Keywords
Neuroestrogens; Catfish; Brain; Estradiol-17β; Testosterone
Introduction
Sex steroid hormones are critical for the development, growth, differentiation and functions of the central nervous system in all vertebrate species. Traditionally, the brain and spinal cord have been considered the targets of the sex steroids produced by the gonads [1-5]. However, the studies of Baulieu and co-workers provided evidence contrary to this view. They demonstrated that the concentrations of several steroids including pregnenolone (Δ5P), dehydroepiandrosterone (DHEA) and their sulphate esters were much higher in the central and peripheral nervous systems than in the plasma [6]. De novoneurosteroidogenesis from cholesterol is considered a conserved property across vertebrates [7-10]. The presence of various steroidogenic enzymes has now been visualized by immunohistochemistry and in situ hybridization, either in neurons or in glial cells in the brain of various representative vertebrate species, and the occurrence of the corresponding enzymatic activities has been ascertained through biochemical approaches [1,10-13]. A hypothesis that locally produced E2 in the brain modulates neuronal function as a neurotransmitter or neuromodulators has been proposed [14,15]. This hypothesis is based on the rapid timing of E2 synthesis in the brain and the rapid action of E2 including sex behaviour in quails and rats [16]. Androgens and estrogens also share common actions in a wide variety of non-mammals; it seems likely that central aromatization has an important role in regulating reproduction throughout the vertebrate series. A large number of studies have been presented that in vertebrates, neuroendocrine control of reproduction is mediated partly by androgen-derived estrogens in the brain [17].
Cytochrome P450 aromatase (P450arom) is the crucial ratelimiting enzyme that catalyzes the conversion of androgens to estrogens. It is the main regulator of local and systemic estrogen levels in the body, and plays integral roles in reproduction, sexual differentiation and male/female behaviour [18]. Teleosts are known for exhibiting unusually high neural cyp19a1b mRNA activity levels [19-21]. In teleosts the process of aromatization, leading to the local production of estrogens, occurs in several areas of the brain [8,9,22,23]. A detailed study on the localization of aromatase activity in the brain of the catfish revealed its presence in regions known to be involved in the regulation of reproduction [24]. Thus, the identification of neurosteroids has led to the notion that the brain can itself synthesize steroids for use as messengers. In contrast to mammals, aves and amphibians, most teleost species have two different Cyp19 genes that encode two functionally distinct aromatase isoforms, namely-cyp19a1a and cyp19a1b. Cyp19a1a is the dominant ovarian isoform, whereas cyp19alb is mostly expressed in the brain [8,9,25].
For the investigation, the air breathing stinging catfish Heteropneustes fossilis(Bloch) (Order: Siluriformes; Family: Heteropneustidae) was chosen. The catfish is an economically important edible fish. Its flesh is high on protein and low on fat content. Besides, they can be cultured to certain extent in swamps and derelict water bodies, which makes this fish ideal for commercial aquaculture on a large scale. The catfish has been investigated extensively for reproductive endocrinology and endocrinology research in India [26]. It is an annual spawner; gonad recrudescence starts with the rise in photoperiod and temperature from February to June, spawning occurs during the monsoon rainfall in July-August, followed by gonadal regression and a prolonged resting phase (November-January). Little is known about the localisation, distribution and expression of P450 aromatase in the whole brain and its regions of the adult catfish and E2 and testosterone. We examined (a) sex and seasonal variations in aromatase transcripts in the brain regions during preparatory and prespawning phases, (b) aromatase immunolocalization and in situ localisation in the brain and (c) sex and seasonal variations in brain estradiol -17β and testosterone.
Material and Methods
Animal collection and acclimation
Adult Heteropneustes fossilis is a freshwater, air breathing catfish whose reproductive cycle is divided into five phases: resting (Nov-Jan 10.5 L:13.5 D,18 ± 2°C), preparatory-early vitellogenic to vitellogenic (Feb-April, 11.5 L:12.5 D, 22 ± 2°C), prespawning- late vitellogenic (May-June, 13.5 L:10.5 D, 28 ± 2°C), spawning (July-August, 13.0 L:11.0D, 29 ± 2°C) and postspawning (Sep-Oct, 11.5 L:12.5, 22 ± 2°C) phases. Adult male and female catfish (40-50 g) were purchased from a local fish market in Chaukaghat, Varanasi. The fish were collected in January (resting), April (preparatory), May (prespawning), July (spawning) and September (post-spawning) phases. They were maintained in tanks (1X 1X 0.2 M) with circulating water under natural conditions for 48 hours after arrival to overcome stress due to transportation and fed daily with boiled goat liver ad libitum. All experiments were performed in accordance with the guidelines of the Animal Ethics Committee of Banaras Hindu University, Varanasi and all care was taken to prevent cruelty of any kind.
Chemicals and reagents
In the present study the following molecular biology kits and reagents were used : Revert-Aid Hminus first-strand cDNA synthesis kit (Fermentas, Hanover, MD, USA), DNase I, RNasefree (Ambion Inc., Austin, TX, USA), RNA later (Ambion Inc., Austin, TX, USA),RNeasy lipid tissue mini kit (Qiagen, Hilden, GmBH, Germany) and 2X PCR master mix (Fermentas, Hanover, MD, USA). The primers used in the present study were synthesized by integrated DNA Technologies (Coralville, IA, USA). Estradiol (FR E-2000) and, Testosterone (AA E-1300) were purchased from Labor Diagnostika Nord GmbH & Co.KG; Germany. Diethyl ether SRL (Sisco Research Laboratories Pvt. Ltd, Mumbai, India). Degassed and filtered nanopure water (Barnstead International, Dubuque, and IO, USA) and HPLC grade methanol (SRL), Mumbai, India was used for ELISA. Phenol, Chloroform, 75% ethanol, Agarose, tris base, glacial acetic acid, EDTA-Na2 and other chemicals were of molecular grade and locally purchased from Sigma-Aldrich Ltd., St. Louis, MO, USA.
Experimental design
Total RNA isolation and cDNA synthesis
After the acclimatization, both male and female catfish Heteropneustes fossilis (40-50 g) of all reproductive phases were killed by decapitation between 9 a.m. to 11 a.m. Whole brain and its regions such as olfactory bulb, telencephalon (minus olfactory tract and bulb), hypothalamus, pituitary and medulla oblongata were dissected [27] and were stored in RNA later solution for RNA isolation.
Whole brain (n = 5 per group) weighing 80-90mg) and pooled brain regions were used for total RNA extraction. For the pooling of brain regions each sample was constituted as follows: olfactory bulb (n = 10, 20 mg) pituitary (n = 20, 20 mg), hypothalamus (n = 3, 80-70 mg), telencephalon (n = 3, 60-70 mg) and medulla oblongata (n = 3, 60-70 mg). For each group, the sample size was n = 5. The tissues were homogenized using a T10 basic ULTRA-TURRAX homogenizer (IKA, Steaufen, Germany) in 1 mL QIAZOL (Qiagen) buffer and total RNA was isolated with RNeasy Lipid Tissue Mini Kit (Qiagen) and treated with 2U DNase I RNase-free (Ambion) for 30 min at 37°C, following the manufacturer’s protocol. The total RNA yield was determined in a Nano Drop (ND-1000 Spectrophotometer, NanoDrop Technologies, Rockland, DE, USA) and quality assessed by formamide RNA gel electrophoresis. The RNA samples with an A260/A280 ratio of 1.8-2.0 were used in the cDNA synthesis reaction.
For reverse transcription (RT), 5 μg of total RNA of each sample was reverse transcribed in a 20 μL reaction with the Revert Aid H Minus First Strand cDNA Synthesis Kit (Thermo Scientific, Lithuenia, EU), following the manufacturer’s protocol. Briefly, in a sterile nuclease-free tube on ice, the following reagents were added: total RNA template (5 μg), 1 μL of random hexamer primer (100 pM) and nuclease-free water to 12 μL. After mixing gently and centrifuging briefly, the mixture was incubated at 65°C for 5 min. Then it was chilled on ice, spinned down and the vial placed back on ice to add 4 uL of 5X reaction buffer, 1.0 μL of RiboLock RNase Inhibitor (20U/ μL), 2 μL of 10 mM dNTP Mix and 1.0 μL of Revert Aid H minus M-MuLV Reverse Transcriptase (200U/μL). The mixture was incubated for 5 min at 25°C, followed by 60 min at 42°C. The reaction was terminated by heating at 70°C for 5 min. cDNA was stored at -80°C. Transcripts encoding the brain aromatase were amplified from 1 μL cDNA using 10 pM each of a set of the gene-specific primers in 12.5 μL 2X PCR Master mixes to make 25 μL of the reaction mixture. Details of the genespecific primers for catfish brain aromatase (gene specific primers were designed with the sequences of hfcyp19b, Accession No. JN399998.2) and catfish H. Fossilis β-actin (Accession No.FJ409641.2) from Gene Bank were given in Table 1.
| Adaptation |
|
Sequence (5'-3') |
|
| Specific Primer |
H. fossilis |
Forward GTGCTGATCCAGCCTGACGTGTTCTT, |
248 bp |
| |
|
Reverse TGTCAGGAGCAGCAGCGATTACCATCTC |
|
| Probe |
|
Forward GCTGACCTACAGAAGTTGCCAGT |
722 bp |
| |
|
Reverse TCCCACGTACACAGGATTGA |
|
| DNA Control |
β-actin |
Forward TGGCCGTGACCTGACTGAC |
|
| |
|
Reverse CCTGCTCAAAGTCAAGAGCGAC |
157 bp |
Table 1: Primer used for cloning, sequencing and expression analysis of H. fossilis Brain aromatase and β-actin
The thermal conditions were 94°C for 5 min, 35 cycles of 94°C for 50 s, 55-57°C for 1 min and 72°C for 1 min, followed by 72°C for 7 min. The PCR products were resolved on 2% agarose gel and then stained with ethidium bromide to visualize the bands. Images of the gels were taken with Typhoon FLA 7000, and the signal intensity was quantified with Image Quant TL Plus image analysis software (GE Healthcare). A negative control was run without the addition of the reverse transcriptase enzyme for a subset of RNA samples to ascertain the specificity of the reaction. The PCR cycle for each gene was validated through an amplification cycle curve (data not shown). The cycle number was chosen from the exponential (log) phase of the PCR prior to the plateau phase (Figure 1).
