Keywords
Colostrum; Liver fibrosis; Pro-oxidants and antioxidants
Introduction
Multiple studies have helped to establish an important role of oxidative stress in occurrence of various pathologies and its participation in the body's adaptation mechanisms [1-5]. However, it is quite difficult to establish a fine border between physiological and pathological functions of resultants of free radical reactions. In addition to comprehension of pathogenesis mechanisms, it is also important for understanding of participation of free radical reactions resultants in physiological processes regulation.
We believe that the best approach to solution of that problem is the use of appropriate biological models and natural substances with antioxidant properties. The liver fibrosis may be used as the model.
It has previously been shown that a multiple sequential administration of copper sulphate to experimental animals was accompanied with development of liver fibrosis, the formation of which occurred against the backdrop of oxidative stress [6]. As we know, liver fibrosis is induced by a variety of physical and chemical factors, medicinal drugs, alcohol [7-9]. At the present time, liver pathologies represent a significant proportion of disability and mortality factors [10] and development of tools and methods to eliminate these pathologies is a relevant objective. Components of colostrum may be used as regulatory factors, including those for the antioxidant system.
It is known that the colostrum has a unique composition of various biologically active compounds [11-13] and is used in treatment of Alzheimer's disease, multiple sclerosis, Crohn's disease, rheumatoid arthritis, and others [14-16]. It was of interest to investigate a possibility for elimination (treatment) of the liver fibrosis with components of bovine colostrum and to define the role of prooxidant-antioxidant system in manifestation of this disease. Along with this, the colostrum contains so-called "heavy" immunoglobulins that cause allergic reactions [17].
Hence lipids and high molecular weight proteins were removed from the colostrum, and low molecular weight components of colostrum (LMWCC) were obtained, which were used in this study. We have previously received a biological substance "Fungidol" or "Mix-factor" of Sacharomyces cerevisue and Pleurotus osteatus cell components, which possesses pronounced anti-oxidant properties on the model of Cu-induced fibrosis [18].
The idea for this paper was that if liver fibrosis is accompanied with development of oxidative stress, and colostrum components enhance the antioxidant activity of the system and eliminate the oxidative stress, elimination of the morphological manifestations which are typical for this pathology can be expected as well. Through a comparative analysis of the effects of "Mix-factor" and LMWCC on the same properties of the pro-antioxidant system, you can establish the specificity of action of those low molecular weight substances.
The paper identifies some somatometric performance of experimental animals (body weight, rectal temperature, ability to perform a task), performance ability, content of lipid hydroperoxides, glutathione peroxidase activity in blood serum, mitochondria, and cytosol of the liver, as well as liver functional activity (content of cholesterol, triacylglycerols, creatinine, and albumin) in animals with a Cu-induced fibrosis.
Method of Investigations
Materials and methods
Experiments were carried out on adult male (3 months of age.) Wistar line, which were divided into 4 experimental groups: control group of animals-intact; group of animals with Cu-induced liver fibrosis; group of animals with fibrosis, which additionally administered per os “Mix factor" in a dose of 0.1 mg/100 g body weight; and a group with fibrosis, which additionally administered per os in different doses LMWCC. Each group consisted of 8-10 animals. The induction of fibrosis and the introduction of biological substances ("Mix Factor" and LMWCC) was carried out according to the scheme shown in Figure 1.
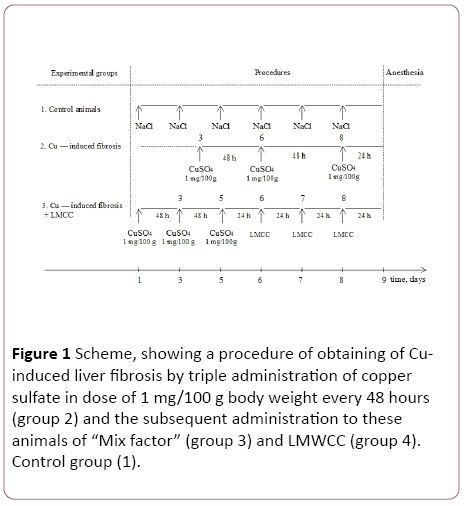
Figure 1: Scheme, showing a procedure of obtaining of Cuinduced liver fibrosis by triple administration of copper sulfate in dose of 1 mg/100 g body weight every 48 hours (group 2) and the subsequent administration to these animals of “Mix factor” (group 3) and LMWCC (group 4). Control group (1).
Identification of some somatometric performance of experimental animals
Experimental animals were regularly weighed at the same time before feeding on 1-th, 3th, 7th, 11th, 13th and 15th days. The changes of temperature determined at the same days using MicroTherma 2T Hand Held Thermometer (Braintree Scientific, Inc., USA). For determination of the ability of experimental animals to do work we used the test "swimming with the load" [19].
