Keywords
Endometrial cancer, E-cadherin, Adherens junction, Histological grade, Prognosis
Introduction
Loss of cell polarity due to disruption of tissue architecture is a hallmark of cancer [1]. Mammalian epithelial tumours lose polarity as they progress toward malignancy, but whether polarity loss might causally contribute to cancer has remained unclear [2-4]. Endometrial cancer is classified into two groupstype I and type II based on pathological histology, as well as molecular pathogenesis and clinical profiles [5]. The endometrial type I cancer is estrogen-dependent of low-grade endometrioid histology and arises in the background of endometrial hyperplasia, its precursor lesion [5]. The type 1 cancer usually occurs in the pre and peri-menopausal women and strongly linked to obesity [5]. The endometrial type II cancer is estrogen-independent tumor with high-grade histology, i.e., high-grade endometrioid, serous and clear cell.
The epithelial-mesenchymal-transition is a major histological feature of human cancer. It has been reported in development of endometrial cancer and its clinical behavior [6,7]. The integrity of adherens junction is critical for maintenance of tissue architecture [8]. E-cadherin is a major component of adherens junction and its loss of expression has been reported to be involved in epithelialmesenchymal- transition [9,10]. Here, we investigated whether loss of E-cadherin is involved in endometrial cancer development.
Methods
Patient characteristics and tumour materials
We analyzed E-Cadherin (CDH-1) expression in 152 cases of endometrial cancer, who underwent surgery at Teikyo University Hospital from January 2003 to December 2012 using immunohistochemical staining. E-Cadherin expression was compared with clinicopathological factors. All patients provided informed consent to participate in this study. Hematoxylin and eosin (H&E)-stained slides of these cases were reviewed. The clinical and pathological characteristics of the cases were obtained from their clinical charts.
Immunohistochemistry (IHC)
Four micrometer-thick paraffin sections from representative tumour blocks were screened for E-Cadherin protein expression using Envision FLEX (DAKO, Glostrup, Denmark). After deparaffinization and warm bath processing, the sections were activated with citric acid buffer and endogenous peroxidase activity was removed by 3% H2O2. After blocking, the sections were incubated for 30 min with primary monoclonal mouse anti- E-cadherin antibody (36B5, NCL-E-Cad, dilution: 1:50; Novocastra, Newcastle, UK), primary monoclonal mouse anti-p53 antibody (DO-7, M7001, dilution: 1:50; DAKO), primary monoclonal mouse anti-estrogen receptor antibody (1D5, M7047, dilution: 1:50; DAKO), or primary monoclonal mouse anti-progesterone receptor antibody (PgR636, M3569, dilution: 1:800; DAKO). The sections were then incubated with a secondary antibody (anti-rabbit, antimouse: DAKO) for 30 minutes. Antibody binding was visualized using a 3,3′-diaminobenzidine solution (DAKO) for 10 minutes. Finally, the tissues were counterstained by standard H&E staining and mounted using a conventional mounting medium. All steps of IHC were performed at room temperature. An additional section was used as a case specific negative control without incubation with the primary antibody. As a positive control, normal endometrial tissue was investigated. The evaluation of E-Cadherin IHC was performed by light microscopy. The patients with endometrial cancer were divided into 3 groups according to the previous report [11]. It was evaluated as homogeneous group when immunostaining of E-Cadherin was observed more than 70% of epithelial cells for each tissue section in (with strong membrane immunostaining), and normal endometrium showed the pattern. It was evaluated as heterogeneous expression when immunostaining of less than 70% of epithelial cells was observed. It was evaluated as negative when immunostaining of no epithelial cells was observed. Homogeneous group was defined as normal expression. Heterogeneous and negative groups were defined as down-regulation.