Figure 1: Showing 248 bp of Brain aromatase amplicon size, with DNA ladder as marker
Synthesis of probes for in situ hybridization
Cloning of brain aromatase: Brain aromatase gene was amplified using gene specific primers (Table 1), which amplified 722 bp of the coding region. The amplicon was cloned into TA cloning vector pTZ57R/T using The InsTAclone™ PCR cloning Kit (Thermo Scientific, Lithuenia, EU). Briefly, the protocol included overnight ligation of the 722bp purified PCR product with the vector pTZ57R/T using T4 DNA ligase, DH5α competent cell preparation by the calcium chloride method and transformation of the competent cells with the ligation mix. After transformation, bacteria were plated into LB agar plate having ampicilin for selection of transformed bacterial colonies and X-Gal/IPTG for blue-white screening of recombinant colonies.
Screening for recombinant clones: Plasmids were prepared from the white recombinant colonies using alkaline lysis method. The orientation of ligation of the PCR product with respect to the T7 promoter sequence was known by PCR on the extracted plasmids and T7 promoter primer and brain aromatase forward primer. A positive amplification indicated reverse orientation of the brain aromatase coding strand with the T7 promoter sequence. Such plasmids were selected for generation of riboprobes.
Synthesis of brain aromatase (Hfcyp19a1b) sense and antisense probes: The selected plasmids were used as template for PCR amplification with T7 promoter primer and cyp19a1b UTR Forward primer. The resulting amplicon was purified using Nucleopore PCR clean-up gel extraction kit (Genetix) and used as template for in vitro transcription of antisense riboprobes using MAXIscript® T7 in-vitro transcription kit (Ambion). In vitro transcription was carried out in a 20 μL reaction volume using T7 polymerase and 1 μL of 10 mM stock of each ATP, CTP, GTP, UTP and Digoxigenin-11- UTP (Roche) (3.5 mM stock) , used as the labelled UTP. The reaction was allowed to proceed at 37°C for 1 hr. 1 μL of the resulting reaction was checked by agarose gel electrophoresis to confirm the formation of the riboprobe and stored at -80°C, to be used for in-situ hybridization. Sense probes were generated in the same way with the in vitro transcription reaction having the PCR product using the primer sets of hfcyp19a1b RP and T7 promoter primer as template.
Whole mount in-situ hybridization (WISH): Excised brain and olfactory bulb were fixed overnight at 4°C in freshly prepared 4% paraformaldehyde (PFA) in PBS. Tissues were then washed three times for 10 min each in PBS-T (0.01% tween-20 in PBS) on an orbital shaker, dehydrated through a methanol series, and stored in 100% methanol at -20°C prior to further processing. After rehydration, tissues were permealized in proteinase K (10 μg/mL), diluted in PBS-T to facilitate infiltration of the probe. After the proteinase K treatment, the tissues (whole brain and olfactory bulb) re-fixed in 4% PFA for 10 min. After re-fixation, the tissues were washed five times with PBS-T for 10 min each while on an orbital shaker. All these were carried out at room temperature (RT). The tissues were then placed in the hybridization buffer (50% deionized formamide, 5X SSC, 0.1% Tween 20, 9.2 M citric acid, 50 μg/mL heparin, and 0.5 mg/mL tRNA) in 2 mL microfuge tube in a water bath set at 70°C to pre-hybridize for 2 hr. Subsequently, the tissues were incubated overnight with probes diluted with hybridization buffer (1:500 dilutions) at 65°C in water bath. After the hybridization, the tissues were washed once in pre-heated hybridization buffer for 10 min. This was followed by stringency washes carried out at 65°C, where the tissues were incubated with a series of solutions having successively lower salt concentration viz. Pre-heated hybridization buffer (Hyb) diluted in 2X SSC-T (75% , 50% and finally 25% Hyb/2X SSC-T), each for 15 min. Now, all samples were incubated for 15 min in 2X SSC-T. This was followed by two 30 min incubation in 0.2X SSC-T. Following these incubations at the hybridization temperature, the samples were taken through a graded series of 0.2X SSC-T diluted in MAB-T (maleic acid buffer containing maleic acid and NaCl, pH-7.5/0.01% Tween-20) i.e., 75%, 50% and 25% 0.2X SSC-T/ MAB-T) at RT. All tissues were subsequently washed twice in MAB-T (1X) at RT for 10 min each. This was followed by blocking in MAB-T/2% blocking reagent (Roche)/10% sheep serum for 2 hrs at RT. Now, the tissues were incubated with anti- Digoxigenin- AP Fab fragments (Roche), diluted 1:2000 in MAB-T/2% blocking reagent/10% sheep serum) at 4°C overnight. After overnight incubation with the antibody, the tissues were rinsed thrice in MAB-T. This was followed by three washes in MAB-T for 1 hr each while on an orbital shaker. Now, the tissues were washed twice with alkaline phosphatase buffer (100 mM Tris, pH 9.5,100 mM NaCl), for 1 hr each. Subsequently, the tissues were incubated in a 1% NBT/BCIP stock solution (Roche) in alkaline phosphatase buffer and the reaction was allowed to proceed in dark for 2 hrs. For stopping the reaction, the tissues were washed in alkaline phosphatase buffer twice for 5 min each. Then, it was washed three times in PBS-T for 1 hr each in the dark. After this, the tissues were cleared in glycerol and photographed, under a stereo zoom binocular. The tissues were stored in glycerol.
in situ hybridization of tissue sections
Tissue processing, block preparation and sectioning:All solutions and reagents were prepared in DEPC treated water to maintain RNAse free conditions (0.1% DEPC in water at 37°C overnight and then autoclaved to remove DEPC). Paraffin sections (8 μM thick cut in transverse and sagittal plane) were deparaffinised withXylene, rehydrated through graded ethanol (100-40%) for 5 min each. After 5 min in PBS, the sections were treated with proteinase K at 40 μg/mL concentration for 7 min. This was followed by a 5 min wash in PBS and post fixation of the sections in 4% PFA in PBS for 20 min. Next, slides were washed in PBS for 5 min, followed by 2X SSC for 5 min. Then sections were quickly dehydrated in graded series of alcohol (40-100%). The slides were allowed to air dry for a short time in dust free condition before putting the probe. 100 μL of probe diluted (1:100) in hybridization buffer (50% deionized formamide/1X salts having NaCl, tris base, tris HCL, NaH2PO4, Na2HPO4, EDTA/10% dextran sulfate/1 mg/mL yeast t-RNA/1X Denhardt’s reagent) was placed in each slide and cover slip was put in such a way so as to spread the solution over the entire slide. The sections were allowed to hybridize at 65°C overnight in moist chambers made by keeping tissue papers soaked in moist chamber solution in tightly sealed box. The humidity level inside the hybridization oven was maintained. After overnight hybridization slides were washed in washing solution of 50% formamide/1X SSC/0.1% Tween 20, 3 times for 30 min each at 65°C. Next, slides were washed twice in MABT buffer (Maleic acid buffer with Tween 20) for 30 min each at room temperature. The sections were then blocked in blocking solution (2% blocking reagent, Roche/10% sheep serum prepared in MABT) for 1.5 hr at room temperature. After blocking, 100 μL of 1:2000 diluted antidigoxigenin AP antibody (Roche) (diluted in a solution of 2% blocking reagent and 1% sheep serum in MABT) was applied to the slides and coverslipped to spread the solution evenly. The antibody incubation was allowed for overnight at room temperature in a moist chamber. Overnight antibody incubation was followed by washing in MABT solution 5 times, 30 min each. This was followed by a 15 min wash in alkaline phosphatase buffer (100 mM tris pH = 9.5, 100 mM NaCl). A 1% solution of the NBT/BCIP stock solution (Roche) prepared in the alkaline phosphatase buffer was applied to the slides and the reaction was allowed to proceed in dark for 2-4 hrs. The reaction was stopped by a 5 min wash in PBS, followed by fixation in 4% PFA for 20 min. The slides were washed in PBS and mounted in 80% glycerol. Tissues were microscopically photographed under Leica DM 2000 microscope and images were taken with Leica digital camera (DFC 295) with 3- megapixel.