An experimental model of induction of fibrosis and a selection of doses of biologically active substances
Previously, it was shown that a three-fold serial intraperitoneal administration of copper sulfate to animals at the dose of 1 mg/100 g of body weight that is 33% of the lethal dose with an interval of 48 hours between injections was accompanied with development of liver fibrosis [20]. Doses of "Mix-factor" and LMWCC were chosen with a somatometric indicators assessment and by reduction in mortality of experimental animals. It was found that a prominent therapeutic effect without causing lethality is caused by the dose of 0.1 ml/100 g of body weight for both "Mix-factor" and for LMWCC.
Analytical definitions
Before killing, animals were anesthetized. Blood was collected into dry centrifuge tubes, incubated 30 min at 20°C, and centrifuged for 15 min at 1500 g. The protein concentration was determined by the Lowry method [21].
When slaughtering animals, blood was collected and serum was prepared. We recovered liver by perfusion with cold saline, homogenized in 0.25 M sucrose solution containing 10 mM EDTA at pH 7.4 4°C to 6°C.
The mitochondria were isolated by differential centrifugation as described [22]. Microsomal fraction was pelleted by centrifugation at 90,000 g for 90 minutes, and the supernatant used as the cytosol fraction. Determination of lipid hydroperoxides were carried out as described [23], and glutathione peroxidase activity were determined by method [24].
Determination of content of cholesterol, triacylglycerols, creatinine and albumin were determined in the blood serum using biochemical analyzer Beckman Coulter AU480 (Germany).
Statistical analysis
The results obtained were processed statistically using the Wilcoxon-Mann-Whitney and Student's t-test, using the software package "Statistika V.6". Results were considered as statistically significant at p<0.05.
Results of the Study
Influence of LMWCC and the "Mix-factor" on some somatometric indicators of animals with Cu-induced fibrosis
Change in body weight depends on diet, age, genetic features, and all those factors which directly or indirectly affect the rate of metabolism. In particular, pathologies that affect these indicators will influence the dynamics of growth as well (Figure 2).
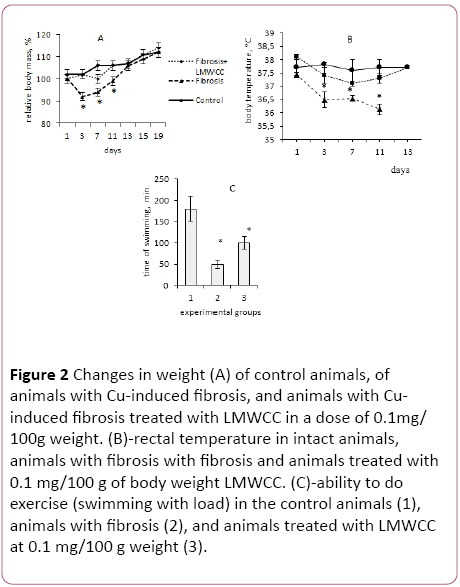
Figure 2: Changes in weight (A) of control animals, of animals with Cu-induced fibrosis, and animals with Cuinduced fibrosis treated with LMWCC in a dose of 0.1mg/ 100g weight. (B)-rectal temperature in intact animals, animals with fibrosis with fibrosis and animals treated with 0.1 mg/100 g of body weight LMWCC. (C)-ability to do exercise (swimming with load) in the control animals (1), animals with fibrosis (2), and animals treated with LMWCC at 0.1 mg/100 g weight (3).
The intensity of growth of 3-month old males from the 1st to the 15th day of observations, which corresponded to the time of fibrosis induction, followed by administration of biologically active substances, was in line with standard indicators. During that period, their weight increased by 12%, which was taken as 100%.
Administration of copper sulfate to animals at the dose of 1 mg/100 g of body weight, which corresponds to 33% of the lethal dose, resulted in a sharp and significant weight loss. Already on the 3rd day after administration of copper sulfate, they lost 10 g of their weight (Figure 2A). After repeated administration of copper sulfate the process of weight loss is less pronounced, indicating the induction of adaptive response in those animals. These results confirm a previously established fact of induction of the hormesis effect to that toxicant [25].
In that case, the animals against the backdrop of copper sulphate introduction were receiving LMWCC at the dose of 0.1 mg/100 g of body weight, then on day 3 a slight growth retardation was observed in them, and they lost only 2% to 3% of the initial body weight later on the 7th day, and after that they did not differ from intact control in the growth intensity (Figure 2A).
If the animals received a twice lower dose of LMWCC (0.05 g/100 g of body weight), they gained weight slower from the 7th to the 19th day than at the dose of (0.1 g/100 g of body weight). These results strongly suggest that LMWCC at the dose of 0.1 g/100 g of body weight eliminated an inhibitory effect of copper sulphate on the dynamics of growth of the animals.
In the next series of experiments, the change of rectal temperature in experimental animals was determined as an indicator of changes in the overall metabolism in those animals. The body temperature in the group of intact animals during the period 1 to 13 day of the experiment remained the same, or rather kept within the physiological range (Figure 2B).
At the same time, the body temperature dropped to 1°C in animals with Cu-induced fibrosis, and this decrease was stable for at least 11 days from the first administration of copper sulfate (Figure 2B). If the animals with fibrosis received LMWCC at the dose of 0.1/100 g of body weight, their body temperature was not significantly different from the intact control and was significantly higher than in the animals with fibrosis (Figure 2B).