Statistical analysis
JMP 12 (SAS Institute, Tokyo, Japan) was used for statistical analysis. To analyse correlations between categorized variables, multi-field tables were calculated and interpreted using the Pearson χ2-test of independence. Risk ratios were estimated for clinical and pathological factors. Survival curves were estimated with Kaplan-Meier methods, and the respective curves were tested for significant differences by a log-rank test. Hazard ratios were estimated by a Cox proportional hazards model. The level of statistical significance was set at p<0.05.
Results
E-cadherin expression in normal and malignant endometrial tissues
E-cadherin expression was observed at the membrane in the normal endometrial tissues (Figure 1A). Nest, we analyzed the expression of E-cadherin in 152 endometrial cancer tissues. The membrane-bound expression of E-cadherin in the endometrial cancer was evaluated as homogeneous group (Figure 1B). It was evaluated as heterogeneous expression when immunostaining of less than 70% of epithelial cells was observed (Figure 1C). It was evaluated as negative when immunostaining of no epithelial cells was observed (Figure 1D). The patients with homogeneous E-cadherin expression were categorized as a normal E-cadherin expression group. The patients with heterogeneous or negative expression of E-cadherin were categorized as a E-cadherin downregulation group.
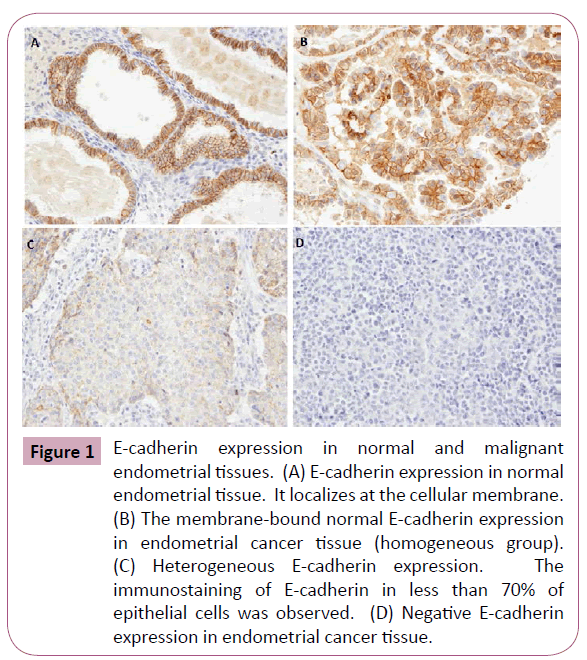
Figure 1: E-cadherin expression in normal and malignant endometrial tissues. (A) E-cadherin expression in normal endometrial tissue. It localizes at the cellular membrane. (B) The membrane-bound normal E-cadherin expression in endometrial cancer tissue (homogeneous group). (C) Heterogeneous E-cadherin expression. The immunostaining of E-cadherin in less than 70% of epithelial cells was observed. (D) Negative E-cadherin expression in endometrial cancer tissue.
Relationship between E-cadherin expression and clinicopathological factors
E-cadherin down-regulation was observed significantly in endometrial cancer with high-grade histology (Table 1). It was frequently observed in cases with advanced clinical stage, deep myometrial invasion, and p53 mutation, but the difference did not reached to the statistical significance (Table 1). Loss of E-cadherin showed no obvious relationship with age at onset, lymph-nodal metastasis, vessel involvement, and estrogen and progesterone receptor expression (Table 1). Next, we evaluated whether E-cadherin down-regulation is involved in prognosis of patients with endometrial cancer. Patients with E-cadherin downregulation showed poorer overall and progression-free survival (Figures 2 and 3).