Mallory triple staining and immunocytochemistry
For Mallory triple staining, brain tissues were fixed in Bouin's solution (saturated picric acid: formaldehyde: acetic acid 15:5:1) for 24 h, embedded in paraffin and serially cut at 6 μm (Leica RM2035 microtome). Sections were stained with Mallory’s Trichrome. Histological observations were carried out by using a Leica DMRE microscope. For immunocytochemistry, brains were excised and fixed in Bouin’s fluid without acetic acid and processed for paraffin embedding. Sections were taken at 8 μm and were mounted on gelatin-coated slides. The sections were deparaffinized and hydrated using different downgrades of ethanol and then washed in the phosphate buffer saline (PBS) buffer (pH 7.4) for 5 min. Immunohistochemistry was performed according to the Vectastain ABC kit with some modifications, as mentioned below. The hydrated sections were immersed in 0.3% H2O2 in methanol for 45 min at room temperature to remove endogenous peroxidase activity, for elimination of non-specific binding in dark humid chamber. The sections were rinsed with PBS, three times and then blocked with blocking serum at room temperature for 1 hour. The sections were incubated with primary antibodies (1: 500; polyclonal anti-aromatase generated in tilapia) at 4°C overnight in a closed humid chamber. After overnight incubation sections were rinsed in PBS four times, biotinylated HRP conjugated-secondary antibody was applied to the sections for 45 minutes at room temperature, followed by washing with PBS four times. The sections were incubated with streptavidin- biotin horseradish peroxidase complex for 30 min at room temperature. The sections were washed with PBS three times, followed by incubation with diaminobenzidine (DAB) substrate solution. When sufficient color intensity was developed (5 minutes), then sections were washed and dehydrated with various grades of alcohol (from 50% to absolute alcohol). Then brain sections were cleared inXylene for 4 minutes and then mounted in DPX. As control, the immunostaining was done without adding primary antibody. In the control studies, no positive staining was observed. The sections were examined under a Leica DM 2000 microscope and images were taken.
Steroid hormone extraction and estimation
For steroid extraction, whole brain (n = 5) pooled brain regions: hypothalamus (n = 5), telencephalon (n = 5), medulla oblongata (n = 5), pituitary (n = 10) and olfactory bulb (n = 10) were used to make a sample (group size n = 5). The tissues were homogenized separately or group-wise in 4 vol of cold PBS (0.02 M, Phosphate buffer saline, pH 7.4) with an ultrasonic homogenizer (XL-2000 Microson, Misomix, USA) at 0°C for 5-10 sec. The homogenate was centrifuged at 5000 g for 20 min at 4°C. Blood plasma and homogenates of brain and its regions were extracted with 3 volume of diethyl ether, three times. The ether phase was collected, pooled, evaporated and dried under N2 gas and stored at -20C till further processed for steroid estimation. After the steroid extraction samples were processed for estimation of steroid hormones (testosterone, estradiol), using the LDN GmBH, ELISA kit according to the manufacturer’s protocol and absorbance was taken at 450 nm using a Multiscan microplate reader using a Multiscan EX (Thermo Lab system) or (Thermo Electron Corporation, USA) ELISA reader. The standard curve was plotted and readings were taken with the help of the software.
Testosterone (T) ELISA: The assay was carried out using an ELISA kit for testosterone. Briefly, 50 μL each of testosterone (T) standard (0, 0.1, 0.5, 2.0, 6.0, 20.0 ng/mL) and reconstituted samples were transferred to the antitestosterone IgG-coated plate. The immunoreactions was started by adding 50 μl of testosterone-HRP conjugate solution into each well, followed by incubation at 37°C for 1 h on a plate shaker. The content from each plate was removed and washed with 300 μL of distilled water, 3 times. Water was completely drained out from each well. Next, 100 μl TMB substrate was dispensed into each well and incubated at room temperature for 15-20 m in dark on a plate shaker. Color development was stopped by adding 50 μL of stop solution (1 M sulphuric acid). Absorbance was taken at 450 nM using a Multiscan microplate reader (Thermo Electron Corporation, USA).
Estradiol-17β (E2) ELISA: E2 was assayed using an ELISA kit according to the manufacturer’s instructions. Briefly, samples were reconstituted and 50 μL sample and E2 standard (0, 20, 100, 300, 800 and 3200 pg/mL) each was pipetted into the anti-E2 Immunoglobulin G (IgG-coated plate wells. The immunoreactions was started by adding 100 μL of E2-HRP conjugate solution to each well, followed by incubation at 37°C for 1 h on a plate shaker. The content from each plate was removed and washed with 300 μl of wash buffer, 3 times. Wash buffer was completely drained out from each well. Next 150 μL tetramethylbenzidine (TMB) substrate was dispensed into each well and incubated at room temperature for 10-15 min on a plate shaker. Color development was stopped by adding 50 μL of stop solution (1 M sulphuric acid). Absorbance was taken at 450 nM using a Multiscan microplate reader (Thermo Electron Corporation, USA).
Validation of steroid hormone assays: The responseconcentration curves for E2 and testosterone were linear over 20-3200 pg/mL, 0.1-20 ng/mL, respectively. The sensitivity of the E2 and testosterone assays was 10 pg/mL, 0.022 ng/mL, respectively. Cross reactivity of the E2 antibody was, estradiol-17β-100%, estriol-1.6%, estrone-1.3%, progesterone-0.1%, cortisol-0.1%, testosterone-0%, Deoxycorticosterone-0% and 17, 20β-DP-0%. Cross reactivity of the testosterone antibody was, testosterone-100%, 5α- DHT-5.2%, androstenedione-1.4%, androstanediol-0.8%, progesterone-0.5%, androsterone-0.1%, E2-0.001% and 17, 20β-DP-0%. Known concentrations of E2, testosterones were processed in the same manner as samples. Percentage recovery was: E2 (77.417 pg/mL added) 98.2 ± 1% (n = 5), testosterone (1.1 ng/mL added) 94 ± 3% (n = 5). The coefficients of inter and intra-assay variations were 9.2%, 9.9% for E2, 7.7%, 7.3% for testosterone.
Statistical analysis
The data of gene expression of the cyp19a1b transcripts were normalized with β-actin (mean ± SEM, n = 5). Data were tested for statistical significance using the statistical Package for the Social Sciences software program (version 10.0; SPSS). The data were analyzed by one or two-way analysis of variance (ANOVA; p < 0.001), followed by Tukey’s or Newman-Keuls’ test, to compare differences between the groups at a significance level of p < 0.05.
Results
Sex and seasonal variation in brain aromatase (cyp19a1b) transcript level
Brain aromatase cyp19a1b showed significant dimorphic and seasonal variations in the catfish brain (Figures 2A and 2B). Sexually dimorphic expression of cyp19a1b was observed in the brain with females having greater expression than males, (P < 0.001). The mRNA level was highest in the preparatory phase (April) in females and low in other phases. In male, it was highest in the preparatory phase and decreased in the prespawning phase. The levels in the resting and prespawning phases are not significantly different. In female, the level was maximum in preparatory phase, lowest in prespawning phase.
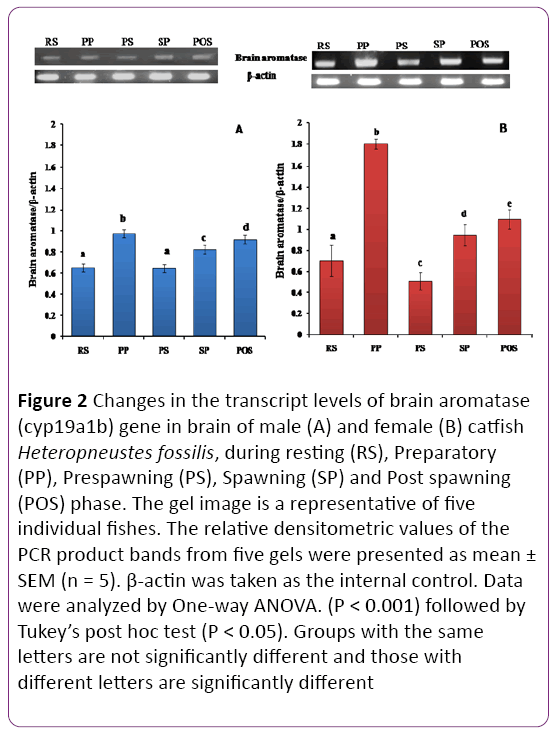
Figure 2: Changes in the transcript levels of brain aromatase (cyp19a1b) gene in brain of male (A) and female (B) catfish Heteropneustes fossilis, during resting (RS), Preparatory (PP), Prespawning (PS), Spawning (SP) and Post spawning (POS) phase. The gel image is a representative of five individual fishes. The relative densitometric values of the PCR product bands from five gels were presented as mean ± SEM (n = 5). β-actin was taken as the internal control. Data were analyzed by One-way ANOVA. (P < 0.001) followed by Tukey’s post hoc test (P < 0.05). Groups with the same letters are not significantly different and those with different letters are significantly different
Sex and seasonal variations in brain regions
Transcript abundance of cyp19a1b was investigated in both male and female brain regions during the preparatory (Figures 3A and 3B) and prespawning (Figures 4A and 4B) phases of the reproductive cycle. There was a significant sex-dependent brain regional difference in the preparatory phase (Figures 3A and 3B; P < 0.001). In males, the olfactory bulb and medulla oblongata showed the highest transcript abundance in comparison to other brain regions. The pituitary recorded the lowest level, followed by hypothalamus and telencephalon. In females, the expression pattern was quite different. The expression was the highest in the pituitary and lowest in the olfactory bulb and moderate in the hypothalamus, telencephalon and medulla oblongata (P < 0.05).
