R., Sridhar, C.N., Wheeler, C.J., Tsai,Y.J., Border, R. et al. Enhanced gene delivery and mechanism studies with a novel series of cationic lipid formulations. J Biol Chem. 1994; 269: 2550-2561.
- Allen, T.M., Ryan, J.L., Papahadjopoulos, D. Gangliosides reduce leakage of aqueous-space markers from liposomes in the presence of human plasma. Biochim Biophys Acta. 1985; 818: 205-210.
- Gabizon, A., Papahadjopoulos, D. Liposome formulations with prolonged circulation time in blood and enhanced uptake by tumors. Proc Natl Acad Sci, USA, 1988; 85: 6949-6953.
- Papahadjopoulos, D., Allen, T.M., Gabizon, A., Mayhew, E., Matthay, K., Huang, et al. Sterically stabilized liposomes: improvements in pharmacokinetics and antitumor therapeutic efficacy. Proc Natl Acad Sci, USA , 1991; 88: 11460-11464.
- Klibanov, A.L., Maruyama, K., Beckerleg, A.M., Torchilin, V.P., Huang, L. Activity of amphipathic poly(ethylene glycol) 5000 to prolong the circulation time of liposomes depends on the liposome size and is unfavorable for immunoliposome binding to target. Biochim Biophys Acta. 1991; 1062: 142-148.
- Lasic, D.D., Martin, F.J., Gabizon, A., Huang, S.K., Papahadjopoulos,D. Sterically stabilized liposomes: a hypothesis on the molecular origin of the extended circulation times. Biochim Biophys Acta. 1991; 1070: 187-192.
- Szoka, F.C. Liposomal drug delivery: current status and future prospects. In: Wilschut, J., Hoekstra, D. (Eds.), Membrane Fusion, Marcel Dekker, New York, 1991; 845-890.
- Gregoriadis, G. Engineering liposomes for drug delivery: progress and problems. Trends Biotechnol. 1995; 13: 527-537.
- Lasic, D.D., Papahadjopoulos, D. Liposomes revisited. Science. 1995; 267: 1275-1276.
- Killion, J.J., Fidler, I.J. Systemic targeting of liposome encapsulated immuno-modulators to macrophages for treatment of cancer metastasis. Immuno methods. 1994; 4: 273-279.
- Allen, T.M., Mehra, T., Hansen, C., Chin, Y.C. Stealth liposomes: an improved sustained release system for 1- beta-D- arabinofuranosylcytosine. Cancer Res. 1992; 52: 2431- 2439.
- Dedrick, R.L., Myers, C.E., Bungay, P.M., DeVita,V.T. Jr. Pharmacokinetic rationale for peritoneal drug administration in the treatment of ovarian cancer. Cancer Treat Rep. 1978; 62: 1-11.
- Markman, M., Hakes, T., Reichman, B., Hoskins, W., Rubin, S., Jones, W., et al. Intra-peritoneal therapy in the management of ovarian cancer. Yale J Biol Med. 1989; 62: 393-403.
- Allison, A.C., Gregoriadis, G. Liposomes as immunological adjuvants. Nature. 1974; 252: 252-255.