Consequently, a relationship between weight loss and decrease in rectal body temperature was observed for animals with Cu-induced liver fibrosis. This can be interpreted as a general metabolic inhibition by copper ions in these animals. Three administrations of LMWCC at intervals of 24 hours between doses to animals with liver fibrosis, eliminated a decrease of temperature and reduced the rate of body weight loss in those animals. If this is the case, it can be expected that it will affect the animals' ability to perform work.
In the next series of experiments the ability of these groups of animals to perform work was determined in the test of swimming with a load. It was found that performance in groups of animals with fibrosis was significantly lower than that of the control animals. Administration of LMWCC significantly increased their performance ability (Figure 2C). These results support the conclusion about the ability of the LMWCC to eliminate the inhibition of the total metabolism of copper ions.
Lipid hydroperoxides content and glutathione peroxidase activity in the serum of experimental animals
The content of lipid hydroperoxides (LH) in the blood serum of animals with fibrosis was increased by 90% in comparison with the control (Figure 3).
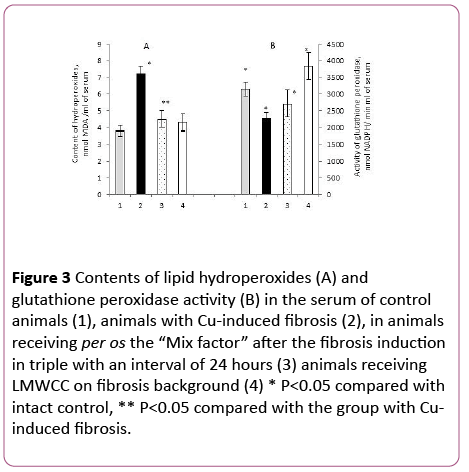
Figure 3: Contents of lipid hydroperoxides (A) and glutathione peroxidase activity (B) in the serum of control animals (1), animals with Cu-induced fibrosis (2), in animals receiving per os the “Mix factor” after the fibrosis induction in triple with an interval of 24 hours (3) animals receiving LMWCC on fibrosis background (4) * P<0.05 compared with intact control, ** P<0.05 compared with the group with Cuinduced fibrosis.
These results are consistent with a previously established fact about manifestation of an oxidative stress against the backdrop of Cu-induced fibrosis [6].
In the case with animals with liver fibrosis that received the “Mix-factor”, their lipid hydroperoxides content in the serum was lower by 37% in comparison to those with fibrosis and little different from the intact control (Figure 3A). Administration of LMWCC to animals with fibrosis at the dose of 0.1 mg/100 g of body weight also resulted in decrease of lipid hydroperoxides content in the serum by 40% (Figure 3A).
Therefore, both the “Mix-factor” and LMWCC reduce a development of oxidative stress, which was induced by copper ions. Such antioxidant effect of the “Mix-factor” and LMWCC can be achieved by various mechanisms and one of them is an increase in activity of antioxidant enzymes.
Earlier studies demonstrated that the most pronounced correlation between the content of Lipid Hydroperoxides and antioxidant enzymes was shown by glutathione peroxidase (GP) [26]. It was found that administration of the "Mix-factor" to animals with induced fibrosis slightly increased GP activity, and the activity of GP against the backdrop of LMWCC increased by 68% in comparison with the group of animals with fibrosis (Figure 3B).
At the same time the GP activity after the administration of LMWCC and the "Mix-factor" was not different from the intact control (Figure 3B). Therefore, the "Mix-factor" and especially LMWCC increased GP activity against the backdrop of fibrosis and, as a consequence, reduced the lipid hydroperoxides content in the serum.
Influence of LMWCC on the antioxidant system suggests that biologically active substances can be attributed to natural antioxidants. In this regard, it was necessary to establish a dose-dependent influence of LMWCC on the content of lipid hydroperoxides and the GP activity.
After administration of LMWCC to the animal group with fibrosis at the dose of 0.01 mg/100 g of body weight the lipid hydroperoxides content decreased by 43% in comparison with the group of animals with fibrosis without admission of LMWCC. Increase of the doses up to 0.05 mg/100 g and 0.1 mg/100 g of body weight produced the same effect. On the contrary, the dose of 1 mg/100 g of body weight was accompanied by an increase of lipid hydroperoxides content in the serum by 109% in respect to low doses (Figure 4).
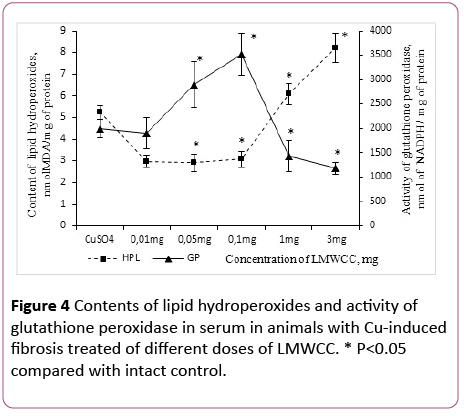
Figure 4: Contents of lipid hydroperoxides and activity of glutathione peroxidase in serum in animals with Cu-induced fibrosis treated of different doses of LMWCC. * P<0.05 compared with intact control.