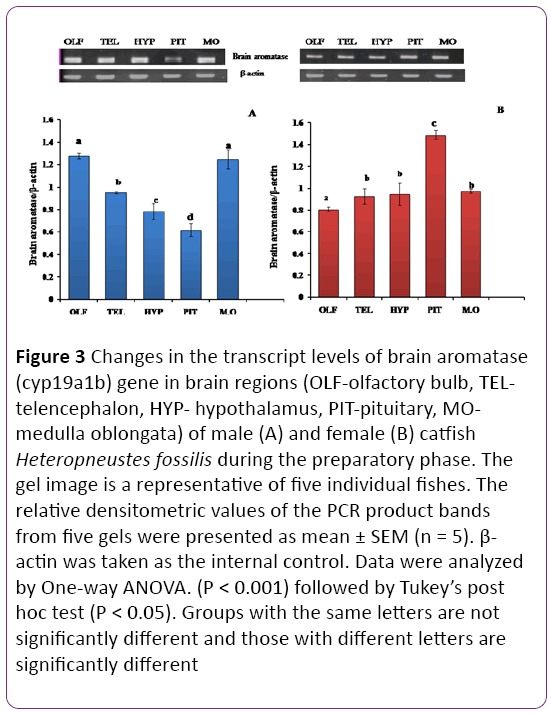
Figure 3: Changes in the transcript levels of brain aromatase (cyp19a1b) gene in brain regions (OLF-olfactory bulb, TELtelencephalon, HYP- hypothalamus, PIT-pituitary, MOmedulla oblongata) of male (A) and female (B) catfish Heteropneustes fossilis during the preparatory phase. The gel image is a representative of five individual fishes. The relative densitometric values of the PCR product bands from five gels were presented as mean ± SEM (n = 5). β- actin was taken as the internal control. Data were analyzed by One-way ANOVA. (P < 0.001) followed by Tukey’s post hoc test (P < 0.05). Groups with the same letters are not significantly different and those with different letters are significantly different
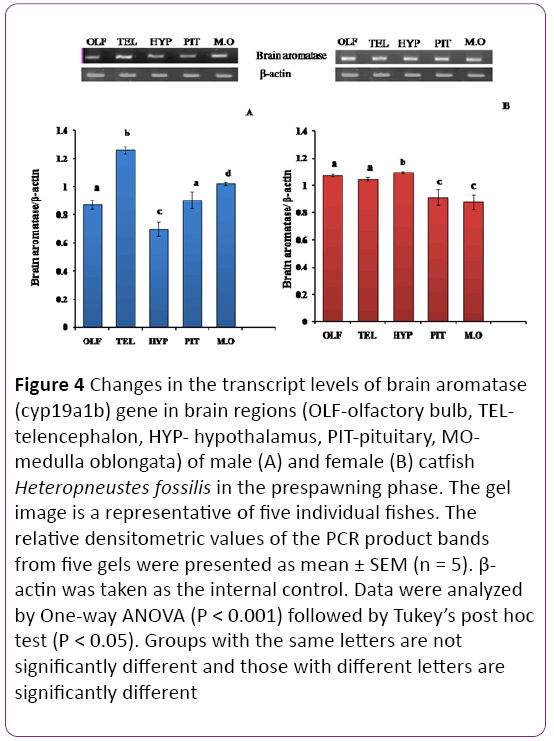
Figure 4: Changes in the transcript levels of brain aromatase (cyp19a1b) gene in brain regions (OLF-olfactory bulb, TELtelencephalon, HYP- hypothalamus, PIT-pituitary, MOmedulla oblongata) of male (A) and female (B) catfish Heteropneustes fossilis in the prespawning phase. The gel image is a representative of five individual fishes. The relative densitometric values of the PCR product bands from five gels were presented as mean ± SEM (n = 5). β- actin was taken as the internal control. Data were analyzed by One-way ANOVA (P < 0.001) followed by Tukey’s post hoc test (P < 0.05). Groups with the same letters are not significantly different and those with different letters are significantly different
In the prespawning phase the telencephalon showed the highest transcript abundance and the pituitary showed the lowest expression in males; other regions expressed inbetween range (Figures 4A and 4B; P < 0.001). In females, the expression was high in the olfactory bulb, telencephalon and hypothalamus and low in the pituitary and medulla oblongata.
Localization of brain aromatase by immunocytochemistry
In the Mallory’s trichrome stain, basophilic (blue) and acidophilic (magenta) cells were identified (Figures 5A-5D). Gonadotrophs (blue cells) are distributed in the proximal pars distalis (PPD) along with thyrotropes and somatotropes. By using polyclonal antibodies raised against ovarian aromatase in tilapia, aromatase protein was detected in the proximal pars distalis (PPD) region of the pituitary; however there was no immunostaining in control (Figures 5E and 5F).
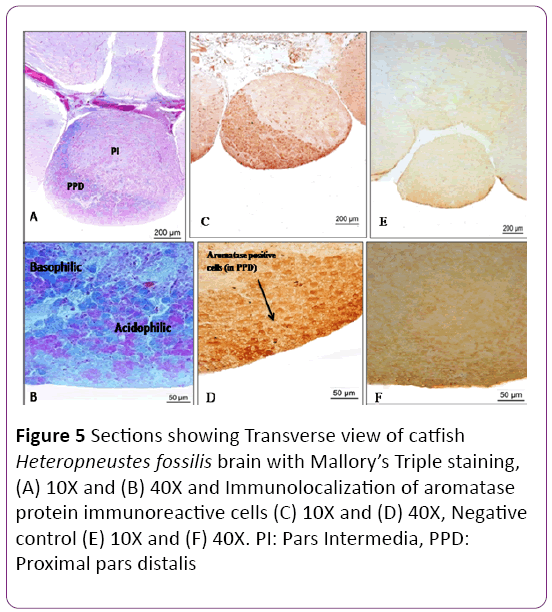
Figure 5: Sections showing Transverse view of catfish Heteropneustes fossilis brain with Mallory’s Triple staining, (A) 10X and (B) 40X and Immunolocalization of aromatase protein immunoreactive cells (C) 10X and (D) 40X, Negative control (E) 10X and (F) 40X. PI: Pars Intermedia, PPD: Proximal pars distalis
Whole-mount brain in situ hybridization of cyp19a1b
A positive signal (a blue precipitate) was detected in the microphotographs of whole-mount in situ hybridization of cyp19a1b mRNA in the brain of female catfish. Cyp19a1b was strongly expressed in the ventral region of telencephalon of forebrain and olfactory bulb (Figure 6A (ventral view) and 6B). Strong signal of cyp19a1b was detected in the olfactory bulb and telencephalon, area ventralis telencephali (Vs) and pars ventralis (Vv). In the diencephalon, expression of cyp19a1b was detected in the preoptic area and periventricular hypothalamus. Transcript abundance of cyp19a1b was readily detected in the pituitary (proximal pars distalis, PPD) of both male and female but there were no apparent differences associated with sex (data not shown). A sense probe was also taken to compare the detection of brain aromatase gene in the whole brain. There was no signal detected with the sense probe of brain aromatase gene (Figures 6C and 6D).
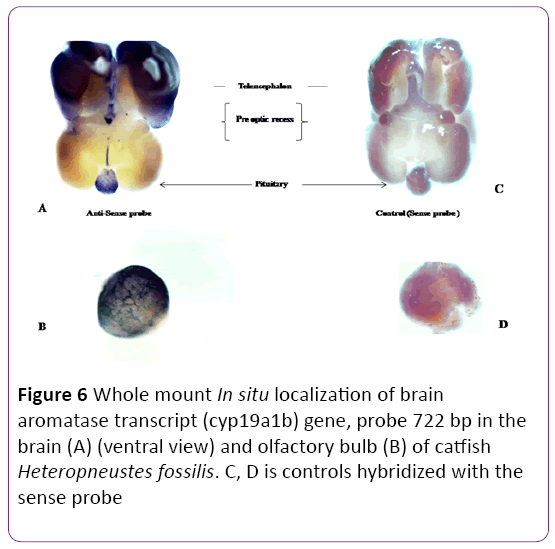
Figure 6: Whole mount In situ localization of brain aromatase transcript (cyp19a1b) gene, probe 722 bp in the brain (A) (ventral view) and olfactory bulb (B) of catfish Heteropneustes fossilis. C, D is controls hybridized with the sense probe
Section in situ hybridization
There are four zones of the optic tectum, superficial white and grey zone (SWGZ), central zone (CZ), deep white zone (DWZ) and periventricular grey zone (PGZ) (Figure 7). High abundance of cyp19a1b transcript was detected in the periventricular grey zone of the optic tectum, where non neuronal cells, called tanycyte or ependymo-glial cells are present (Figures 7A and 7B). In the preoptic area and along the third ventricle of the hypothalamus expression of cyp19a1b was observed in moderate level near the nucleus lateralis tuberis (NLT) and nucleus recessus lateralis region (NRL) (Figures 8A-8D). There was no signal in the cerebellum and a low expression was noticed in the brain stem. In the hypothalamus (Figures 8A and 8B) and medulla oblongata (Figures 8C and 8D), a strong signal of cyp19a1b was expressed. Intense signal was observed in proximal pars distalis region of pituitary (Figures 9A and 9C) , with negative control (Figures 9B and 9D) In general, transcript abundance of cyp19a1b was highest in the olfactory bulb, ventricular lining of the telencephalon and hypothalamus and also in the medulla oblongata. There was no signal detected with sense probe of brain aromatase gene in brain sections (Figures 7C and 7D) and (Figures 9B and 9D).