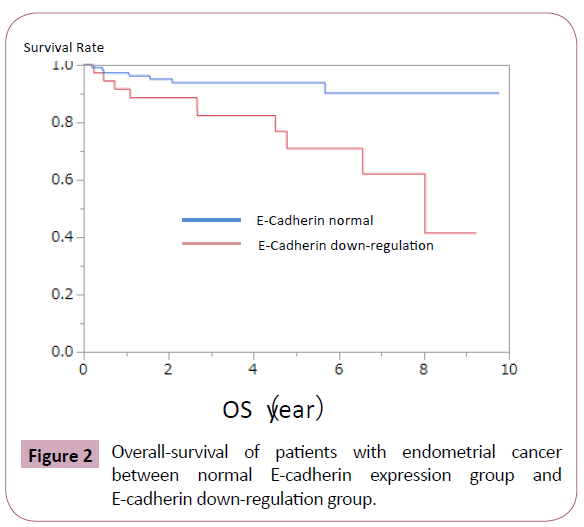
Figure 2: Overall-survival of patients with endometrial cancer between normal E-cadherin expression group and E-cadherin down-regulation group.
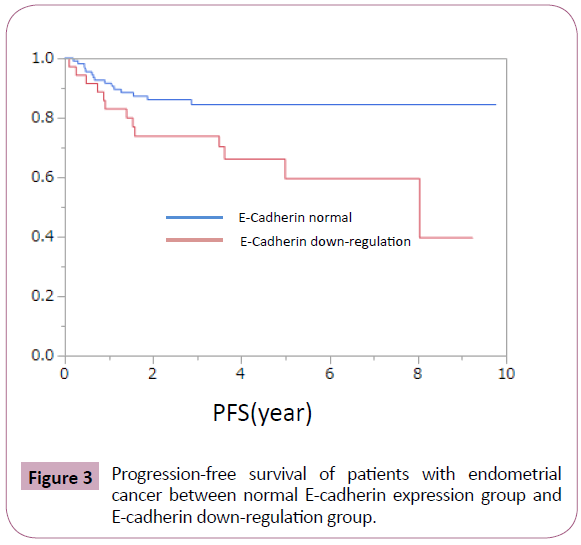
Figure 3: Progression-free survival of patients with endometrial cancer between normal E-cadherin expression group and E-cadherin down-regulation group.
Discussion
The relationship between loss of epithelial polarity and development of malignant tumor has long been known, but it has not been revealed whether loss of tissue architecture have a causative role in tumor development and growth [2]. Adherens junction has an essential role in establishment of cell polarity and tissue architecture [8,9]. Many junctional protein complexes are involved in construction of the adherens junction [9]. E-cadherin has a critical role in establishment of integral adherens junction [8,9]. Loss of E-cadherin expression leads to epithelial-mesenchymal-transition, which is a representative malignant phenotype of human cancer [6,7].
We evaluated E-cadherin expression in 152 endometrial cancer cases. Our analysis revealed that loss of E-cadherin is linked to the high-grade histology (Table 1). Endometrial cancers are subdivided into 2 groups according to clinicopathological factors. Although the type II endometrial cancers contribute only 10% of endometrial cancer incidence, they cause about 50% of disease recurrence [12]. The 5-year survival rate of endometrial cancer is 96% if the cancer diagnosed at a local stage, but decreases to 17% if diagnosed at an advanced stage [13]. Deep myometrial invasion, nodal involvement and distant metastasis worsen prognosis of endometrial cancer [14]. Awareness of the biomarkers that predict prognosis in endometrial cancer is warranted. Recent study showed the genomic features of endometrial cancer permit a reclassification of endometrial cancer patients for their prognosis [15]. The overexpression of p53 based on genetic mutation is a molecular signature of type II endometrial cancer [16,17]. The previous studies and ours suggest the possibilities that loss of E-cadherin is also molecular signature of type II endometrial cancer [11,18-25]. The prognosis of patients with type I endometrial cancer is relatively excellent, whereas prognosis of patients with type II cancer is poor [5]. Survival of patients with loss of E-cadherin was poorer than that with normal E-cadherin expression (Figures 2 and 3). These data also support the possibility that loss of E-cadherin is a key molecular event during development of type II endometrial cancer. Loss of E-cadherin has been reported to be associated with aggressive behavior and metastasis in many human cancers [1,26,27]. These data support the critical significance of loss of tissue architecture in cancer development and tumor progression.