In that case, if the group of animals with fibrosis was treated with LMWCC at the dose of 3 mg/100 g, the lipid hydroperoxides content in the serum increased by 56% in comparison with those with fibrosis and by 114% in comparison with the intact control (Figure 4).
At the same time the GP activity in the serum was unchanged with administration of LMWCC at the dose of 0.01 mg/100 g in comparison with the fibrosis and was lower than the intact control by 39% (Figure 4). Increasing in the dose of LMWCC up to 0.05 mg/100 g to 0.1 mg/100 g was accompanied by an increased activity of the GP, but at the doses of 1.3 mg/100 g its activity decreased sharply (Figure 4). Thus, it was even lower than that of fibrosis for the dose of 3 mg/100 g of body weight by 41% and by 62% in comparison with the untreated control (Figure 4).
Consequently, there is an S-shaped relationship between the activity of glutathione peroxidase and lipid hydroperoxides content in the serum, antioxidant activity is witnessed at low doses of LMWCC (0.01-0.1) mg/100 g of body weight), and prooxidant activity at higher ones (1-3) mg/100 g of body weight).
As it is known, the main place of LH formation in the cell is mitochondria [27]. In the next series of experiments the LH content and the GP activity in liver mitochondria were determined. The content of lipid hydroperoxides and the activity of glutathione peroxidase in animals with induced fibrosis, and after the effect of the "Mix-factor" and LMWCC in liver mitochondria
The LH content in liver mitochondria of rat with Cu-induced fibrosis was increased by 83% as compared to liver mitochondria in the intact animals (Figure 5).
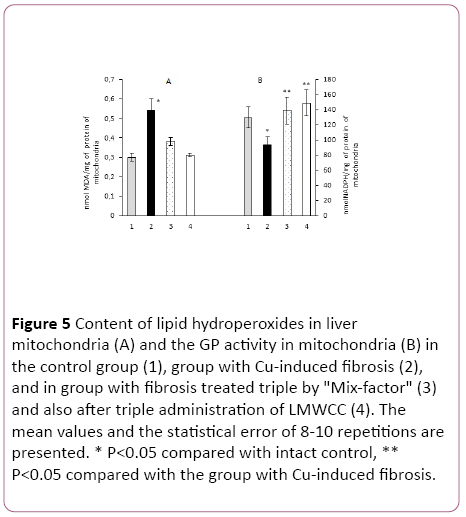
Figure 5: Content of lipid hydroperoxides in liver mitochondria (A) and the GP activity in mitochondria (B) in the control group (1), group with Cu-induced fibrosis (2), and in group with fibrosis treated triple by "Mix-factor" (3) and also after triple administration of LMWCC (4). The mean values and the statistical error of 8-10 repetitions are presented. * P<0.05 compared with intact control, ** P<0.05 compared with the group with Cu-induced fibrosis.
If the animals with fibrosis received "Mix-factor" at the dose of 0.1 mg/100 g of body weight, the LH content in mitochondria was reduced by 45%, and after administration of LMWCC to such animals with fibrosis by 71% in comparison with the group of animals with fibrosis, that matched the values in the serum (Figure 5).
GP activity in mitochondria with fibrosis with an administered "Mix-factor" was higher by 48% in comparison to those with fibrosis, and in the case of LMWCC by 59%. Activity of LH in groups of animals treated with the "Mix-Factor" and LMWCC did not differ among them (Figure 5).
If we evaluate the antioxidant activity of liver mitochondrial system for the ratio of Glutathione Peroxidase/Lipid Hydroperoxides, administration of the "Mix-factor" to animals with fibrosis was accompanied by an increase by 2.1 times and after LMWCC by 2.8 times, which corresponded to that in the blood serum.
At the same time, the "Mix-Factor" and LMWCC had no effect on activity of another antioxidant enzyme-glutathione reductase (GR).
These results may indicate that the investigated low molecular weight substances of colostrum have a "selective" effect on the elements of antioxidant protection and form a specific pattern of its many elements (Table 1).
| Variant of experiment |
Activity of glutathione reductase. nmol NADPH / min x mg of protein of mitochondria |
| Cu-inducted fibrosis |
34.7 ± 5.9 n=8 |
| Fibrosis after administration of «Mix-factor» |
32.1 ± 5.0 n=8 |
| Fibrosis after administration of LMWCC |
31.2 ± 2.7 n =10 |
Table 1: The activity of glutathione reductase in animals with Cu-induced fibrosis, induced fibrosis treated "Mix Factor" and with induced fibrosis treated with LMWCC. Presents the average values of 8-10 repetitions.
To confirm the effect of the test substances on GP and glutathione reductase in the next series of experiments their activity in the cytosolic fraction of the liver was determined.