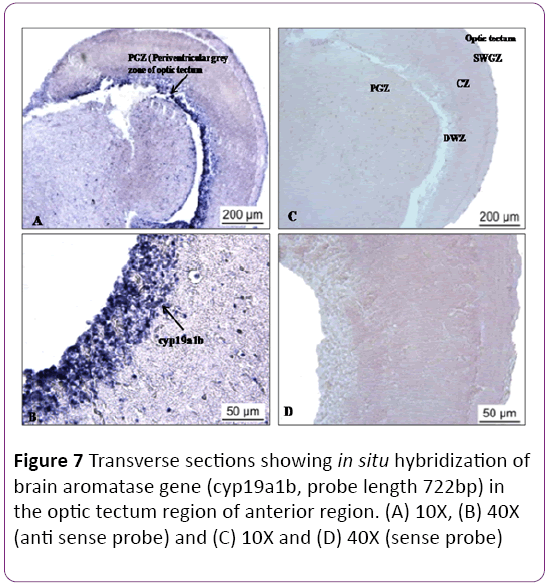
Figure 7: Transverse sections showing in situ hybridization of brain aromatase gene (cyp19a1b, probe length 722bp) in the optic tectum region of anterior region. (A) 10X, (B) 40X (anti sense probe) and (C) 10X and (D) 40X (sense probe)
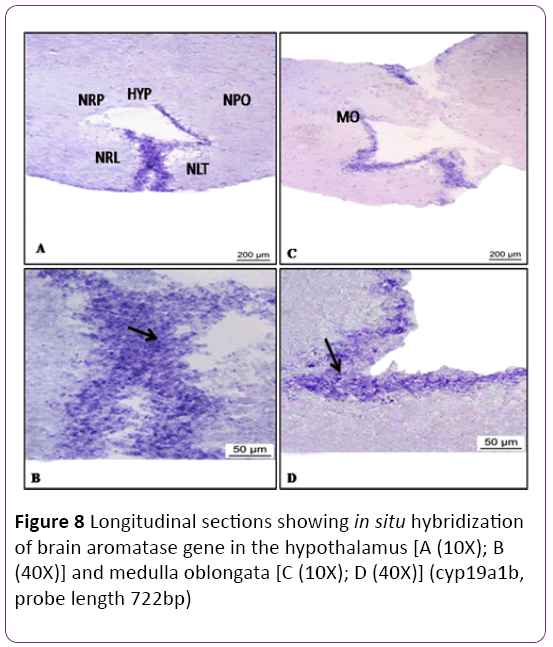
Figure 8: Longitudinal sections showing in situ hybridization of brain aromatase gene in the hypothalamus [A (10X); B (40X)] and medulla oblongata [C (10X); D (40X)] (cyp19a1b, probe length 722bp)
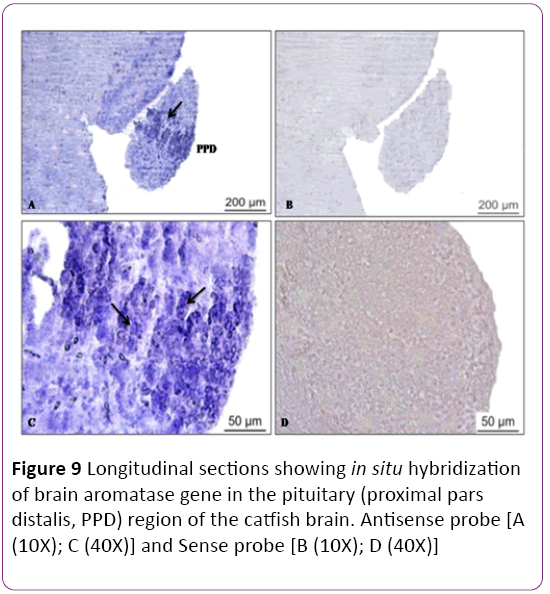
Figure 9: Longitudinal sections showing in situ hybridization of brain aromatase gene in the pituitary (proximal pars distalis, PPD) region of the catfish brain. Antisense probe [A (10X); C (40X)] and Sense probe [B (10X); D (40X)]
Sex and seasonal variations in brain T and E2 levels
Testosterone (T) level showed an overall significant seasonal variation (Figure 10A P < 0.001), with no significant changes between the sexes. The T level was higher in males in the preparatory and prespawning phases but lowest was in spawning phase, however in female the T level was increased from the resting phase to the prespawning phase, decreased in the spawning phase. Highest level of T was in the postspawning phase in female.
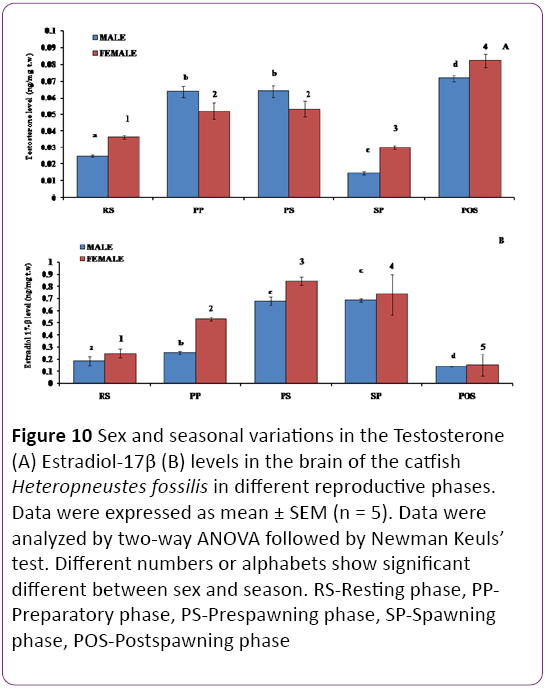
Figure 10: Sex and seasonal variations in the Testosterone (A) Estradiol-17β (B) levels in the brain of the catfish Heteropneustes fossilis in different reproductive phases. Data were expressed as mean ± SEM (n = 5). Data were analyzed by two-way ANOVA followed by Newman Keuls’ test. Different numbers or alphabets show significant different between sex and season. RS-Resting phase, PPPreparatory phase, PS-Prespawning phase, SP-Spawning phase, POS-Postspawning phase
There is an overall significant variation in E2 level in both sexes of the catfish during different reproductive phases (Figure 10B; P < 0.001). E2 levels were significantly higher in females than males in the preparatory and prespawning phases. In both sexes, the E2 level increased from the resting to prespawning phases and decreased through the spawning phase to the lowest level in the postspawning phase (P < 0.05).
Sex and seasonal variations of T level in brain regions
Olfactory bulb: The T level showed both seasonal and sex variations (Figure 11A, P < 0.001). The T level was significantly higher in females compared to males in the resting to prespawning phases, similar in the spawning phase and higher in males in the postspawning phase. In females, a highly significant seasonal pattern with the peak level in the prespawning phase was noticed. However, in males, there was no significant change in the T level from the preparatory to postspawning phases. The lowest level was noticed in the resting phase.
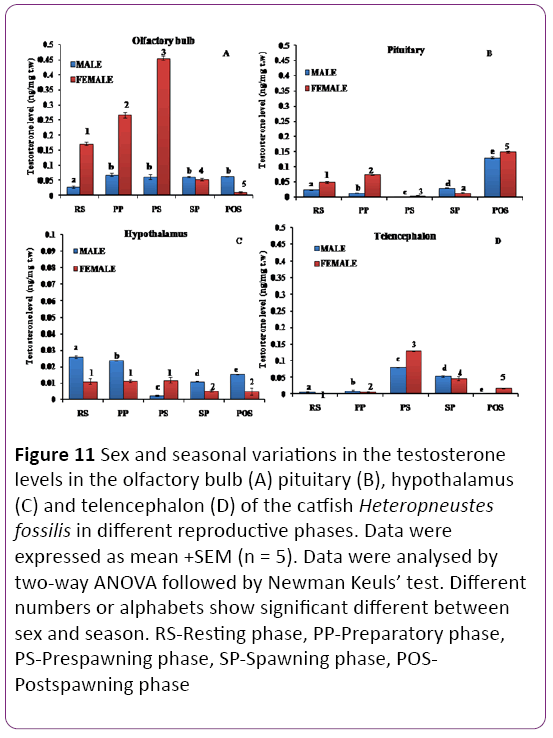
Figure 11: Sex and seasonal variations in the testosterone levels in the olfactory bulb (A) pituitary (B), hypothalamus (C) and telencephalon (D) of the catfish Heteropneustes fossilis in different reproductive phases. Data were expressed as mean +SEM (n = 5). Data were analysed by two-way ANOVA followed by Newman Keuls’ test. Different numbers or alphabets show significant different between sex and season. RS-Resting phase, PP-Preparatory phase, PS-Prespawning phase, SP-Spawning phase, POSPostspawning phase
Pituitary: There was a significant dimorphic variation in testosterone level (Figure 11B; P < 0.001) in the different reproductive phases. The testosterone level was the highest in both males and females in the postspawning season and the lowest in the prespawning season.
Hypothalamus: The T level showed significant sexual dimorphic variations (Figure 11C; P < 0.001) during the reproductive cycle. The T level was higher in males in all phases except the prespawning phase in which females showed higher levels. The T level was the highest in the resting phase, decreased in the preparatory to give the lowest level in the prespawning phase and increased in spawning postspawning phases in males. In females, the T level was higher and remained unchanged in the resting, preparatory and prespawning and declined in the spawning and postspawning phases.
Telencephalon: There were significant changes in T level in the telencephalon (Figure 11D; P < 0.001). The T level was the lowest in both sexes in the resting and preparatory phases, recorded the highest level in the prespawning phase to decline in the spawning and postspawning phases. A dimorphic variation was prominent in the prespawning and postspawning phases with the females having higher levels.
Medulla oblongata: The T level showed significant sexual dimorphic variations (Figure 13A; P < 0.001). In both male and female catfish, the T level was the highest in the postspawning phase and the lowest in the resting phase. In female catfish, the T level did not vary in the preparatory, prespawning and spawning phases, however in males significant changes were observed.