| Clinicopathological factors |
Total |
E-Cadherin normal |
E-Cadherin down regulation |
P-value |
| Overall survival |
|
|
|
0.0019 |
| Progression free survival |
|
|
|
0.0093 |
| Age |
| <50 |
46 |
32(69.6%) |
14(30.4%) |
0.308 |
| ?50 |
106 |
82(77.4%) |
24(22.6%) |
|
| Stage |
| pT1-2 |
123 |
96(78.0%) |
27(22.0%) |
0.0738 |
| pT3-4 |
29 |
18(62.1%) |
11(37.9%) |
|
| Lymphonodal metastasis |
| pN0 |
88 |
66(75.0%) |
22(25%) |
0.3933 |
| pN1 |
27 |
18(66.7%) |
9(33.3%) |
|
| Distant metastasis |
| M0 |
140 |
107 (76.4%) |
33 (23.6%) |
0.1647 |
| M1 |
12 |
7 (58.3%) |
5 (41.7%) |
|
| Vessel involvement |
| - |
93 |
73 (78.5%) |
20 (21.5%) |
0.2116 |
| + |
59 |
41 (69.5%) |
18 (30.5%) |
|
| Myometrial invasion |
| <1/2 |
91 |
73 (80.2%) |
18 (19.8%) |
0.0695 |
| ?1/2 |
61 |
41 (67.2%) |
20 (32.8%) |
|
| Histlogical grade |
| Low grade |
90 |
74 (82.2%) |
16 (17.8%) |
0.0132 |
| High grade |
62 |
40 (64.5%) |
22 (53.5%) |
|
| Estrogen receptor |
| - |
26 |
17 (65.4%) |
9 (34.6%) |
0.2136 |
| + |
126 |
97 (77.0%) |
29 (33.0%) |
|
Table 1: E-cadherin in endometrial cancer.
Conclusion
Our study revealed that loss of E-cadherin is linked to highgrade histology and poor prognosis. It shed light on molecular mechanism of endometrial carcinogenesis. The development of cancer is very complicated and based on multiple steps. Further study will be necessary to fully understand the detail molecular events during endometria carcinogenesis.
8390
References
- Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144:646-674.
- Bilder D (2004) Epithelial polarity and proliferation control: links from the Drosophila neoplastic tumor suppressors. Genes & development 18:1909-1925.
- Bilder D (2001) Cell polarity: squaring the circle. Current biology 11:132-135.
- Humbert PO, Grzeschik NA, Brumby AM, Galea R, Elsum I, et al. (2008) Control of tumourigenesis by the Scribble/Dlg/Lgl polarity module. Oncogene 27:6888-6907.
- Bokhman JV (1983) Two pathogenetic types of endometrial carcinoma. Gynecol Oncol 15:10-7.
- Takai M, Terai Y, Kawaguchi H, Ashihara K, Fujiwara S, et al. (2014) The EMT (epithelial-mesenchymal-transition)-related protein expression indicates the metastatic status and prognosis in patients with ovarian cancer. J Ovarian Res7:76.
- Zhou XM, Zhang H, Han X (2014) Role of epithelial to mesenchymal transition proteins in gynecological cancers: pathological and therapeutic perspectives. Tumour Biol35:9523-9530.
- Harris TJ (2012) Adherens junction assembly and function in the Drosophila embryo. Int Rev Cell Mol Biol 293:45-83.
- Meng W, Takeichi M (2009) Adherens junction: molecular architecture and regulation. Cold Spring Harb Perspect Biol 1:002899.
- Singh M, Darcy KM, Brady WE, Clubwala R, Weber Z, et al (2011) Cadherins, catenins and cell cycle regulators: impact on survival in a Gynecologic Oncology Group phase II endometrial cancer trial. Gynecol Oncol 123:320-328.
- Carico E, Atlante M, Giarnieri E, Raffa S, Bucci B, et al. (2010) E-cadherin and alpha-catenin expression in normal, hyperplastic and neoplastic endometrium. Anticancer Res 30:4993-4997.