Currently there are 7 known isoforms of GP that localize in various cell compartments. These isoforms vary in activity and a degree of response to different exposures [28,29].
It turned out that after administration of "Mix-factor" and LMWCC to animals with Cu-induced fibrosis the glutathione peroxidase activity increased equally by 38% and 36% and the glutathione reductase activity remained unchanged in comparison to the group of animals with fibrosis (Figure 6).
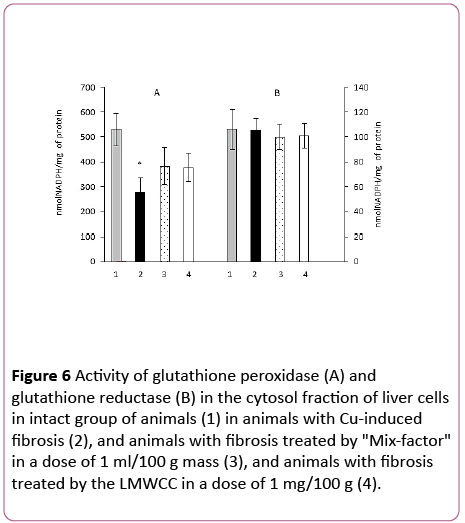
Figure 6: Activity of glutathione peroxidase (A) and glutathione reductase (B) in the cytosol fraction of liver cells in intact group of animals (1) in animals with Cu-induced fibrosis (2), and animals with fibrosis treated by "Mix-factor" in a dose of 1 ml/100 g mass (3), and animals with fibrosis treated by the LMWCC in a dose of 1 mg/100 g (4).
Although an increase in the GP activity in the cytosol fraction manifested at a lesser extent than in the serum and mitochondria, it also took place, while the glutathione reductase activity remained unchanged.
Therefore, small doses (0.1 mg/100 g of body weight) of "Mix-factor" and LMWCC increased the activity of glutathione peroxidase localized in the blood serum, mitochondria and cytosol fraction of the liver and did not affect the activity of glutathione reductase and did not reduce lipid hydroperoxides that occurred with recovery of the body weight and temperature of animals with Cu-induced fibrosis.
Characteristics of some of morphological and biochemical indicators of liver functional activity in Cu-induced fibrosis
For over 50 years, the most reliable indicator in determining the stage of fibrosis has been a morphological analysis of the liver. But because the liver biopsy is associated with a risk of hypotension, internal bleeding, damage to the biliary system and the frequency of hospitalization after biopsy is 1% to 5%, with complications up to 0.57% of the cases [30], noninvasive methods of analysis are used more often.
The main group of noninvasive methods of analysis is related to assessment of the functional activity of the liver. The AAR, Shasta index, BARD score and others are the most frequently used tests. Most of the tests include: an activity of aspartate, and of aminotransferases, a content of albumin, cholesterol, etc. However, all these functional tests can usually detect a progressive fibrosis or cirrhosis.
Morphological changes in the liver were observed within 24 hours after the last administration of copper sulfate. Thus, hepatocytes with elements of necrosis and apoptosis were diffusely encountered. The most pronounced changes were observed in the morphology of the capsule. It was severely thickened with a large number of fibroblasts, lymphocytes, monocytes, etc. Vascular endothelium was damaged; there was a hemorrhage in the liver parenchyma and a collagen accumulation (Figure 7).
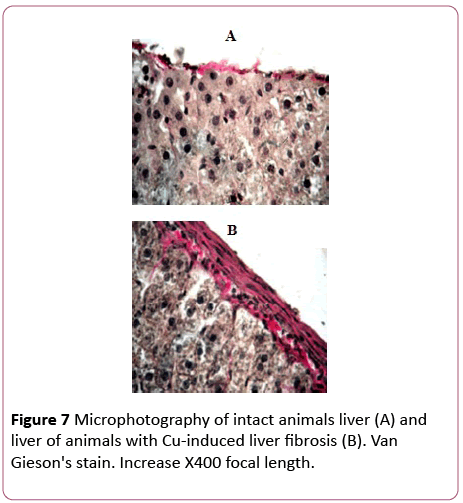
Figure 7: Microphotography of intact animals liver (A) and liver of animals with Cu-induced liver fibrosis (B). Van Gieson's stain. Increase X400 focal length.
A collagen content was increased by 30% in comparison with the control. Therefore, triple administration of copper sulphate to animals was accompanied with morphological changes that are characteristic of an inflammatory process, and a slight increase in collagen content that indicates a development of an early stage liver fibrosis [31].
In the next series of experiments indicators of a hepatic functional activity after three administration of copper sulfate were determined.