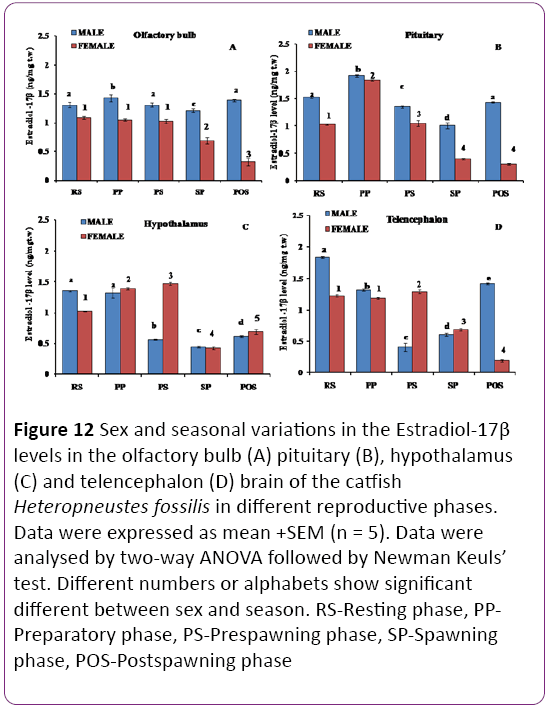
Figure 12: Sex and seasonal variations in the Estradiol-17β levels in the olfactory bulb (A) pituitary (B), hypothalamus (C) and telencephalon (D) brain of the catfish Heteropneustes fossilis in different reproductive phases. Data were expressed as mean +SEM (n = 5). Data were analysed by two-way ANOVA followed by Newman Keuls’ test. Different numbers or alphabets show significant different between sex and season. RS-Resting phase, PPPreparatory phase, PS-Prespawning phase, SP-Spawning phase, POS-Postspawning phase
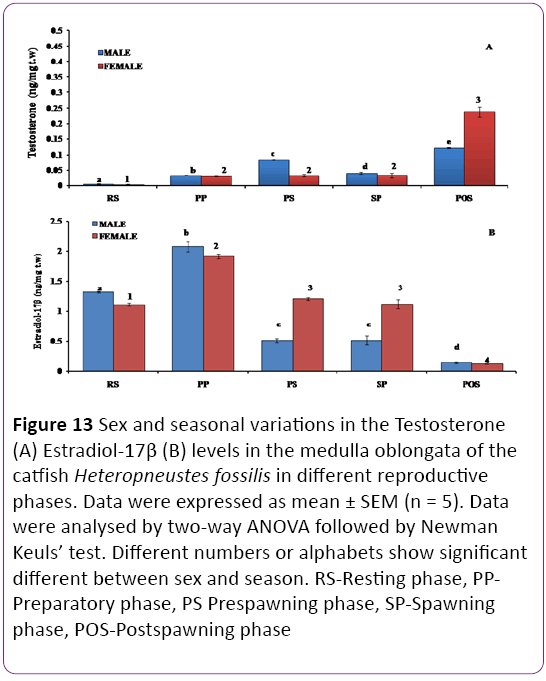
Figure 13: Sex and seasonal variations in the Testosterone (A) Estradiol-17β (B) levels in the medulla oblongata of the catfish Heteropneustes fossilis in different reproductive phases. Data were expressed as mean ± SEM (n = 5). Data were analysed by two-way ANOVA followed by Newman Keuls’ test. Different numbers or alphabets show significant different between sex and season. RS-Resting phase, PPPreparatory phase, PS Prespawning phase, SP-Spawning phase, POS-Postspawning phase
Sex and seasonal variations in E2 level
Olfactory bulb: The E2 level in olfactory bulb showed significant seasonal and sexual variations (Figure 12A, P < 0.001). Male catfish showed the highest level in all phases than females with the highest level in the preparatory phase and it varied significantly or insignificantly in other phases. In females, the E2 level was higher and insignificant different in the resting, preparatory and prespawning phases and decreased in the spawning phase to give the lowest values in the postspawning phase.
Pituitary: The E2 level showed dimorphic changes (Figure 12B; P < 0.001) with males having significantly higher levels except in the preparatory phase. In both sexes, the E2 level was the highest in the preparatory phase and decreased to the lowest level in the spawning phase (males) or postspawning phase (females).
Hypothalamus: The E2 level showed significant sexual dimorphic variations (Figure 12C; P < 0.001). The levels were higher in females (preparatory, prespawning and postspawning phases), or similar (spawning phase) or lower (resting phase). In females, the level increased from the resting phase to the prespawning phase and decreased in the spawning phase to increase in the postspawning phase. In males, the levels remained high in the resting and preparatory phase and decreased in the prespawning and spawning phase to increase in the postspawning phase.
Telencephalon: There were significant sexual dimorphic changes in the E2 level (Figure 12D; P < 0.001). The E2 level is significantly higher in males (resting, preparatory and postspawning phases) and lower in the prespawning and spawning phases when compared to the females. In males, the E2 level was the highest in the resting phase and decreased to the lowest level in the prespawning phase to increase again in the spawning and postspawning phase.
In females, the E2 level did not vary in the resting and preparatory phases, transiently increased in the prespawning phase and decreased drastically in the spawning and postspawning phases.
Medulla oblongata: The E2 level showed significant sexual dimorphic variations (Figure 13B; P < 0.001) with significantly higher levels in females in the prespawning and spawning phases. In both sexes, the highest concentration was noticed in the preparatory season and the lowest in the postspawning season. In both sexes, there was no significant change in the E2 concentration during the prespawning and spawning seasons.
Discussion
The present study shows that the catfish brain is potentially a steroidogenic organ having the ability to produce neuroestrogens, as evident from the demonstration of testosterone (substrate), E2 (product) and P450 aromatase, the enzyme responsible for aromatization of testosterone to E2. Aromatases (cyp19a1a and cyp19a1b) are crucial for regulation of estrogen levels and are key players in sexual differentiation and reproduction in teleosts.
Sex and seasonal variation of aromatase (cyp19a1b) transcript level in the brain and its regions
In our study, brain cyp19a1b transcript level peaked in the early vitellogenic stage and declined in the late vitellogenic phase in females. Males showed a similar pattern. A similar result of seasonal variation in cyp19a1a (ovarian) transcripts peaking in vitellogenic oocytes was observed in zebrafish [28]. With respect to the brain type (cyp19a1b), the transcript levels exhibited seasonal variation peaking in the preparatory phase, although aromatase activity was low when compared with that in the ovary [29]. Similar seasonal pattern of cyp19a1b transcript levels was reported in goldfish and channel catfish with mRNA levels peaking prior to the spawning phase [28,30,31]
Generally, female brain showed more transcript levels of cyp19a1b than males. Female goldfish, longhorn sculpin (Myoxcephalus octodecimspinous) and stickleback (Gasterosteus aculeatus) have higher aromatase activity in the telencephalon and preoptic area (POA), respectively than males, while male and female European sea bass show the opposite relationship [32-36]. In medaka (Oryzias latipes), females have higher activity levels in homogenates of brain slices that contain periventricular hypothalamic areas, while males have higher levels in slices which contain the posterior preoptic area and suprachiasmatic nucleus (Melo and Rams dell. 2001). In zebrafish, although quite variable, most males have higher aromatase mRNA in the telencephalon and hypothalamus compared to females [28], and brain aromatase may play a role in sex differentiation [37]. Similarly, male pejerrey (Odontesthes bonariensis) has higher mRNA expression in forebrain and midbrain homogenates [38]. The sexually dimorphic expression of cyp19a1b in the catfish brain suggests a sex-specific role for cyp19a1b, perhaps related to gonadotropin secretion and sexual behaviours. In agreement with this suggestion, a study in medaka and zebrafish has indicated there is a rapid and ephemeral change in cyp19a1b mRNA expression associated with female spawning [28]. It is also known that mice with impaired aromatase function in the brain were deficient in male sexual behaviour. Collectively these observations imply that aromatase (primarily cyp19a1b in fish) plays a crucial role in the reproductive functions of the brain in both sexes.
In channel catfish, synchronous seasonal pattern of cyp19a1b was related to regulating the gonadotropin levels directly or indirectly by local production of estrogen in brain, thus implying a plausible role of brain aromatase in the brainpituitary- gonadal axis [31]. This is in contrast to goby fish, where significant correlations between isoforms-specific transcript levels and aromatase activity were observed in the ovary but not in the brain [39]. Brain type aromatase (cyp19a1b) in goldfish was reported to be involved in regulating seasonal reproductive cyclicity [30], implying both isoforms of aromatase gene are related to steroidal events involved in reproduction and sexual differentiation. Very high expression of cyp19a1b in H. fossilis brain tissue implies that this gene is directly responsible for estrogen synthesis in the brain. Previous studies indicate that estrogen in the brain of teleosts contributes to several neural functions, including continuous neurogenesis, migration, sexual plasticity and regeneration of the brain in teleosts [30].