- Bansal N, Yendluri V, Wenham RM (2009) The molecular biology of endometrial cancers and the implications for pathogenesis, classification, and targeted therapies. Cancer control : journal of the Moffitt Cancer Center 16:8-13.
- Jemal A, Siegel R, Xu J, Ward E (2010) Cancer statistics, 2010. CA: a cancer journal for clinicians 60:277-300.
- Barrena Medel NI, Herzog TJ, Deutsch I, Burke WM, Sun X, et al. (2011) Comparison of the prognostic significance of uterine factors and nodal status for endometrial cancer. American journal of obstetrics and gynecology 204:1-7.
- Kandoth C, Schultz N, Cherniack AD, Akbani R, Liu Y, et al. (2013) Integrated genomic characterization of endometrial carcinoma. Nature 497:67-73.
- Kovalev S, Marchenko ND, Gugliotta BG, Chalas E, Chumas J, et al. (1998) Loss of p53 function in uterine papillary serous carcinoma. Hum Pathol 29:613-619.
- Zheng W, Khurana R, Farahmand S, Wang Y, Zhang ZF, et al. (1998) p53 immunostaining as a significant adjunct diagnostic method for uterine surface carcinoma: precursor of uterine papillary serous carcinoma. Am J Surg Pathol22:1463-1473.
- Wincewicz A, Baltaziak M, Kanczuga-Koda L, Lesniewicz T, Rutkowski R, et al. (2010) Aberrant distributions and relationships among E-cadherin, beta-catenin, and connexin 26 and 43 in endometrioid adenocarcinomas. Int J Gynecol Pathol29:358-365.
- Sakuragi N, Nishiya M, Ikeda K, Ohkouch T, Furth EE, et al. (1994) Decreased E-cadherin expression in endometrial carcinoma is associated with tumor dedifferentiation and deep myometrial invasion. Gynecol Oncol 53:183-189.
- Ahmed AR, Muhammad EM (2014) E-cadherin and CD10 expression in atypical hyperplastic and malignant endometrial lesions. J Egypt Natl Canc Inst26:211-217.
- Holcomb K, Delatorre R, Pedemonte B, McLeod C, Anderson L, et al. (2002) E-cadherin expression in endometrioid, papillary serous, and clear cell carcinoma of the endometrium. Obstet Gynecol 100:1290-1295.
- Ohishi Y, Kurihara S, Takeuchi T, Aman M, Kaku T, et al. (2012) E-cadherin nuclear staining is useful for the diagnosis of ovarian adult granulosa cell tumor. Hum Pathol43:808-817.
- Varras M, Skafida E, Vasilakaki T, Anastasiadis A, Akrivis C, et al. (2013) Expression of E-cadherin in primary endometrial carcinomas: clinicopathological and immunohistochemical analysis of 30 cases. Eur J Gynaecol Oncol 34:31-35.
- Koyuncuoglu M, Okyay E, Saatli B, Olgan S, Akin M, et al. (2012) Tumor budding and E-Cadherin expression in endometrial carcinoma: are they prognostic factors in endometrial cancer? Gynecol Oncol 125:208-213.
- Ramalingam P, Masand RP, Euscher ED, Malpica A (2015) Undifferentiated Carcinoma of the Endometrium: An Expanded Immunohistochemical Analysis Including PAX-8 and Basal-Like Carcinoma Surrogate Markers. Int J Gynecol Pathol.
- Kim SA, Inamura K, Yamauchi M, Nishihara R, Mima K, et al. (2016) Loss of CDH1 (E-cadherin) expression is associated with infiltrative tumour growth and lymph node metastasis. Br J Cancer 114: 199-206.
- Di Bartolomeo M, Pietrantonio F, Pellegrinelli A, Martinetti A, Mariani L, et al. (2015) Osteopontin, E-cadherin, and beta-catenin expression as prognostic biomarkers in patients with radically resected gastric cancer. Gastric Cancer 11: 1-9.