The content of cholesterol, triacylglycerol, albumin, and creatinine were similar in all animal groups that were studied (Table 2). These results indicate that the functional activity of the liver after 3 successive injections of copper sulfate, and also the administration of LMWCC did not differ from the control, even against the backdrop of morphological changes and increased production of connective tissue in the liver.
| Group |
Parameter |
| Cholesterol |
Triacylglycerides |
Creatinine |
Albumin |
| Intact |
1.6 ± 0.2 |
0.77 ± 0.13 |
69.7 ± 15.1 |
32.3 ± 0.4 |
| Fibrosis |
1.2 ± 0.4 |
0.79 ± 0.27 |
50.0 ± 3.9 |
29.3 ± 2.6 |
| Fibrosis with administration of LMWCC |
1.5 ± 0.5 |
1.0 ± 0.48 |
93.0 ± 20.0 |
33.5 ± 4.0 |
| Fibrosis with administration ofMix factor |
1.2 ± 0.2 |
0.91 ± 0.84 |
49.0 ± 2.6 |
30.3 ± 2.3 |
Table 2: Contents of various biochemical parameters in experimental groups of animals.
Therefore, the experimental animals had initial stages of development of liver fibrosis.
Discussion
The results showed that 1) already 24 hours after the last administration of triple administration of copper sulfate at the dose of 33% of the lethal one the oxidative stress is induced at the liver level and of the body in general; b) the growth of animals is inhibited and their body temperature is lowered by 1°C, and an ability of the animals to perform work is reduced; c) a collagen content is increased and the morphology of the liver changes, which is characteristic of initial stages of development of fibrosis; d) wherein the main functional characteristics of the liver remain at the level of the control animals.
2) per os administration of LMWCC and the "Mix-factor" at the dose of 0.1 mg/100 ml and 0.1mg/100 g of body weight three times with intervals of 24 hours between the doses to animals with fibrosis at early stages of its development was accompanied with increase of activity of the antioxidant enzyme-glutathione peroxidase in the serum, liver mitochondria, and to a lesser extent in liver cytosol fraction; b) elimination of oxidative stress (the content of hydroperoxides is not different from the control; c) normalization of growth indicators and a body temperature and partly of animal performance capability.
3) Low molecular weight components obtained from various sources (from cow colostrum or from Pleurotus ostreatus fungi) provide an offset in the balance of prooxidant ↔ antioxidant system toward antioxidants, and eliminate morpho-physiological manifestations that are characteristic of liver fibrosis.
For many years, it has been believed that fibrosis is an irreversible pathological condition that is accompanied with development of connective tissue, loss of functional activity of the body and development of liver cirrhosis.
Currently, there are works where a possibility of the treatment of fibrosis was demonstrated, i.e., it may be reversible [32]. However, a successful treatment of this pathology is not always achieved. We believe it depends on the timing of the induction of the fibrosis stage. As it is known, clinicians distinguish 4 stages in development of fibrosis [33]. We have developed a model of Cu-induced fibrosis, according to a functional activity of the liver, the morphological characteristics of that organ, and activity of the proantioxidant system can be attributed to initial stages of development of this pathology.
These results suggest that body intoxication from copper ions is accompanied by severe systemic response which manifests itself at various levels of the organization. The relationship between these levels is complex and diverse. In particular, there is no direct connection between a manifestation of oxidative stress and an inhibition of body weight growth intensity and the body temperature. Since the inhibition of the total metabolism, as evidenced by a decrease in body temperature, should be accompanied with a decrease in respiration rate, then, as a consequence, free radical production should decrease, and in this case, there was a significant increase (90%) of lipid hydroperoxides against the backdrop of general metabolism inhibition. This situation can be explained by a pronounced inhibition by copper ions of not only the general metabolism, but the activity of antioxidant enzymes as well, which was accompanied by a significant increase in a number of products of free radical reactions due to lower respiration rate and overall metabolism.
Such metabolic and physiological situation can be explained by the fact that immediately after administration (24 hours after the last dose) by means of binding to proteins the copper ions inhibit the activity of not only the antioxidant enzymes, but the action of a wide range of other proteins, which may bring to inhibition of protein synthesis [34], resulting in a "aversion" to food, disability of the digestive system or contractibility.
From then on, there is formation of a new metabolic pattern which is "directed" to the survival of the organism in such extreme conditions, i.e., they have the adaptive nature of the reaction.
Firstly, this is due to a diverse regulatory role of free radical products.
So, an increase in free radical reactions products is accompanied not only by oxidative stress, but also by activation of cellular immunity that is essential for survival of the organism.
It is known that phagocytic cells such as neutrophils, "use" an oxidative stress as a method for inactivation of bacterial cells. An increase in phagocytic activity of neutrophils was observed against the backdrop of Cu-induced liver fibrosis, which was shown in [6].
Secondly, products of free radical reactions activate stellate cells which play a central role not only in development of fibrosis, but in subsequent choice of a response strategy to inflammation. It is exactly the stellate cells that produce connective tissue matrix in liver under fibrosis and they also produce matrix metalloproteinases that are able to ensure the hydrolysis of connective tissue after the acute phase [9].
Available data suggest that it is possible to provide a selection of one or another direction for inflammatory processes development by regulation of the function of stellate cells.
Of course, stellate cells are sensitive to a large number of pro-inflammatory cytokines (IL-1, TNF2, NO, TgFg3-1, platelet activating factor, etc.), and other regulatory molecules.