In brain regions from anterior to posterior, a significant variation in transcript abundance of cyp19a1b gene was noticed between the preparatory phase and prespawning phases in both sexes. During the preparatory phase, higher transcript abundance of cyp19a1b was observed in the olfactory bulb and medulla oblongata of males followed by the telencephalon, hypothalamus and pituitary. On the other hand in females, the highest abundance was in the pituitary, moderate level in the telencephalon, hypothalamus and medulla oblongata, and the lowest level in the olfactory bulb. During the prespawning season, higher transcript abundance was found in the telencephalon, followed by medulla oblongata, pituitary and olfactory bulb, and the lowest in the hypothalamus of the males. In females, the highest cyp19a1b transcript level was in the olfactory bulb, hypothalamus and telencephalon, and the lowest in the pituitary and medulla oblongata. Thus, the expression of cyp19a1b varied with sex, the brain region as well as with the reproductive phase. The olfactory bulb and telencephalon are concerned with reproductive behaviour of teleosts through pheromonal activity, the hypothalamus is concerned with pituitary regulation and the medulla oblongata is concerned with motor activity and transmission of afferent and efferent information.
Localization of brain aromatase in the brain and its regions
In the present study, whole-mount and section in situ hybridization showed a defined pattern of localization ofcyp19a1btranscripst in the brain. High transcript abundance was demonstrated in the olfactory bulb-ventral telencephalonpreoptic area, periventricular hypothalamus and optic tectum. However, lower expression of brain aromatase was detected in the medulla oblongata with no detection in the cerebellum. Forlano et al. [40] showed in midshipman fish that the distribution of aromatase was consistently found throughout the ventricular areas of the brain and lined the entire periphery of the telencephalon. Aromatase activity was found at relatively high levels in both (tectum and torus semicircularis) of the African catfish (Clarias gariepinus) but absent in the tectum of stickleback.
Our observations are consistent with the studies conducted on pejerrey, Odontesthes bonariensis [38] and bluehead wrasse, Thalassoma bifasciatum [41]. in situ hybridization in rainbow trout [42] has shown clearly that aromatase is expressed in the ependymal layer of the periventricular regions of the forebrain, unlike mammals and birds. This is likely to be true in the zebrafish since a strong signal of cyp19a1bmRNA was reported around the brain ventricles. In contrast to mammals, the brains of teleost fish exhibit high aromatase activity and expression that peak in adult animals with high steroid levels. Callard and his colleagues were the first to report the exceptionally high aromatase activity in the brain of teleost fishes [36,43].
In teleosts cyp19a1b gene is expressed only in radial glial cells of the developing and adult fish [19,40,44]. Aromatase B expression in radial glial cells has been also reported in zebrafish using in situ hybridization and immunohistochemistry and also in transgenic fish [19,44,45].
Such cells are observed in abundance in the pallial and subpallial regions in the preoptic area and mediobasal hypothalamus and also have been observed in the thalamus, the periventricular layer of torus semicircularis, the optic tectum and the fourth ventricle [19,45]. In goldfish brains, aromatase was detected in neurons using a human-specific antibody, but recent data showed that fish specific brain aromatase antibody only stained glial cells and not the neurons in midshipman and rainbow trout, [42]. Radial glial cells, many of which express brain type aromatase sustain high neurogenic activity in adult fish and this proliferation is not restricted to the telencephalon, but has been also seen in all forebrain regions [46]. Continuous neurogenesis is known to occur throughout the fish’s lifetime. Differential expression of aromatase in the medial hypothalamus, preoptic area, septum and amygdala (homologous of the ventral and suprachiasmatic nuclei of ventral telencephalon in fish) is an important component in the development and expression of male reproductive behaviour [47-49]. Aromatase activity is considered an indicator of estrogen synthesis [50] and can be correlated with the expression level of mRNA [30].
In the present study, we showed that cyp19a1b transcript was localised in the proximal pars distalis of the pituitary, which is consistent with the studies in zebrafish [30]. Similarly, aromatase immunoreactivity was also noticed in the PPD. Both the methods seem to stain the same pituitary cells where the majority of the gonadotrophs are located. The results show that the gonadotrophs may be a source of E2, as reported in tilapia [51]. These findings led us to propose that the local production of E2 in the brain and pituitary in the catfish may have important roles in regulating gonadotropin secretion directly or indirectly.
Sex and seasonal variations in E2 and T levels
Brain E2 and T levels significantly fluctuated seasonally and with reproductive state in teleosts, implying an important role in reproductive physiology. In the present study, the steroids showed significant seasonal variations in both male and female brain throughout the reproductive cycle. E2 concentration was higher in the brain in comparison to testosterone, implying a higher rate of aromatization. In both males and females, T level was the highest in the postspawning, preparatory and prespawning seasons and lowest in the spawning and resting phase. E2 level was the highest in female brain in comparison to male and follows a decreased pattern in the order prespawning > spawning > preparatory > resting phase, and the lowest level was observed in the post spawning phase. Similar pattern was observed in males.
Our data of brain regions also showed differential levels of T and E2 showing that these steroids might play an important role in modulation of region-specific reproductive and nonreproductive functions. It is difficult to relate the regional variations to specific functions but the data would provide a basis for future experimental studies. In the present study, cyp19a1b transcripts, E2 and T exhibited sexually dimorphic expression with reproductively active females expressing significantly higher levels, an observation consistent with those in zebrafish [52] and medaka [53,54]. However, some studies exploring differences between corresponding sub-regions of brains have reported higher cyp19a1b expression in males than in females of pejerrey [38], zebrafish [28] and Atlantic halibalt [55]. Nevertheless, the differences across species and between sexes have been attributed to sex-linked seasonal variations of cyp19a1b expression associated with reproductive cycles as aromatase expression varies with the reproductive status of individual fish [56,57]. The significance of E2 in male brain needs future investigations.
Conclusions
In conclusion, the high expression of aromatase gene and steroid hormones (E2 and T), implies that catfish brain is involved in neuroestrogen biosynthesis from testosterone by aromatization. The neuroestrogens may contribute to several neural functions, including continuous neurogenesis, migration, sexual plasticity, regeneration of the brain, regulation sexual and non-sexual behaviour etc. Neuroestrogens may form a functional estrogen milieu taking part in feedback control of neuropeptides and neurotransmitters directly or indirectly involved in gonadotropin secretion. A functional study is required in future to demonstrate the neural functions of the locally produced estrogens.
Conflict of Interest
Authors declare no conflict of interest.
Acknowledgements
SM is a recipient of UGC-BSR Research Fellowships for meritorious students (RFSMS), Department of Zoology, Banaras Hindu University.
This work was partially supported by a UGC and DST -WOS A grant to RC, New Delhi.
8686
References
- Balthazart J, Ball GF (2006) Is brain estradiol a hormone or a neurotransmitter? Trends Neurosci29: 241-249.
- Blazquez M, Piferrer F (2004) Cloning, sequence analysis, tissue distribution, and sex-specific expression of the neural form of P450 aromatase in juvenile seabass (Dicentrarchuslabrax), Mol Cell Endocrinol 219: 83-94.
- Borg B, Timmers RJ, Lambert JG (1987) Aromatase activity in the brain of the three-spined stickleback, Gasterosteusaculeatus. II. Effects of castration in winter.ExpBiol47: 69-71.
- Callard GV, Petro Z, Ryan KJ (1978) Phylogenetic distribution of aromatase and other androgen- converting enzymes in the central nervous system, Endocrinology 103: 2283-2290.
- Callard GV, Petro Z, Ryan KJ (1981)Estrogen synthesis in vitro and in vivo in the brain of a marina teleost (Myoxocephalus), Gen Comp Endocrinol 43: 243-255.
- Callard GV (1983) Androgen and estrogen actions in the vertebrate brain, Am Zool 23: 607-620.
- ChangXT, Kobayashi T, Kajiura H, Nakamura M, Nagahama Y (1997) Isolation and characterization of the cDNA encoding the tilapia (Oreochromisniloticus) cytochrome P450 aromatase (P450arom): changes in P450arom mRNA, protein and enzyme activity in ovarian follicles during oogenesis, J MolEndocrinol 18: 57-66.
- Chaube R, Mishra S (2012)Brain steroid contents in the catfish Heteropneustesfossilis: sex and gonad stage-specific changes, Fish PhysiolBiochem 38: 757- 767.
- Chaube R, Joy KP (2002) Effects of ovariectomy and oestradiol-17β replacement on brain tyrosine hydroxylase in the catfish Heteropneustesfossilis: changes in in vivo activity and kinetic parameters, J Endocrinol 175: 329-342.
- Cheshenko KF, Pakdel H, Segner O, Kah RI, Eggen (2008) Interference of endocrine disrupting chemicals with aromatase CYP19 expression or activity, and consequences for reproduction of teleost fish, Gen Com Endocrinol 155: 31-62.
- Compagnone NA, Mellon SH (2000) Neurosteroids Biosynthesis and functions of these novel neuromodulators, Front Neuroendocrinol 21: 1-56.
- Cornil CA, Dalla C, Papadopoulou-Daifoti Z, Baillien M, Dejace C, et al. (2005) Rapid decreases in preoptic aromatase activity and brain monoamine concentrations after engaging in male sexual behavior, Endocrinology 146: 3809-3820.
- Corpéchot C, Synguelakis M, Talha S, Axelson M, Sjovall J, et al. (1983) Pregnenolone and its sulfate ester in rat brain, Brain Res 270: 119-125.
- Diotel N, Do Rego JL, Anglade I, Vaillant C, Pellegrini E (2011) The brain of teleost fish, a source, and a target of sexual steroids, Front Neurosci 5: 137.
- Diotel N, Le Page Y, Mouriec K, Tong SK, Pellegrini E, et al. (2010) Aromatase in the brain of teleost fish: expression, regulation and putative functions, Front Neuroendocrinol 31:172-192.
- Do Rego JL, Seong JY, Burel D, Leprince J, Luu-The V, et al. (2009)Neurosteroid biosynthesis : enzymatic pathways and neuroendocrine regulation by neurotransmitters and neuropeptides, Front Neuroendocrinol300: 259-301.