However, the available data suggest that one of the factors in starting the cascade mechanisms of regulation of the stellate cells activity is products of free radical reactions.
And finally, a formation of the hormesis effect to copper sulphate may give evidence in favor of an adaptive oxidative stress which occurs in response to administration of copper sulphate [25].
So, if the experimental animals are successively administered copper sulphate three times with intervals of 48 hours at the dose of 33% of the lethal one, they have developed liver fibrosis [6]. If these animals against the backdrop of liver fibrosis are administered a lethal dose of copper sulfate, all animals survive well, whereas in the control group 100% mortality was observed within 1 hour after administration of that toxicant [25]. Therefore, formation of liver fibrosis was accompanied by such reorganization of the system of copper ions deposition (copper binding proteins) and increase in the detoxification function that even a subsequent administration of lethal doses did not lead to death of the animals.
The results and data available in the literature suggest that the most pronounced changes in this series have been associated with activation of the prooxidant system and such reaction possesses a pronounced adaptive nature.
Therefore, multiple sequential administration of copper sulphate to experimental animals was accompanied with its binding with a variety of proteins in the body and in liver cells that have been shown in our work [25], and they form a specific physiological and metabolic pattern that has an adaptive nature. However, in spite of the adaptive nature of those changes, such metabolic system is extremely unstable and in future they will "choose" one of the possible response strategies.
The choice of the strategy for further response will be influenced by a complex of factors. We believe that if a number of products of free radical reactions will decrease after some time, the metabolism will be similar to the metabolism of the intact animals. In the event when an oxidative stress will persist for a long time (presently we do not know this amount of time), the strategy of fibrogenesis will be chosen. This is supported by the results of the present work.
In the event when during that time (24 hours after the last administration of copper sulfate) low molecular weight components of colostrum or low molecular weight components of fungi and yeasts were begun to be administered to the animals it was accompanied with activation of antioxidant enzyme-glutathione peroxidase, reduction of lipid hydroperoxides in mitochondria and serum down to the level of the control group, and the body temperature, the growth rate, and partially the performance ability were restored against this backdrop. Therefore, a physiological and metabolic pattern is not very different from that of the control animals.
This effect of LMWCC and the "Mix-factor", which are derived from different sources on the pro-antioxidant system, can be explained by means of several mechanisms.
Firstly, there are components with antioxidant properties included in the LMWCC and the "Mix-factor", however, to determine their contribution to elimination of the oxidative stress in an in vivo system is extremely difficult. Previously it has been shown that the components of the "Mix-factor" exhibit an antioxidant activity comparable with tocopherol in the in vitro environment [18].
Secondly, there are immunostimulants also among the components of colostrum, which may have a direct effect on the immune system [17].
Finally, low molecular weight components of colostrum, and also of the "Mix-factor" are capable to perform regulatory effects as well as growth factors [18]. We can believe that a biological system response to low molecular weight components will depend not only on their properties and composition, but primarily on a state of the biological system and of course, their concentrations.
As the present paper showed after the introduction of LMWCC in various doses there was a pronounced monomodal dose-dependent response in activity of the antioxidant enzyme-glutathione peroxidase and in the content of lipid hydroperoxides (Figure 4).
Conclusion
The results showed that LMWCC obtained from various sources (from cow colostrum or from Pleurotus ostreatus fungi, or from yeast) activated antioxidant defense enzymes (glutathione peroxidase), eliminated oxidative stress in animals with Cu-induced liver fibrosis. These biochemical changes in the body of animals with fibrosis were accompanied by normalization of physiological characteristics (body temperature, growth rate of animals).
18953
References
- Thanan R, Oikawa S, Hiraku Y, Ohnishi S, Ma N, et al. (2015) Oxidative stress and its significant roles in neurodegenerative diseases and cancer. Int J Mol biol Sci 16: 193-217.
- Kim YW, West XZ, Byzova TV (2013) Inflammation and oxidative stress in angiogenesis and vascular disease. J Mol Med (Berl) 9: 323-328.
- Daffu G, del Pozo CH, O’Shea KM, Ananthakrishnan R, Ramasamy R, et al.(2013) Radical roles for RAGE in the pathogenesis of oxidative stress in cardiovascular diseases and beyond. Int J Mol Sci 14: 19891-19910.
- Cichoż-Lach H, Michalak A (2014) Oxidative stress as a crucial factor in liver diseases. World JGastroenterol 20: 8082-8091.
- Indo HP, Yen HC, Nakanishi I, Matsumoto KI, Tamura M (2015) A mitochondrial superoxide theory for oxidative stress diseases and aging.JClinBiochem Nutr 56: 1-7.
- Bozhkov AI, Nikitchenko YI, Klimova EM, Lenkevich ES, Lebid EN, et al. (2016) Young and old animals use different metabolic strategies to adapt to the Cu-induced fibrosis of the liver. The successes of gerontology 29: 555-566.
- Vinken M, Maes M,Vanhaecke, T, Rogiers V (2013) Drug-induced liver injury: Mechanisms, types and biomarkers. Curr Med Chem 20: 3011-3021.
- Lim JK,Tate JP,Fultz SL, Goulet JL,Conigliaro J, et al.(2014) Relationship between alcohol use categories and noninvasive markers of advanced hepatic fibrosis in HIV-infected, chronic hepatitis C virus–infected, and uninfected patients. Clin Infec Dis 58: 1449-1458.
- Pellicoro A, Ramachandran P, Iredale JP, Fallowfield JA(2014) Liver fibrosis and repair: Immune regulation of wound healing in a solid organ. Nat Rev Immunol 14: 181-194.
- Hernandez-Gea V, Friedman SL (2010) Pathogenesis of liver fibrosis athology: Mechanisms of disease. Annu Rev Pathol 6: 425-456.
- Fox PF, Uniacke-Lowe T, McSweeney PLH, O’Mahony JA ( 2015) Dairy chemistry and biochemistry: Springer international Publishing Switzerland, pp: 567.
- Steele J, Sponseller J, Schmidt D,Cohen O,Tzipori S (2013) Hyperimmune bovine colostrum for treatment of GI infections. J Hum VaccineImmunother 9: 1565-1568.
- McGrath BA, Fox PF, McSweeney PLH, Kelly AL(2016) Composition and properties of bovine colostrum: A review. Dairy SciTechnol 96: 133-158.
- Sacerdote P, Mussano F, Franchi S,Panerai AE, Bussolati G, et al. (2013) Biological components in a standardized derivative of bovine colostrum. J Dairy Sci 96: 1745-1754.
- Rathe M,Müller K,Sangild P (2014) Clinical applications of bovine colostrum therapy. Nutr Rev 72: 237-254.
- Pareek AK, Garg S, Kumar M, Yadav Sm (2015) Colostrum: An essential component for physical and mental growth and its role in disease prevention. Indo Am J Pharm Res5: 1044-1052.
- HurleyWL, Theil PK (2011) Perspectives on immunoglobulins in colostrum and milk. Nutrients 3: 442-474.
- Kurguzova NI, Bozhkov AI, Nikitchenko YV, Al Begai MAY,Goltvyansky AV, et al. (2015) Interconnection of antitoxic and antioxidant systems of the organism under the action of natural low molecular complex-Fungidol. Am J Biomed Life Sci 2: 25-32.
- Dawson CA, Horvath SM (1970) Swimming in small laboratory animals. Med Sci in Sports2: 51-78.
- Bozhkov AI, Kurguzova NI, Krivoruchko TV, Lebed’ EN, Mikhailets AO, et al. (2014) A cyclic feeding regime: A new model in experimental Gerontology. Adv Gerontol4: 252-259.
- Lowry OH, Rosebrough NJ, Farr Al, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265-275.
- KamatchSA (1972) InteractionofCa2+with endoplasmatic reticulum of rat liver:A standart procedure forthe isolation of microsomes. AnalBiochem 48: 53-61.
- Ohkawa H (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95: 351-358.
- Paglia DE, Valentine WN (1967) Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J LaboratClinMed 70: 158-169.
- Bozhkov AI, Sidorov VI, Kurguzova NI, Dlubovskaya VL (2014) Metabolic memory enhances the effect of hormesis to copper ions and has a character of age. Uspekhi gerontologii (in Russian) 1: 72-80.
- Bozhkov, Nikitchenko YV, Lebed’ KN, Linkevych OS, Kurguzova NI, et al. (2016) The cyclic feeding regime induces decaying age-dependent oxidative stress and regulates the cell chain of the immunity. Adv Aging Res5: 151-165.
- Ott M, Gogvadze V, Orrenius S, Zhivotovsky B (2007) Mitochondria, oxidative stress and cell death. Apoptosis 12: 913-922.
- Menshchikova EB, Lankin VZ, Zenkov NK (2006) Oxidative stress. Pro-oxidants and antioxidants, pp: 556.
- Kalinina EV, Chernov NN, Novikov MD (2014) The role of glutathione, glutathione and glutaredoxin in the regulation of redox-dependent processes. Adv Biol Chem 54: 299-348.
- West J, Card TR (2010) Reduced mortality rates following elective percutaneous liver biopsies. Gastroenterology139:1230-1237.
- Bozhkov AI, Linkevych OS, Ivanov EG, Klimova OM, AlBegai MAY (2016) Low molecular weight components of colostrum regulate the activity of cellular component of the immune system in animals with Cu-induced liver fibrosis. Int J Curr Res 8: 44129-44137.
- Friedman SL, Bansal MB (2006) Reversal of hepatic fibrosis-Fact or fantasy?. Hepatology43: 82-88.
- Paradis V, Zalinski S, Chelbi E, Guedj N, Degos F, et al. (2009) Hepatocellular carcinomas in patients with metabolic syndrome often develop without significant liver fibrosis: A pathological analysis. Hepatology 49: 851-859.
- Halliwell B, Gutteridge JMC (2015) Free Radicals in Biology and Medicine. Oxford: Oxford University Press pp:905.