- Do Rego JL, Vaudry H (2015) Comparative aspects of neurosteroidogenesis: From Fish to Mammals, Gen Comp Endocrinol 5: 0-14.
- Forlano PM, Deitcher DL, Myers DA, Bass AH (2001) Anatomical distribution and cellular basis for high levels of aromatase activity in the brain of teleost fish: aromatase enzyme and mRNA expression identify glia as source, J Neurosci 21: 8943-8955.
- Forlano PM, Schlinger BA, Bass AH (2006) Brain aromatase: new lessons from non-mammalian model systems, Front Neuroendocrinol27: 247-274.
- Gelinas D, Pitoc G, Callard G (1998) Isolation of a goldfish brain cytochrome P450 aromatase cDNA: mRNA expression during the seasonal cycle and after steroid treatment, Mol Cell Endocrinol 138: 81-93.
- Gonzalez A, Piferrer F (2003) Aromatase activity in the European sea bass (Dicentrarchuslabrax L.) brain. Distribution and changes in relation to age, sex, and the annual reproductive cycle, Gen Comp Endocrinol 132: 223-230.
- Goto-Kazeto R, Kight KE, Zohar Y, Place AR, Trant JM (2004) Localization and expression of aromatase mRNA in adult zebrafish, Gen Comp Endocrinol 139: 72-84.
- Huddleston GG, Michael RP, Zumpe D, Clancy AN (2003) Estradiol in the male rat amygdala facilitates mounting but not ejaculation, PhysiolBehav 79: 239- 246.
- Karishma KK, Herbert J (2002) Dehydroepiandrosterone (DHEA) stimulates neurogenesis in the hippocampus of the rat, promotes survival of newly formed neurons and prevents corticosterone-induced suppression, Eur J Neurosci 16: 445-453.
- Kazeto Y, Goto-Kazeto R, Place AR, Trant JM (2003) Aromatase (CYP19A2) in zebrafish and channel catfish brains: changes in expression associated with the reproductive cycle and endocrine disrupting chemicals. Fish PhysiolBiochem 28: 29-32.
- Kazeto Y, Trant JM (2005) Molecular biology of channel catfish brain cytochrome P450 aromatase (CYP19A2): cloning, preovulatory induction of gene expression, hormonal gene regulation and analysis of its promoter region, J MolEndocrinol 35: 569-581.
- Lambert JGD, Wilms PG, ZandwijkML, Goos HJTH, van Oordt PGWJ (1982) Localization of aromatase activity in the brain of the rainbow trout (Sulmoguirdneri). In “Reproductive physiology of fish” (C. J. J. Richter and H. J. Th. Goos, Eds.), Pudoc, Wageningenp. 57.
- Lephart ED (1996) A review of brain aromatase cytochrome P450, Brain Res Rev22: 1-26.
- Marsh KE, Creutz LM, Hawkins MB, Godwin J (2006) Aromatase immunoreactivity in the bluehead wrasse brain, Thalassomabifasciatum:immunolocalization and co-regionalization with arginine vasotocin and tyrosine hydroxylase, Brain Res 1126: 91-101.
- Matsuoka MP, Van Nes S, Andersen O, Benfey TJ, Reith M (2006) Real-time PCR analysis of ovary and brain-type aromatase gene expression during Atlantic halibut (Hippoglossushippoglossus) development, Comp BiochemPhysiol 144B: 128-135.
- McEwen BS, Lieberburg I, Chaptal C, Krey LC (1977) Aromatization : important for sexual differentiation of the neonatal rat brain. HormBehav9: 249-263.
- Melamed, P, Gur G, Rosenfeld H, Elizur A, Yaron Z (1999) Possible interactions between gonadotrophs and somatotrophs in the pituitary of tilapia: apparent roles for insulin-like growth factor I and estradiol, Endocrinology 140: 1183-1191.
- Mellon SH, Vaudry H (2001) Biosynthesis of neurosteroids and regulation of their synthesis, Int Rev Neurobiol 46: 33-78.
- Mensah-Nyagan AG, Do-Rego JL, Beaujean D, Luu-The V, Pelletier G, et al. (1999) Vaudry, H, Neurosteroids : expression of steroidogenic enzymes and regulation of steroid biosynthesis in the central nervous system, Pharmacol Rev 51: 63-81.
- Menuet A, Anglade I, Le Guevel R, Pellegrini E, Pakdel F (2003) Distribution of aromatase mRNA and protein in the brain and pituitary of female rainbow trout: Comparison with estrogen receptor alpha, J Comp Neurol 462: 180-193.
- Menuet A, Pellegrini E, Brion F, Gueguen MM, Anglade (2005) Expression and estrogen-dependent regulation of the zebrafish brain aromatase gene J Comp Neurol 485:304-320.
- Naftolin F, Ryan KJ, Davies IJ, Reddy VV, Flores F, et al. (1975) The formation of estrogens by central neuroendocrine tissues, Recent ProgHorm Res31: 295-315.
- Norris DO (1997) Vertebrate Endocrinology, Academic Press, San Diego, CA.
- Nunez BS, Applebaum SL (2006) Tissue-and sex-specific regulation of CYP19A1 expression in the Atlantic croaker (Micropogoniasundulatus), Gen Comp Endocrinol 149: 205-216.
- Okubo K, Takeuchi A, Chaube R, Paul-Prasanth B, Kanda S (2011) Sex differences in aromatase gene expression in the medaka brain, J Neuroendocrinol 23: 412- 423.
- Pasmanik M, Callard G (1985) Aromatase and 5alpha-reductase in the teleost brain, spinal cord, and pituitary gland, Gen Comp Endocrinol60: 244-251.
- Pasmanik M, Callard GV (1988) Changes in brain aromatase and 5-alphareductase activities correlate significantly with seasonal reproductive cycles in goldfish (Carassiusauratus), Endocrinology 122: 1349-1356.
- Patil JG, Gunasekera RM (2008) Tissue and sexually dimorphic expression of ovarian and brain aromatase mRNA in the Japanese medaka (Oryziaslatipes): Implications for their preferential roles in ovarian and neural differentiation and development, Gen Comp Endocrinol, 158: 131-137.
- Pellegrini E, Mouriec K, Anglade I, Menuet A, Le Page Y (2007) Identification of aromatase-positive radial glial cells as progenitor cells in the ventricular layer of the forebrain in zebrafish, J Comp Neurol 501: 150-16.
- Rasheeda MK, Sridevi P, Senthilkumaran B (2010)Cytochrome P450 aromatases: impact on gonadal development, recrudescence and effect of hCG in the catfish, Clariasgariepinus, Gen Comp Endocrinol 167: 234-245.
- Riters LV, Baillien M, Eens M, Pinxten R, Foidart A, et al. (2001) Seasonal variation in androgen-metabolizing enzymes in the diencephalon and telencephalon of the male European starling (Sturnus vulgaris), J Neuroendocrinol13: 985-997.
- Sakamoto H, Ukena K, Tsutsui K (2001) Effect of progesterone synthesized de novo in the developing Purkinje cell on its dendritic growth and synaptogenesis, J Neurosci21: 6221-6232.
- Okubo K, Takeuchi A, Chaube R, Paul-Prasanth B, Kanda S (2011) Sex differences in aromatase gene expression in the medaka brain, J Neuroendocrinol 23: 412-423.
- Saldanha CJ, Remage-Healey L, Schlinger BA (2011) Synaptocrine signaling: steroid synthesis and action at the synapse, Endocr Rev 32: 532-549.
- Sawyer S, Gerstner KA, Callard GV (2006) Real-time PCR analysis of cytochrome P450 aromatase expression in zebrafish: gene specific tissue distribution, sex differences, developmental programming and estrogen regulation, Gen Com Endocrinol 147: 108-117.
- Strobl-Mazzulla PH, Moncaut NP, López, GC, Miranda LA, CanarioAVM, et al. (2005)Brain aromatase from pejerrey fish (Odontesthesbonariensis): cDNA cloning, tissue expression, and immunohistochemical localization, Gen Com Endocrinol143: 21-32.
- Sundararaj BI (1981) Reproductive physiology of teleost fishes: a review of present knowledge and needs for future research. United Nations Development Programme, Food and Agriculture Organization of the United Nations publisher, Rome.
- Timmers RJ, Lambert JG, Peute J, Vullings HG, van Oordt PG (1987) Localization of aromatase in the brain of the male African catfish, Clariasgariepinus (Burchell), by micro dissection and biochemical identification, J Comp Neurol 258: 368-377.
- Tong SK, Mouriec K, Kuo MW, Pellegrini E, Gueguen MM (2009) A cyp19a1bgfp (aromatase B) transgenic zebrafish line that expresses GFP in radial glial cells. Genesis 47: 67-73.
- Trant JM, Gavasso S, Ackers J, Chung C, Place RA (2001) Developmental expression of cytochrome P450 aromatase genes (CYP19a and CYP19b) in zebrafish fry (Daniorerio), J ExpZool 290: 475-483.
- Tsutsui K, Ukena K, Takase M, Kohchi C, Lea RW (1999) Review: neurosteroid biosynthesis in vertebrate brains, Comp BiochemPhysiol C ToxicolPharmacol 124: 121-129.
- Wang Y, Chan F L, Chen S, Leung LK , The plant polyphenolbutein inhibits testosterone-induced proliferation in breast cancer cells expressing aromatase, Life Sci 77: 39-51.


















